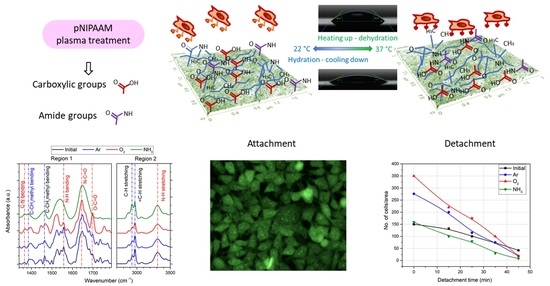
Chemistry-Induced Effects on Cell Behavior upon Plasma Treatment of pNIPAAM
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Material Investigations
2.3. In Vitro Studies
3. Results
3.1. Material Characterization
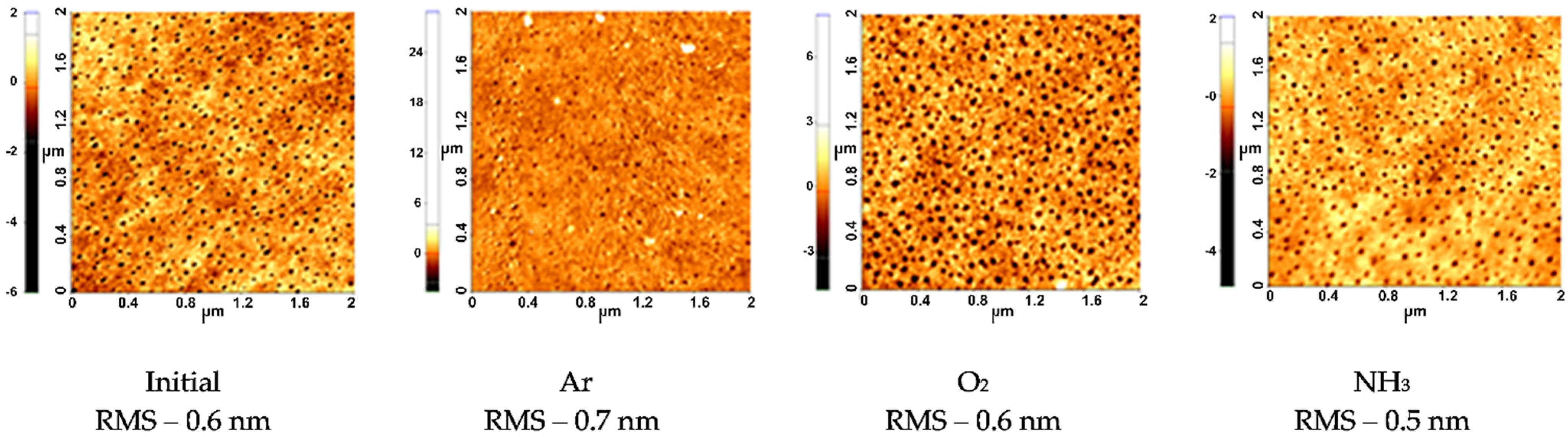
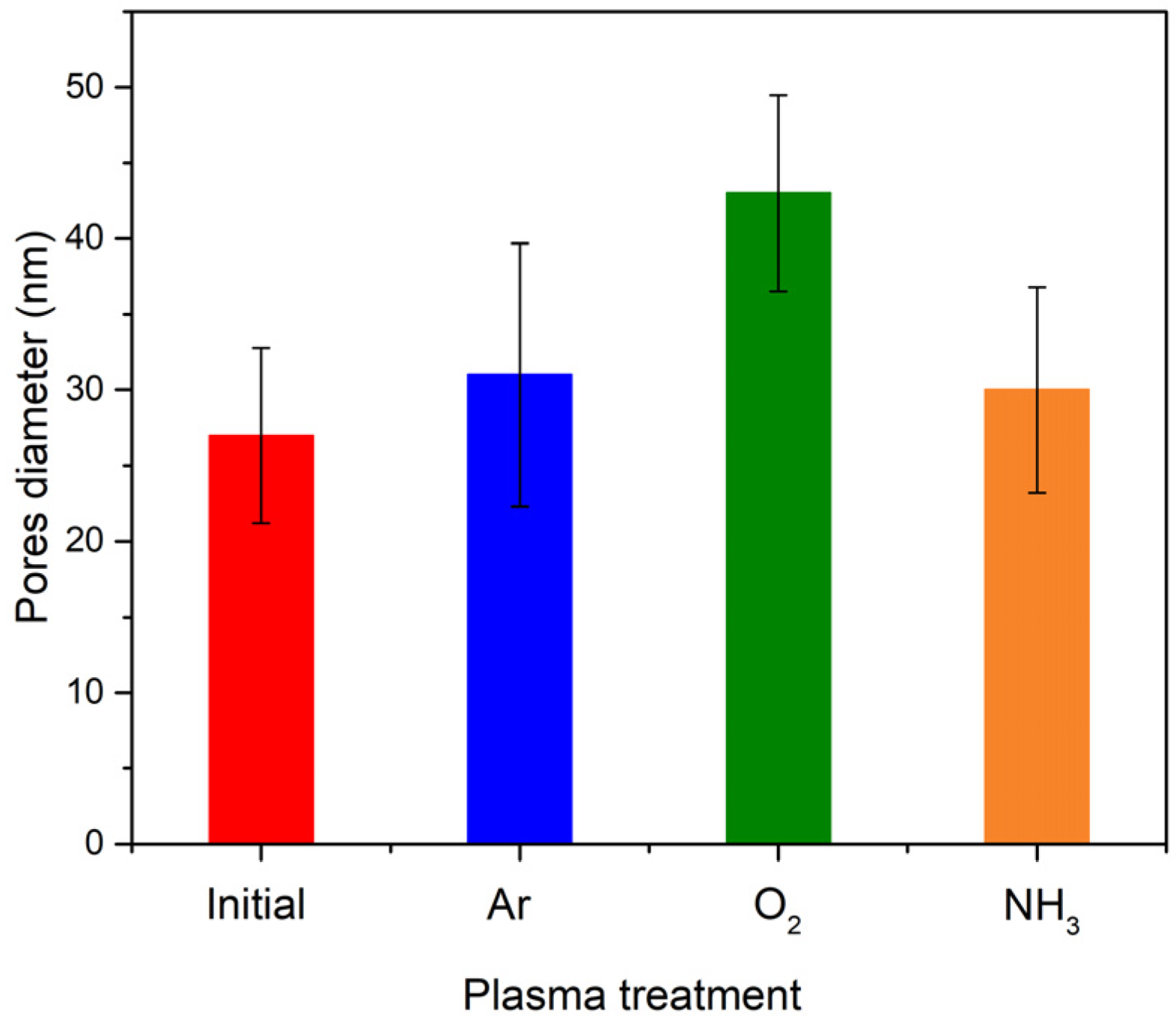
3.1.1. Topographical Characterization of the Plasma Treated pNIPAAM Coatings
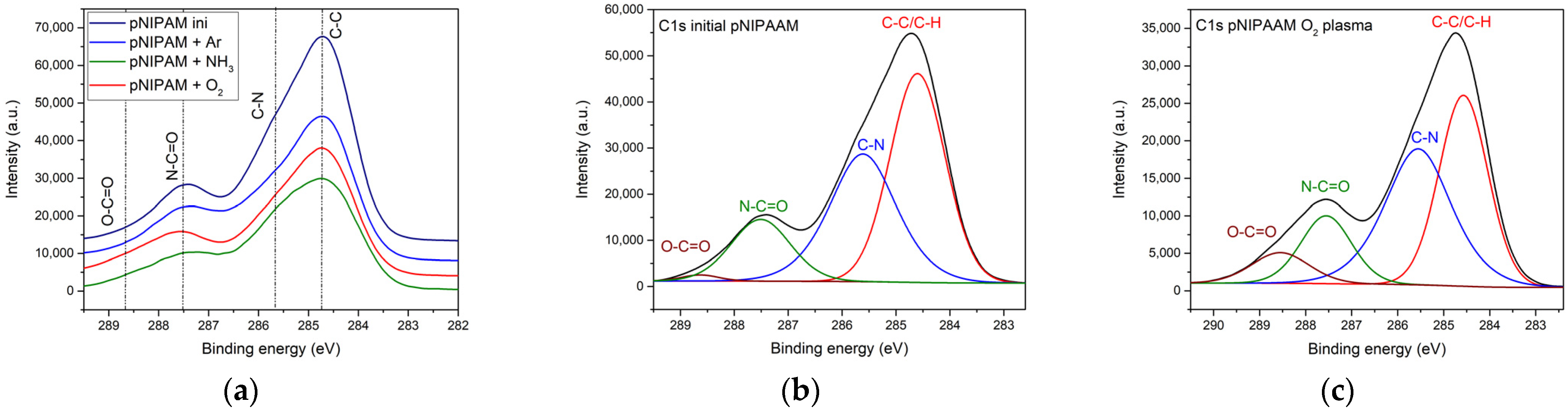
3.1.2. Chemical Composition of the Plasma Functionalized pNIPAAM Coatings
3.2. Investigation of Wettability Behavior with the Temperature
3.3. Biocompatibility Assessment of pNIPAAM Surfaces Treated with Various Gases Plasma
3.3.1. In Vitro Biological Investigations
3.3.2. Cell Detachment Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brun-Graeppi, A.K.A.S.; Richard, C.; Bessodes, M.; Scherman, D.; Merten, O.-W. Thermoresponsive surfaces for cell culture and enzyme-free cell detachment. Prog. Polym. Sci. 2010, 35, 1311–1324. [Google Scholar] [CrossRef]
- Gil, E.S.; Hudson, S.M. Stimuli-responsive polymers and their bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Kumar, A.; Srivastava, A.; Galaev, I.Y.; Mattiasson, B. Smart polymers: Physical forms and bioengineering applications. Prog. Polym. Sci. 2007, 32, 1205–1237. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- Gilsenan, P.M.; Richardson, R.K.; Morris, E.R. Thermally reversible acid-induced gelation of low-methoxy pectin. Carbohydr. Polym. 2000, 41, 339–349. [Google Scholar] [CrossRef]
- Patel, N.G.; Cavicchia, J.P.; Zhang, G.; Newby, B.-M.Z. Rapid cell sheet detachment using spin-coated pNIPAAM films retained on surfaces by an aminopropyltriethoxysilane network. Acta Biomater. 2012, 8, 2559–2567. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, J.E. Solution Properties of Poly(Nisopropylacrylamide). J. Macromol. Sci. Part A Chem. 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Stetsyshyn, J.; Raczkowska, J.; Harhay, K.; Gajos, K.; Melnyk, Y.; Dąbczyński, P.; Shevtsova, T.; Budkowski, A. Temperature-responsive and multi-responsive grafted polymer brushes with transitions based on critical solution temperature: Synthesis, properties, and applications. Coll. Polym. Sci. 2021, 299, 363–383. [Google Scholar] [CrossRef]
- Seuring, J.; Agarwal, S. Polymers with Upper Critical Solution Temperature in Aqueous Solution: Unexpected Properties from Known Building Blocks. ACS Macro Lett. 2013, 2, 597–600. [Google Scholar] [CrossRef]
- Qiu, X.-P.; Tanaka, F.; Winnik, F.M. Temperature-Induced Phase Transition of Well-Defined Cyclic Poly(N-isopropylacrylamide)s in Aqueous Solution. Macromolecules 2007, 200, 7069–7071. [Google Scholar] [CrossRef]
- Weder, G.; Guillaume-Gentil, O.; Matthey, N.; Montagne, F.; Heinzelmann, H.; Voros, J.; Liley, M. The quantification of single cell adhesion on functionalized surfaces for cell sheet engineering. Biomaterials 2010, 31, 6436–6443. [Google Scholar] [CrossRef] [PubMed]
- Stetsyshyn, Y.; Zemla, J.; Zolobko, O.; Fornal, K.; Budkowski, A.; Kostruba, A.; Donchak, V.; Harhay, K.; Awsiuk, K.; Rysz, J.; et al. Temperature and pH dual-responsive coatings of oligoperoxide-graft-poly (N-isopropylacrylamide): Wettability, morphology, and protein adsorption. J. Coll. Interface Sci. 2012, 387, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Ebara, M.; Yamato, M.; Hirose, M.; Aoyagi, T.; Kikuchi, A.; Sakai, K.; Okano, T. Copolymerization of 2-Carboxyisopropylacrylamide with N-Isopropylacrylamide Accelerates Cell Detachment from Grafted Surfaces by Reducing Temperature. Biomacromolecules 2003, 4, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Okano, T.; Sakai, H.; Karikusa, F.; Sawasaki, Y.; Sakurai, Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Macromol. Chem. Rapid Commun. 1990, 11, 571–600. [Google Scholar] [CrossRef]
- Rusen, L.; Dinca, V.; Mustaciosu, C.; Icriverzi, M.; Sima, L.E.; Bonciu, A.; Brajnicov, S.; Mihailescu, N.; Dumitrescu, N.; Popovici, A.I.; et al. Smart Thermoresponsive Surfaces Based on pNIPAm Coatings and Laser Method for Biological Applications. In Modern Technologies for Creating the Thin-Film Systems and Coatings; Nikitenkov, N., Ed.; InTechOpen: London, UK, 2017; Chapter 10; ISBN 978-953-51-3004-8. [Google Scholar] [CrossRef]
- Nash, M.E.; Carroll, W.M.; Nikoloskya, N.; Yang, R.; O′ Connell, C.; Gorelov, A.V.; Dockery, P.; Liptrot, C.; Lyng, F.M.; Garcia, A.; et al. Straightforward, one-step fabrication of ultrathin thermoresponsive films from commercially available pNIPAAM for cell culture and recovery. ACS Appl. Mater. Interface 2011, 3, 1980–1990. [Google Scholar] [CrossRef]
- Reed, J.A.; Love, S.A.; Lucero, A.E.; Haynes, C.L.; Canavan, H.E. Effect of Polymer Deposition Method on Thermoresponsive Polymer Films and Resulting Cellular Behavior. Langmuir 2012, 28, 2281–2287. [Google Scholar] [CrossRef]
- Yang, X.; Sun, Z.; Gao, J.; Yang, C.; Tang, D. Plasma-initiated polymerization of N-isopropylacrylamide and functionalized with dopamine for the adhesion to Hela cells. Polym. Bull. 2020, 77, 963–974. [Google Scholar] [CrossRef]
- Hegemann, D.; Gaiser, S. Plasma surface engineering for manmade soft materials: A review. J. Phys. D Appl. Phys. 2022, 55, 173002. [Google Scholar] [CrossRef]
- Rayatpisheh, S.; Li, P.; Chan-Park, M.B. Argon-Plasma-Induced Ultrathin Thermal Grafting of Thermoresponsive pNIPAm Coating for Contractile Patterned Human SMC Sheet Engineering. Macromol. Biosci. 2012, 12, 937–945. [Google Scholar] [CrossRef]
- Katesripongsa, P.; Trongsatitkul, T. An optimization of microwave-assisted grafting of poly(N-isopropylacrylamide) (PNIPAM) onto nylon-6 porous film for thermo-responsive gating membrane. Mech. Eng. J. 2019, 6, 19–00038. [Google Scholar] [CrossRef]
- Satulu, V.; Ionita, M.D.; Vizireanu, S.; Mitu, B.; Dinescu, G. Plasma Processing with Fluorine Chemistry for Modification of Surfaces Wettability. Molecules 2016, 21, 1711. [Google Scholar] [CrossRef] [PubMed]
- Acsente, T.; Ionita, M.D.; Teodorescu, M.; Marascu, V.; Dinescu, G. Surface modification of polymethylmethacrylate foils using an atmospheric pressure plasma jet in presence of water vapors. Thin Solid Films 2016, 614, 25–30. [Google Scholar] [CrossRef]
- Matei, A.; Schou, J.; Canulescu, S.; Zamfirescu, M.; Albu, C.; Mitu, B.; Buruiana, E.C.; Buruiana, T.; Mustaciosu, C.; Petcu, I.; et al. Functionalized ormosil scaffolds processed by direct laser polymerization for application in tissue engineering. Appl. Surf. Sci. 2013, 278, 357–361. [Google Scholar] [CrossRef][Green Version]
- Shimizu, K.; Fujita, H.; Nagamori, E. Oxygen Plasma-Treated Thermoresponsive Polymer Surfaces for Cell Sheet Engineering. Biotechnol. Bioeng. 2010, 106, 303–310. [Google Scholar] [CrossRef]
- Rusen, L.; Dinca, V.; Mitu, B.; Mustaciosu, C.; Dinescu, M. Temperature responsive functional polymeric thin films obtained by matrix assisted pulsed laser evaporation for cells attachment–detachment study. Appl. Surf. Sci. 2014, 302, 134–140. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.H.; Choi, K.H.; Noh, H.S.; Lee, J.W.; Pearton, S.J. Comparison of dry etching of PMMA and polycarbonate in diffusion pump-based O2 capacitively coupled plasma and inductively coupled plasma. Thin Solid Films 2010, 518, 6465–6468. [Google Scholar] [CrossRef]
- Mitu, B.; Vizireanu, S.; Petcu, C.; Dinescu, G.; Dinescu, M.; Birjega, R.; Teodorescu, V.S. Carbon material deposition by remote RF plasma beam. Surf. Coat. Technol. 2004, 180–181, 238–243. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies; John Wileys and Sons: Chichester, UK, 2001. [Google Scholar]
- Anderson, K.D.; Weber, R.B.; McConney, M.E.; Jiang, H.; Bunning, T.J.; Tsukruk, V.V. Responsive plasma polymerized ultrathin nanocomposite films. Polymer 2012, 53, 4686–4693. [Google Scholar] [CrossRef]
- Pan, Y.V.; Wesley, R.A.; Luginbuhl, R.; Denton, D.D.; Ratner, B.D. Plasma Polymerized N-Isopropylacrylamide: Synthesis and Characterization of a Smart Thermally Responsive Coating. Biomacromolecules 2001, 2, 32–36. [Google Scholar] [CrossRef]
- Xia, F.; Feng, L.; Wang, S.; Sun, T.; Song, W.; Jiang, W.; Jiang, L. Dual-Responsive Surfaces That Switch between Superhydrophilicity and Superhydrophobicity. Adv. Mater. 2006, 18, 432–436. [Google Scholar] [CrossRef]
- Jiao, Y.; Xu, J.; Zhou, C. Effect of ammonia plasma treatment on the properties and cytocompatibility of a poly(L-lactic acid) film surface. J. Biomater. Sci. Polym. Ed. 2012, 23, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.A.; Lucero, A.E.; Cooperstein, M.A.; Canavan, H.E. The effects of cell culture parameters on cell release kinetics from thermoresponsive surfaces. J. Appl. Biomater. Biomech. 2008, 6, 81–88. [Google Scholar] [PubMed]


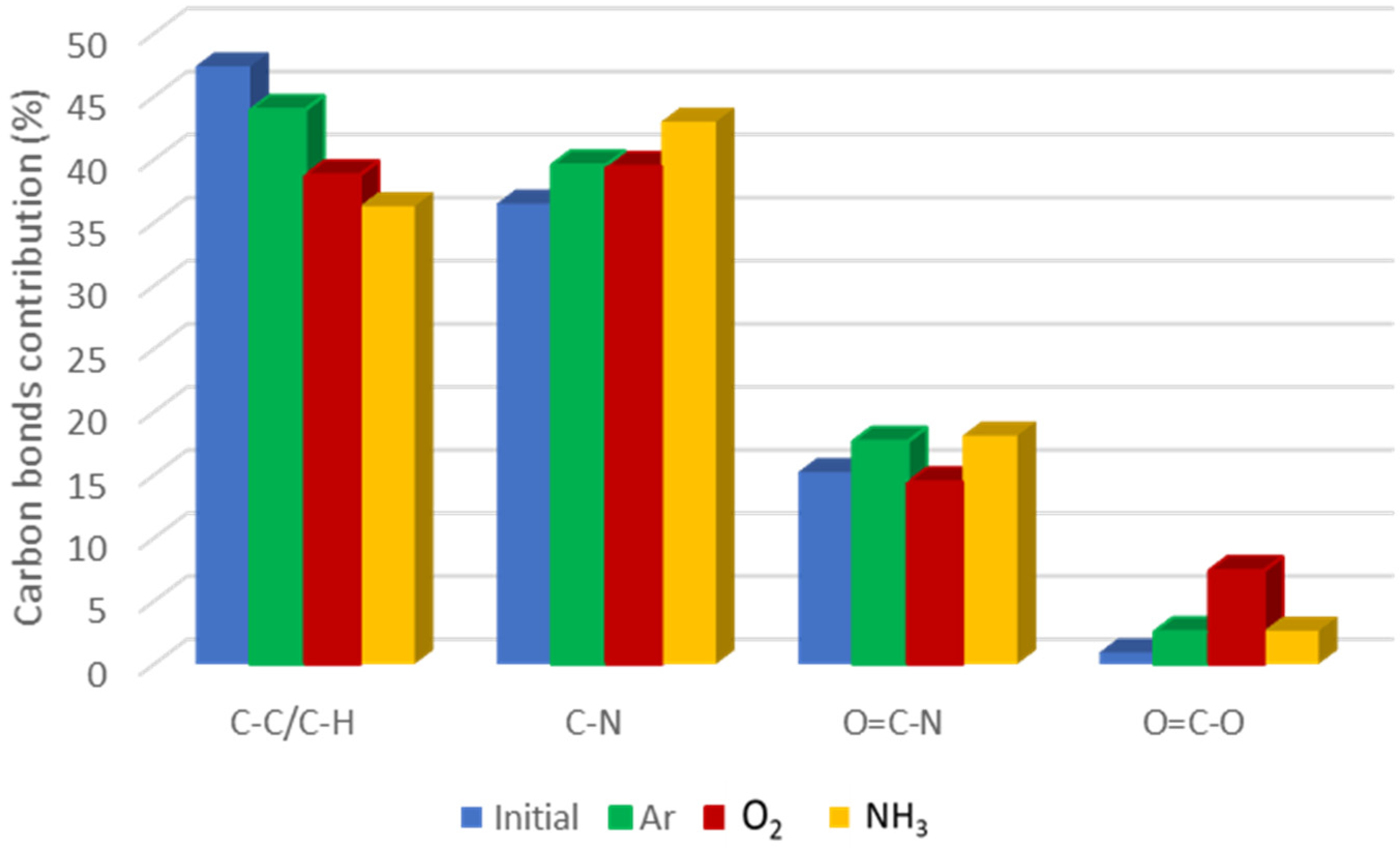

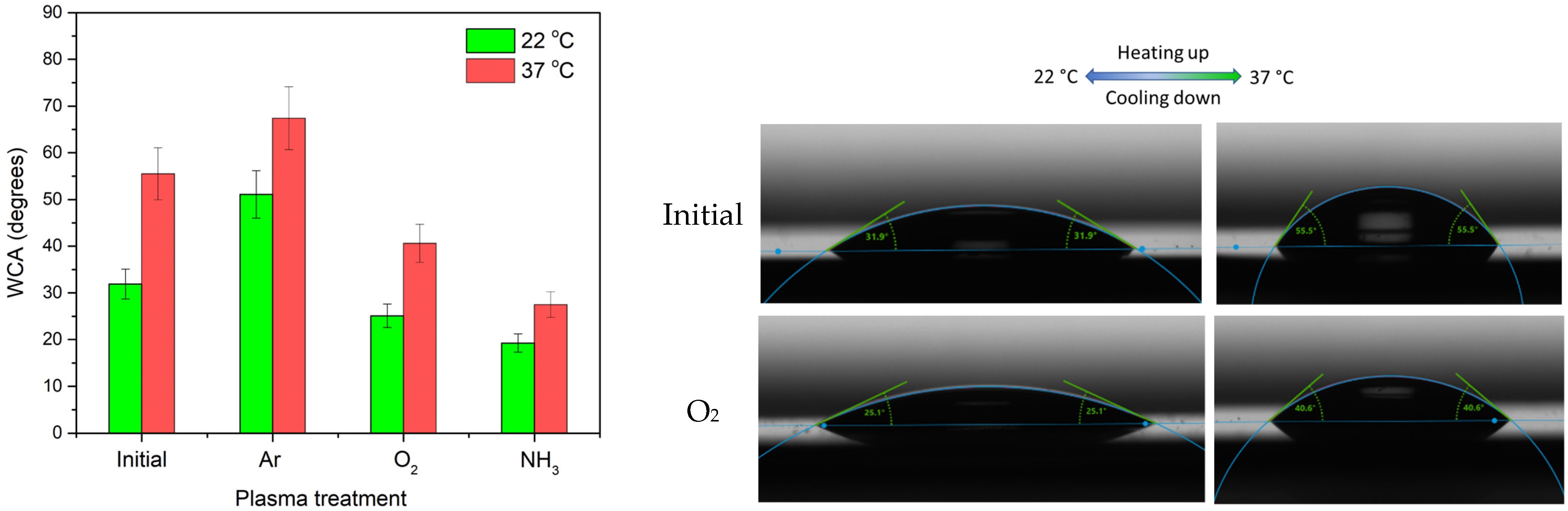


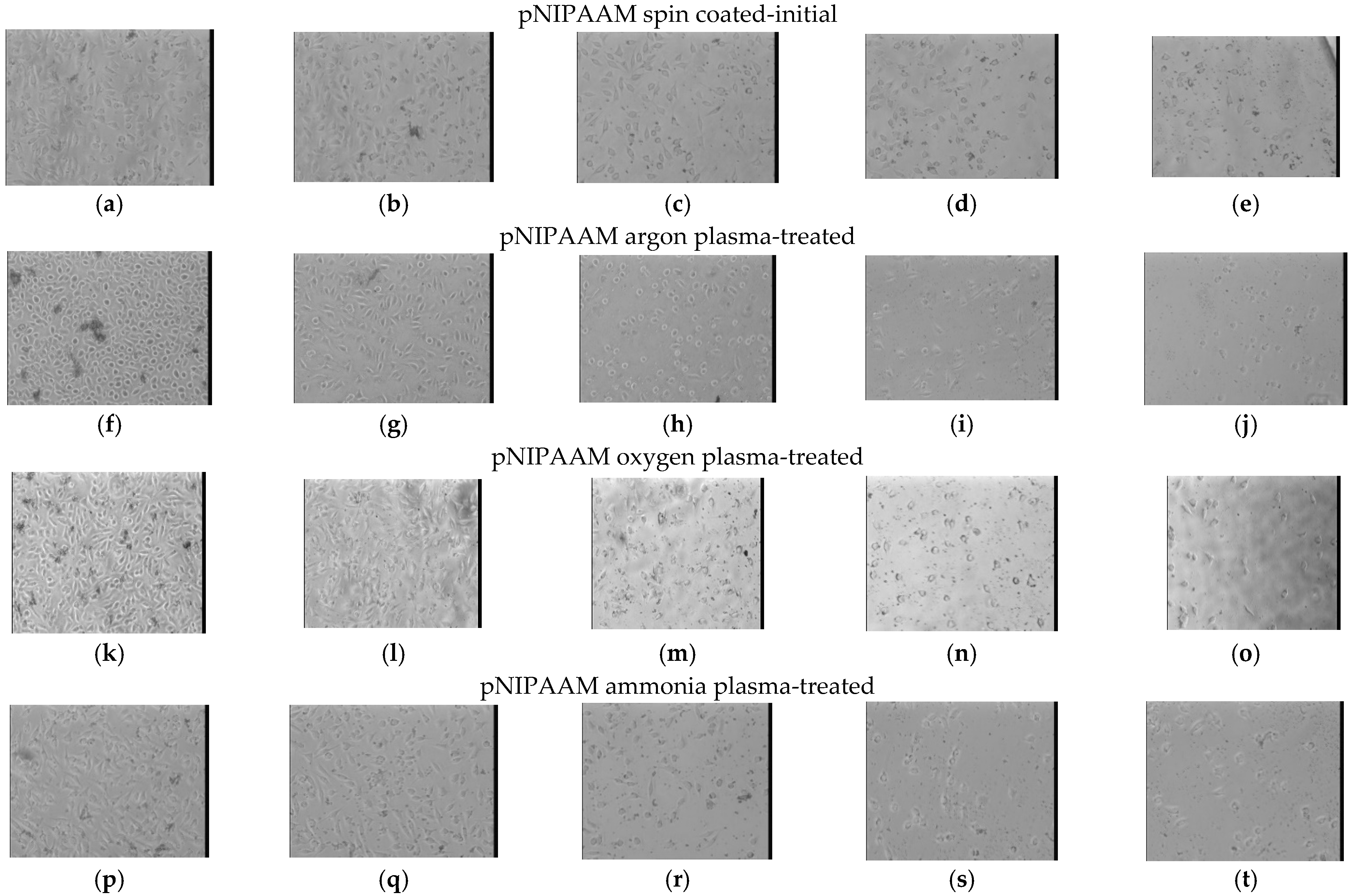

| Chemical Bonds | Wavenumber (cm−1) | |
|---|---|---|
| a | C-N bending | 1367 |
| b | C-CH3 methyl bending II | 1386 |
| c | C-CH3 methyl bending I | 1461 |
| d | N-H bending | 1540 |
| e | N-C=O | 1646 |
| f | O-C=O | 1698 |
| g | CH3 symmetric stretching CH2 symmetric stretching CH3 asymmetric stretching | 2874–2970 |
| h | =C-H stretching | 3070 |
| i | N-H stretching mode | 3308 |
| j | N-H stretching mode | 3435 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satulu, V.; Dinca, V.; Bacalum, M.; Mustaciosu, C.; Mitu, B.; Dinescu, G. Chemistry-Induced Effects on Cell Behavior upon Plasma Treatment of pNIPAAM. Polymers 2022, 14, 1081. https://doi.org/10.3390/polym14061081
Satulu V, Dinca V, Bacalum M, Mustaciosu C, Mitu B, Dinescu G. Chemistry-Induced Effects on Cell Behavior upon Plasma Treatment of pNIPAAM. Polymers. 2022; 14(6):1081. https://doi.org/10.3390/polym14061081
Chicago/Turabian StyleSatulu, Veronica, Valentina Dinca, Mihaela Bacalum, Cosmin Mustaciosu, Bogdana Mitu, and Gheorghe Dinescu. 2022. "Chemistry-Induced Effects on Cell Behavior upon Plasma Treatment of pNIPAAM" Polymers 14, no. 6: 1081. https://doi.org/10.3390/polym14061081
APA StyleSatulu, V., Dinca, V., Bacalum, M., Mustaciosu, C., Mitu, B., & Dinescu, G. (2022). Chemistry-Induced Effects on Cell Behavior upon Plasma Treatment of pNIPAAM. Polymers, 14(6), 1081. https://doi.org/10.3390/polym14061081