Effect of Ultrasound Treatment on Barrier Changes of Polymers before and after Exposure to Food Simulants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Packaging Materials and Chemicals
2.2. Sample Preparation
2.3. Ultrasound Treatment
2.4. Film Characterisation
2.4.1. Film Thickness
2.4.2. Water Vapour Permeability
2.4.3. Oxygen Permeability
2.4.4. Overall Migration
2.5. Data Analysis
3. Results and Discussion
3.1. Water Vapour Permeability
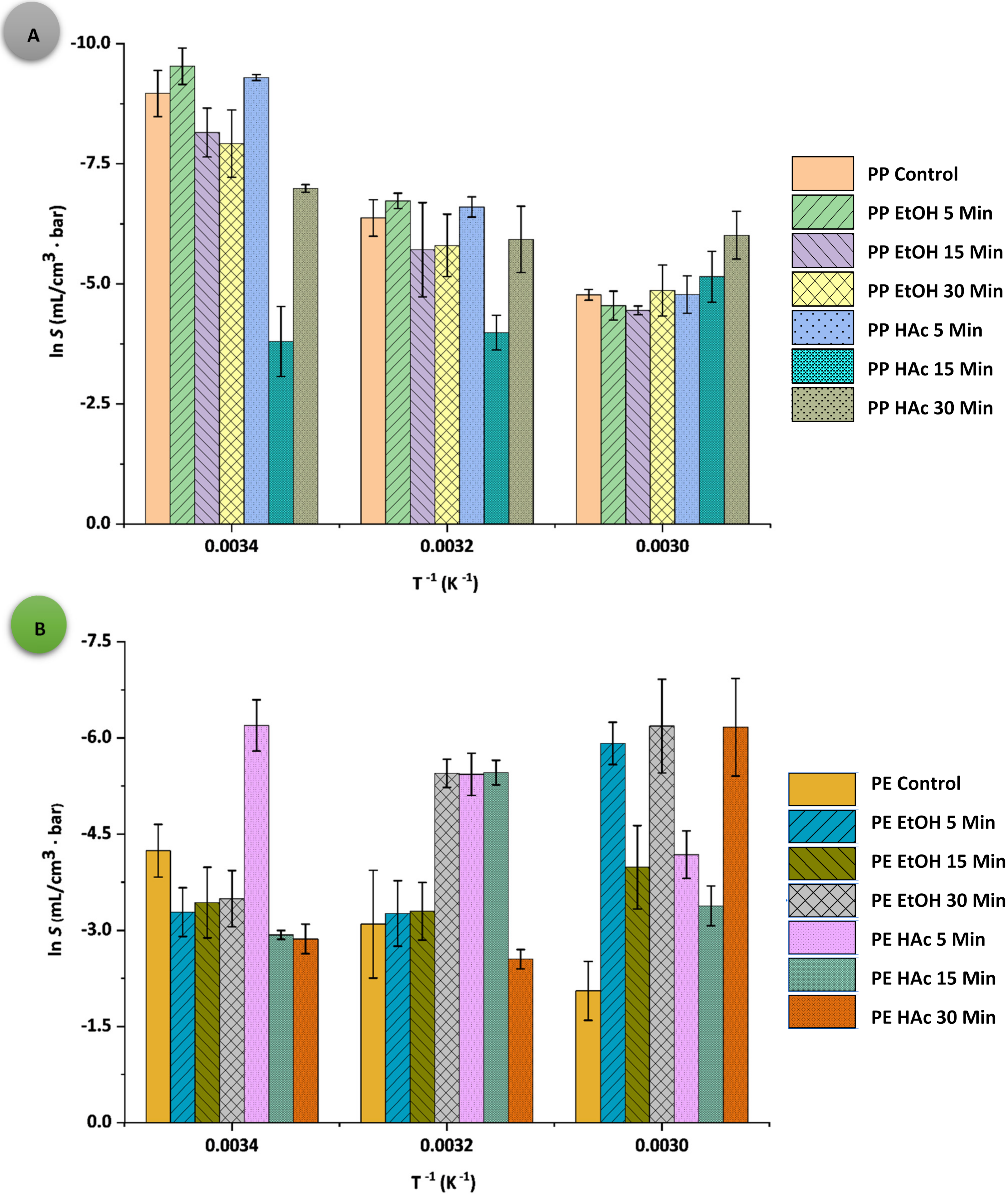
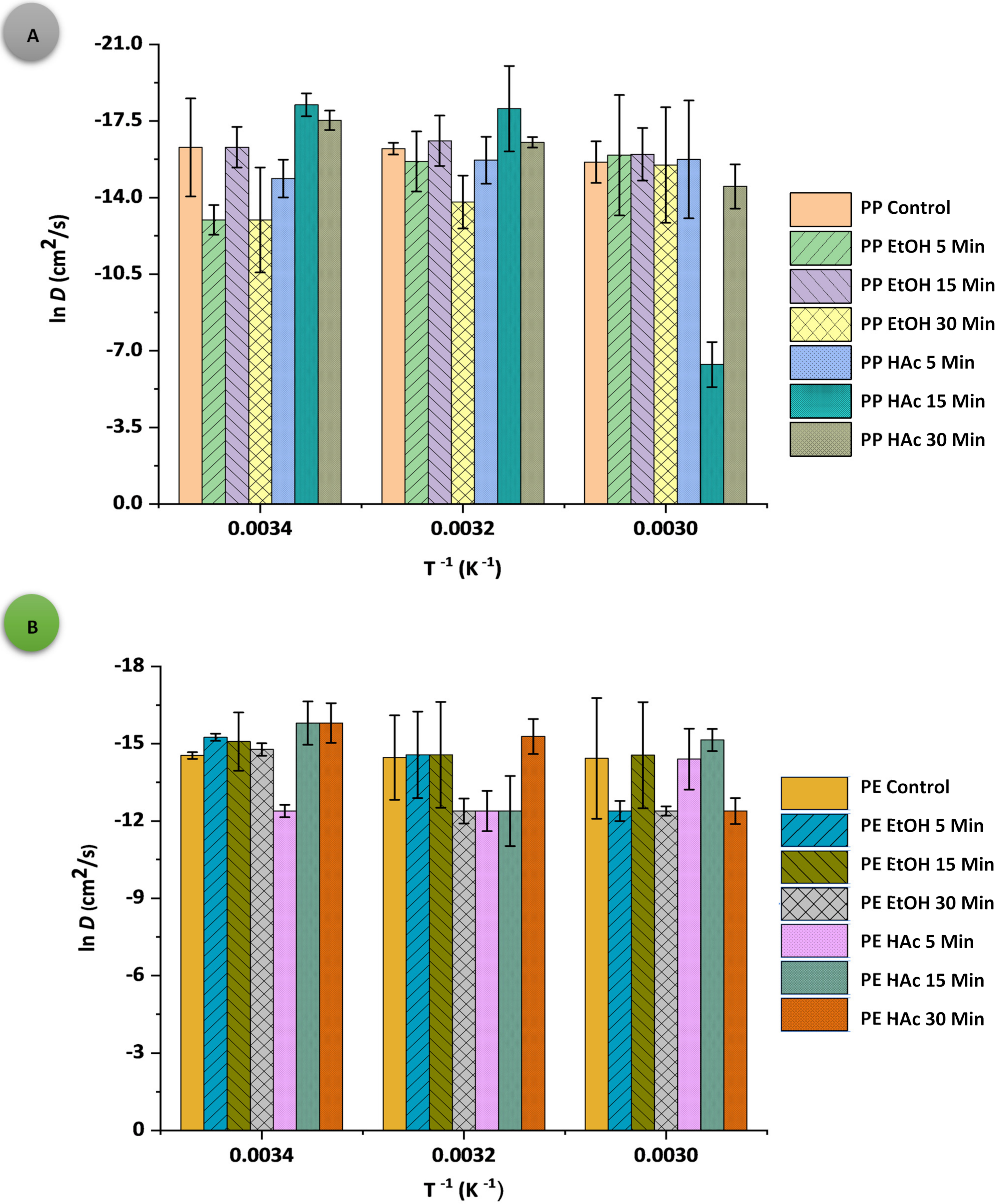
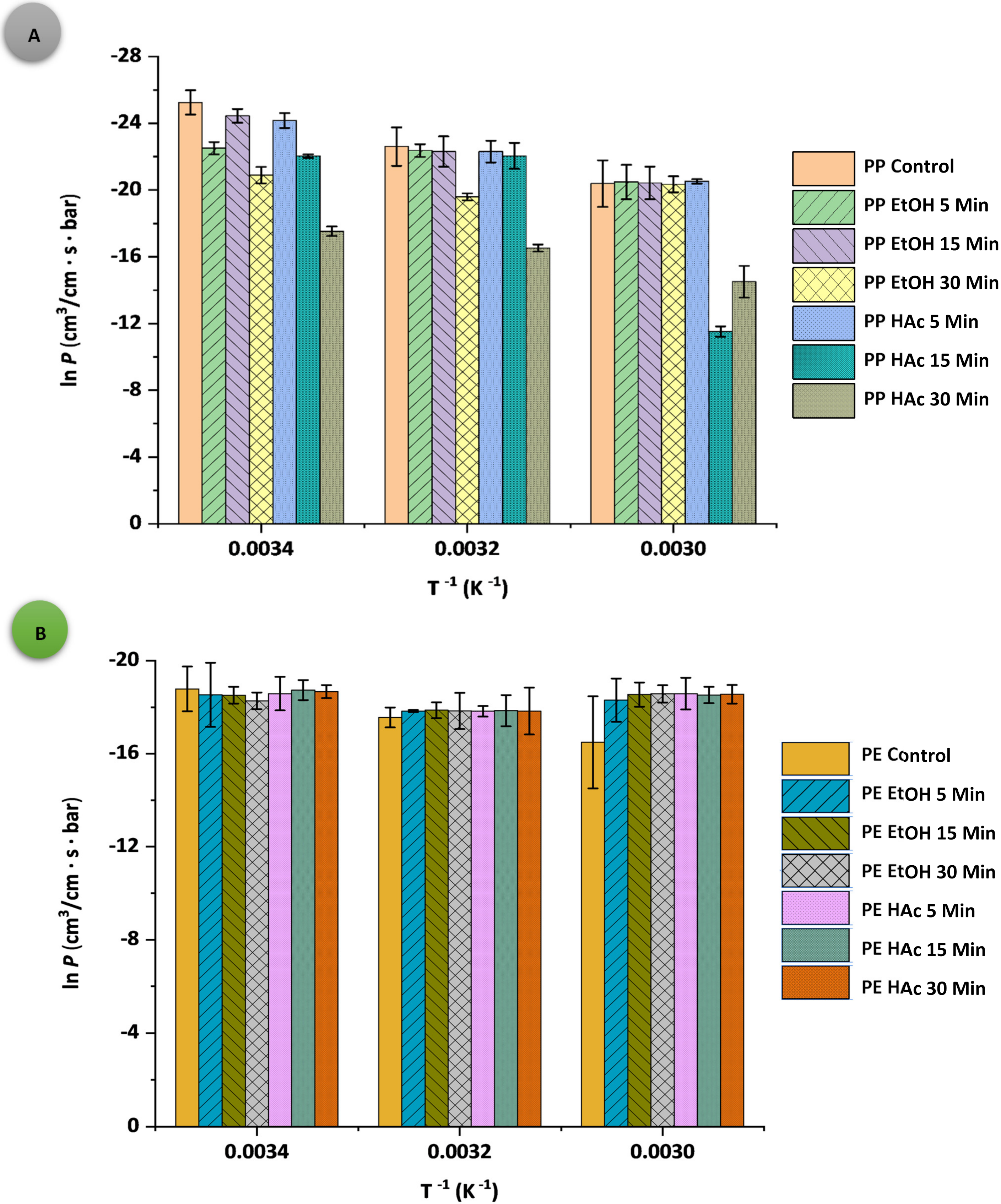
3.2. Oxygen Permeability Parameters
3.3. Overall Migration
4. Conclusions and Future Recommendation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petkoska, A.T.; Daniloski, D.; D’Cunha, N.M.; Naumovski, N.; Broach, A.T. Edible packaging: Sustainable solutions and novel trends in food packaging. Food Res. Int. 2021, 140, 109981. [Google Scholar] [CrossRef]
- Daniloski, D.; Petkoska, A.T.; Lee, N.A.; Bekhit, A.E.-D.; Carne, A.; Vaskoska, R.; Vasiljevic, T. Active edible packaging based on milk proteins: A route to carry and deliver nutraceuticals. Trends Food Sci. Technol. 2021, 111, 688–705. [Google Scholar] [CrossRef]
- Chang, Y.; Kang, K.; Park, S.-J.; Choi, J.C.; Kim, M.; Han, J. Experimental and theoretical study of polypropylene: Antioxidant migration with different food simulants and temperatures. J. Food Eng. 2019, 244, 142–149. [Google Scholar] [CrossRef]
- Ščetar, M.; Kurek, M.; Jambrak, A.R.; Debeaufort, F.; Galić, K. Influence of high power ultrasound on physical-chemical properties of polypropylene films aimed for food packaging: Barrier and contact angle features. Polym. Int. 2017, 66, 1572–1578. [Google Scholar] [CrossRef]
- Patel, R.M.; Karjala, T.P.; Savargaonkar, N.R.; Salibi, P.; Liu, L. Fundamentals of structure–property relationships in blown films of linear low density polyethylene/low density polyethylene blends. J. Plast. Film Sheeting 2019, 35, 401–421. [Google Scholar] [CrossRef]
- Irwanto, D.; Pidhatika, B.; Nurhajati, D.W.; Harjanto, S. Mechanical properties and crystallinity of linear low density polyethylene based biocomposite film. Majalah Kulit Karet dan Plastik 2020, 35, 93–98. [Google Scholar] [CrossRef]
- Klepac, D.; Ščetar, M.; Kurek, M.; Mallon, P.E.; Luyt, A.S.; Galić, K.; Valić, S. Oxygen permeability, electron spin resonance, differential scanning calorimetry and positron annihilation lifetime spectroscopy studies of uniaxially deformed linear low-density polyethylene film. Polym. Int. 2013, 62, 474–481. [Google Scholar] [CrossRef]
- Ronca, S. Polyethylene. In Brydson’s Plastics Materials, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 247–278. [Google Scholar]
- Ahmadi, E.; Sareminezhad, S.; Azizi, M.H. The effect of ultrasound treatment on some properties of methylcellulose films. Food Hydrocoll. 2011, 25, 1399–1401. [Google Scholar] [CrossRef]
- Alarcon-Rojo, A.D.; Carrillo-Lopez, L.M.; Reyes-Villagrana, R.; Huerta-Jiménez, M.; Garcia-Galicia, I.A. Ultrasound and meat quality: A review. Ultrason. Sonochem. 2019, 55, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, M.; Yang, C.-H. Application of ultrasound technology in processing of ready-to-eat fresh food: A review. Ultrason. Sonochem. 2020, 63, 104953. [Google Scholar] [CrossRef] [PubMed]
- Kirsh, I.; Frolova, Y.; Bannikova, O.; Beznaeva, O.; Tveritnikova, I.; Myalenko, D.; Romanova, V.; Zagrebina, D. Research of the Influence of the Ultrasonic Treatment on the Melts of the Polymeric Compositions for the Creation of Packaging Materials with Antimicrobial Properties and Biodegrability. Polymers 2020, 12, 275. [Google Scholar] [CrossRef] [Green Version]
- Reichert, C.L.; Bugnicourt, E.; Coltelli, M.-B.; Cinelli, P.; Lazzeri, A.; Canesi, I.; Braca, F.; Martínez, B.M.; Alonso, R.; Agostinis, L.; et al. Bio-Based Packaging: Materials, Modifications, Industrial Applications and Sustainability. Polymers 2020, 12, 1558. [Google Scholar] [CrossRef]
- Zink, J.; Wyrobnik, T.; Prinz, T.; Schmid, M. Physical, Chemical and Biochemical Modifications of Protein-Based Films and Coatings: An Extensive Review. Int. J. Mol. Sci. 2016, 17, 1376. [Google Scholar] [CrossRef] [PubMed]
- Ščetar, M.; Kurek, M.; Jambrak, A.R.; Debeaufort, F.; Galić, K. Effect of high power ultrasound on physical–chemical properties of polypropylene films aimed for food packaging: Structure and surface features. Polym. Bull. 2019, 76, 1007–1021. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Lan, W.; Qin, W. Development of ultrasound treated polyvinyl alcohol/tea polyphenol composite films and their physicochemical properties. Ultrason. Sonochem. 2019, 51, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of Protein-Based Films and Coatings for Food Packaging: A Review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, Y.; Cheng, W.; Feng, X.; Gao, C.; Wu, D.; Meng, L.; Zhang, Y.; Tang, X. Fabrication, structure and properties of pullulan-based active films incorporated with ultrasound-assisted cinnamon essential oil nanoemulsions. Food Packag. Shelf Life 2020, 25, 100547. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.W. Effect of ultrasonic wave on the degradation of polypropylene melt and morphology of its blend with polystyrene. Polymer 2002, 43, 2585–2589. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, B.; Wang, J.; Qin, Y. Structural changes and nano-TiO2 migration of poly (lactic acid)-based food packaging film contacting with ethanol as food simulant. Int. J. Biol. Macromol. 2019, 139, 85–93. [Google Scholar] [CrossRef]
- ASTM. ASTM E96-80 Standard test methods for water vapor transmission of materials. In ASTM Book of Standards; ASTM International: Philadelphia, PA, USA, 1980; pp. 811–818. [Google Scholar]
- Basiak, E.; Debeaufort, F.; Lenart, A. Effect of oil lamination between plasticized starch layers on film properties. Food Chem. 2016, 195, 56–63. [Google Scholar] [CrossRef] [PubMed]
- ISO 15105-1. In Plastics–Film and Sheeting–Determination of Gas-Transmission Rate–Part 1: Differential-Pressure Methods; International Organization for Standardization: Geneva, Switzerland, 2007.
- Brugger Feinmechanik GmbH. Gas Permeability Testing; Brugger Feinmechanik GmbH: Munich, Germany, 2003. [Google Scholar]
- BS EN 1186-1. In Materials and Article in Contact with Foodstuffs—Plastics. Part 1: Guide to the Selection of Conditions and Test Methods for Overall Migration; European Committee for Standardization: Brussels, Belgium, 2002.
- EN 1186-5. In Materials and Article in Contact with Foodstuffs—Plastics. Part 5: Test Methods for Overall Migration into Aqueous Food Simulants by Cell; European Committee for Standardization: Brussels, Belgium, 2002.
- Wang, Z.; Zhou, J.; Wang, X.-X.; Zhang, N.; Sun, X.-X.; Ma, Z.-S. The effects of ultrasonic/microwave assisted treatment on the water vapor barrier properties of soybean protein isolate-based oleic acid/stearic acid blend edible films. Food Hydrocoll. 2014, 35, 51–58. [Google Scholar] [CrossRef]
- Holden, P.S.; Orchard, G.A.J.; Ward, I.M. A study of the gas barrier properties of highly oriented polyethylene. J. Polym. Sci. Polym. Phys. Ed. 1985, 23, 709–731. [Google Scholar] [CrossRef]
- Lagnika, C.; Adjovi, Y.C.S.; Lagnika, L.; Gogohounga, F.O.; Do-Sacramento, O.; Koulony, R.K.; Sanni, A. Effect of Combining Ultrasound and Mild Heat Treatment on Physicochemical, Nutritional Quality and Microbiological Properties of Pineapple Juice. Food Nutr. Sci. 2017, 08, 227–241. [Google Scholar] [CrossRef] [Green Version]
- Gholizadeh, M.; Razavi, J.; Mousavi, S. Gas permeability measurement in polyethylene and its copolymer films. Mater. Des. 2007, 28, 2528–2532. [Google Scholar] [CrossRef]
- Daniloski, D.; Petkoska, A.T.; Galić, K.; Scetar, M.; Kurek, M.; Vaskoska, R.; Kalevska, T.; Nedelkoska, D.N. The effect of barrier properties of polymeric films on the shelf-life of vacuum packaged fresh pork meat. Meat Sci. 2019, 158, 107880. [Google Scholar] [CrossRef]
- Hanika, M.; Langowski, H.-C.; Moosheimer, U.; Peukert, W. Inorganic Layers on Polymeric Films—Influence of Defects and Morphology on Barrier Properties. Chem. Eng. Technol. 2003, 26, 605–614. [Google Scholar] [CrossRef]
- Lin, Y.; Dias, P.; Chen, H.; Chum, S.; Hiltner, A.; Baer, E. Oxygen permeability of biaxially oriented polypropylene films. Polym. Eng. Sci. 2008, 48, 642–648. [Google Scholar] [CrossRef]
- Siracusa, V. Food Packaging Permeability Behaviour: A Report. Int. J. Polym. Sci. 2012, 2012, 302029. [Google Scholar] [CrossRef]
- Price, G.; White, A.J.; Clifton, A.A. The effect of high-intensity ultrasound on solid polymers. Polymer 1995, 36, 4919–4925. [Google Scholar] [CrossRef]
- Klepac, D.; Scetar, M.; Baranović, G.; Galić, K.; Valić, S. Influence of high doses γ-irradiation on oxygen permeability of linear low-density polyethylene and cast polypropylene films. Radiat. Phys. Chem. 2014, 97, 304–312. [Google Scholar] [CrossRef]
- Leterrier, Y. Durability of nanosized oxygen-barrier coatings on polymers. Prog. Mater. Sci. 2003, 48, 1–55. [Google Scholar] [CrossRef] [Green Version]
- Mrkić, S.; Galić, K.; Ivanković, M.; Hamin, S.; Ciković, N. Gas transport and thermal characterization of mono-and di-polyethylene films used for food packaging. J. Appl. Polym. Sci. 2006, 99, 1590–1599. [Google Scholar] [CrossRef]
- Michaels, A.S.; Bixler, H.J. Solubility of gases in polyethylene. J. Polym. Sci. 1961, 50, 393–412. [Google Scholar] [CrossRef]
- Seymour, R.B.; Carraher, C.E. Diffusion and Permeation of Gas and Vapors in Polymers. In Structure—Property Relationships in Polymers; Springer: Boston, MA, USA, 1984; pp. 107–111. [Google Scholar]
- Hong, S.-I.; Krochta, J.M. Oxygen barrier performance of whey-protein-coated plastic films as affected by temperature, relative humidity, base film and protein type. J. Food Eng. 2006, 77, 739–745. [Google Scholar] [CrossRef]
- Dole, P.; Feigenbaum, A.E.; De La Cruz, C.; Pastorelli, S.; Paseiro, P.; Hankemeier, T.; Voulzatis, Y.; Aucejo, S.; Saillard, P.; Papaspyrides, C. Typical diffusion behaviour in packaging polymers – application to functional barriers. Food Addit. Contam. 2006, 23, 202–211. [Google Scholar] [CrossRef]
- Gulmine, J.; Janissek, P.; Heise, H.; Akcelrud, L. Degradation profile of polyethylene after artificial accelerated weathering. Polym. Degrad. Stab. 2003, 79, 385–397. [Google Scholar] [CrossRef]
- Fasihnia, S.H.; Peighambardoust, S.H.; Peighambardoust, S.J.; Oromiehie, A.; Soltanzadeh, M.; Peressini, D. Migration analysis, antioxidant, and mechanical characterization of polypropylene-based active food packaging films loaded with BHA, BHT, and TBHQ. J. Food Sci. 2020, 85, 2317–2328. [Google Scholar] [CrossRef]
- Arutchelvi, J.; Sudhakar, M.; Arkatkar, A.S.; Doble, M.; Bhaduri, S.; Uppara, P.V. Biodegradation of polyethylene and polypropylene. Indian J. Biotechnol. 2008, 7, 9–22. [Google Scholar]
- Alariqi, S.A.; Kumar, A.P.; Rao, B.; Singh, R. Biodegradation of γ-sterilised biomedical polyolefins under composting and fungal culture environments. Polym. Degrad. Stab. 2006, 91, 1105–1116. [Google Scholar] [CrossRef]
- Bae, B.; Chun, B.-H.; Kim, D. Surface characterization of microporous polypropylene membranes modified by plasma treatment. Polymer 2001, 42, 7879–7885. [Google Scholar] [CrossRef]
- Belmonte, G.K.; Charles, G.; Strumia, M.C.; Weibel, D.E. Permanent hydrophilic modification of polypropylene and poly(vinyl alcohol) films by vacuum ultraviolet radiation. Appl. Surf. Sci. 2016, 382, 93–100. [Google Scholar] [CrossRef]
- Hafttananian, N.; Khajavi, M.Z.; Farhoodi, M.; Jahanbin, K.; Pure, A.E. Migration of nano-clay and nano-silica from low-density polyethylene nanocomposites into different food simulants. J. Food Meas. Charact. 2021, 15, 3893–3900. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Zhao, N.; Wang, Z.; Lv, S.; Liu, J.; Chen, L.; Wang, J.; Zhang, Y.; Wang, S. Migration regularity of phthalates in polyethylene wrap film of food packaging. J. Food Sci. 2020, 85, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Welle, F. Chemical Migration from Beverage Packaging Materials—A Review. Beverages 2020, 6, 37. [Google Scholar] [CrossRef]
| Sample: Treatment Conditions | Thickness (μm) | WVP × 10−13 (g·m−1·s−1·Pa−1) |
|---|---|---|
| LLDPE (control) | 51 ± 1.54 ab | 3.51 ± 0.32 c |
| LLDPE: EtOH 5 | 50 ± 1.01 abc | 4.03 ± 0.17 bc |
| LLDPE: EtOH 15 | 52 ± 0.24 a | 4.10 ± 0.20 bc |
| LLDPE: EtOH 30 | 50 ± 0.73 abc | 4.07 ± 0.12 bc |
| LLDPE: HAc 5 | 48 ± 1.01 bc | 10.3 ± 0.53 a |
| LLDPE: HAc 15 | 48 ± 0.98 bc | 3.95 ± 0.08 bc |
| LLDPE: HAc 30 | 51 ± 0.68 ab | 4.28 ± 0.27 b |
| PPAcPVDC (control) | 34 ± 0.51 d | 1.55 ± 0.06 d |
| PPAcPVDC: EtOH 5 | 30 ± 0.98 e | 1.05 ± 0.04 de |
| PPAcPVDC: EtOH 15 | 29 ± 0.45 e | 1.17 ± 0.07 de |
| PPAcPVDC: EtOH 30 | 32 ± 0.14 de | 1.08 ± 0.05 de |
| PPAcPVDC: HAc 5 | 30 ± 0.29 e | 0.99 ± 0.09 de |
| PPAcPVDC: HAc 15 | 30 ± 1.26 e | 0.83 ± 0.03 e |
| PPAcPVDC: HAc 30 | 30 ± 0.53 e | 0.81 ± 0.01 e |
| PPAcPVDC | |||||
|---|---|---|---|---|---|
| Sample: Treatment Conditions | t (°C) | D × 10−11 (cm2·s−1) | S × 10−5 (mL·cm−3∙bar−1) | P × 10−6 (cm3·cm−1·s−1·bar−1) | q (cm3·m−2·d−1·bar−1) |
| PPAcPVDC (control) | 20 | 0.84 ± 1.61 j | 0.13 ± 0.04 b | 1.07 ± 0.94 t | 2.91 ± 0.17 a |
| 40 | 0.89 ± 0.01 j | 1.71 ± 0.12 b | 15.25 ± 1.50 p | 41.30 ± 3.84 a | |
| 60 | 1.65 ± 1.23 g | 8.48 ± 0.24 a | 139.92 ± 9.76 c | 377.00 ± 8.99 a | |
| PPAcPVDC: EtOH 5 | 20 | 23.10 ± 1.53 a | 0.07 ± 0.02 b | 16.74 ± 1.54 o | 28.90 ± 3.68 a |
| 40 | 1.60 ± 3.20 g | 1.20 ± 0.79 b | 19.20 ± 1.26 m | 52.00 ± 2.95 a | |
| 60 | 1.20 ± 1.08 i | 10.60 ± 0.31 b | 127.20 ± 2.28 e | 342.00 ± 16.00 a | |
| PPAcPVDC: EtOH 15 | 20 | 0.83 ± 0.01 j | 0.29 ± 0.02 b | 2.41 ± 0.65 r | 6.53 ± 0.26 a |
| 40 | 0.62 ± 0.03 k | 3.31 ± 0.13 b | 20.55 ± 1.24 l | 55.50 ± 2.76 a | |
| 60 | 1.15 ± 0.31 i | 11.70 ± 0.51 b | 134.55 ± 9.61 d | 364.00 ± 3.29 a | |
| PPAcPVDC: EtOH 30 | 20 | 23.10 ± 1.49 a | 0.36 ± 0.01 b | 84.08 0.84 i | 145.00 ± 4.07 a |
| 40 | 10.20 ± 4.45 c | 3.03 ± 0.01 b | 309.06 ± 1.06 a | 836.00 ± 13.82 a | |
| 60 | 1.88 ± 0.23 f | 7.75 ± 0.15 b | 145.70 ± 5.09 b | 393.00 ± 1.37 a | |
| PPAcPVDC: HAc 5 | 20 | 3.48 ± 0.79 e | 0.10 ± 0.02 b | 3.20 ± 0.24 q | 8.65 ± 0.12 a |
| 40 | 1.51 ± 0.37 h | 1.40 ± 0.02 b | 20.53 ± 4.22 l | 55.30 ± 0.86 a | |
| 60 | 1.45 ± 0.17 h | 8.42 ± 0.05 b | 122.09 ± 0.96 g | 331.00 ± 0.89 a | |
| PPAcPVDC: HAc 15 | 20 | 0.12 ± 0.01 m | 22.40 ± 2.24 b | 26.88 ± 0.11 j | 72.50 ± 1.61 a |
| 40 | 0.14 ± 0.04 m | 18.60 ± 1.60 b | 26.59 ± 0.72 k | 72.00 ± 1.33 a | |
| 60 | 17.10 ± 4.70 b | 5.81 ± 0.13 b | 99.35 ± 0.52 h | 301.33 ± 17.15 a | |
| PPAcPVDC: HAc 30 | 20 | 0.24 ± 0.03 l | 0.92 ± 0.03 b | 2.25 ± 0.53 s | 6.10 ± 0.41 a |
| 40 | 0.66 ± 0.08 k | 2.67 ± 0.27 b | 17.80 ± 0.86 n | 48.20 ± 0.20 a | |
| 60 | 5.00 ± 1.34 d | 2.45 ± 0.28 b | 122.50 ± 1.64 f | 330.00 ± 1.95 a | |
| LLDPE | |||||
|---|---|---|---|---|---|
| Sample: Treatment Conditions | t (°C) | D × 10−11 (cm2·s−1) | S × 10−5 (mL·cm−3·bar−1) | P × 10−6 (cm3·cm−1·s−1·bar−1) | q (cm3·m−2·d−1·bar−1) |
| LLDPE (control) | 20 | 4.83 ± 1.94 e | 14.40 ± 0.01 j | 695.52 ± 2.57 s | 1200.00 ± 6.33 o |
| 40 | 5.24 ± 2.04 d | 45.20 ± 0.45 e | 2368.48 ± 3.97 b | 4100.00 ± 12.62 a | |
| 60 | 5.40 ± 1.17 c | 128.00 ± 5.03 a | 6912.00 ± 7.12 a | 1100.00 ± 1.17 p | |
| LLDPE: EtOH 5 | 20 | 2.39 ± 0.11 i | 37.50 ± 9.89 f | 896.25 ± 11.97 l | 1540.00 ± 2.64 ij |
| 40 | 4.71 ± 1.15 e | 38.30 ± 2.21 f | 1803.93 ± 7.86 d | 3110.00 ± 1.55 c | |
| 60 | 41.70 ± 8.45 a | 2.70 ± 0.70 klm | 1125.90 ± 10.57 i | 1940.00 ± 3.30 g | |
| LLDPE: EtOH 15 | 20 | 2.82 ± 0.43 g | 32.30 ± 1.94 gh | 910.86 ± 2.09 j | 1570.00 ± 7.09 h |
| 40 | 4.71 ± 0.97 e | 37.00 ± 0.66 f | 1742.70 ± 9.71 g | 3010.00 ± 1.63 e | |
| 60 | 4.77 ± 1.95 e | 18.60 ± 5.42 i | 887.22 ± 7.25 m | 1530.00 ± 4.21 jk | |
| LLDPE: EtOH 30 | 20 | 3.83 ± 0.11 f | 30.40 ± 0.25 h | 1164.32 ± 3.25 h | 2010.00 ± 1.70 f |
| 40 | 41.70 ± 4.66 a | 4.30 ± 1.25 kl | 1793.10 ± 5.32 e | 3100.00 ± 0.89 c | |
| 60 | 41.70 ± 1.97 a | 2.06 ± 0.75 lm | 859.02 ± 3.89 o | 1480.00 ± 3.29 l | |
| LLDPE: HAc 5 | 20 | 41.70 ± 7.14 a | 2.04 ± 0.20 m | 850.68 ± 5.42 p | 1460.00 ± 39.85 l |
| 40 | 41.70 ± 8.49 a | 4.37 ± 0.41 k | 1822.29 ± 9.09 c | 3150.00 ± 5.40 b | |
| 60 | 5.56 ± 1.71 b | 15.30 ± 1.82 j | 850.68 ± 23.03 p | 1470.00 ± 2.11 l | |
| LLDPE: HAc 15 | 20 | 1.37 ± 0.45 j | 53.50 ± 2.18 d | 732.95 ± 5.03 r | 1260.00 ± 1.31 m |
| 40 | 41.70 ± 3.30 a | 4.25 ± 0.46 klm | 1772.25 ± 26.72 f | 3050.00 ± 1.13 d | |
| 60 | 2.65 ± 0.33 h | 34.00 ± 1.18 g | 901.00 ± 18.93 k | 1560.00 ± 2.00 hi | |
| LLDPE: HAc 30 | 20 | 1.37 ± 0.97 j | 57.00 ± 1.09 c | 780.90 ± 2.10 q | 1230.00 ± 1.11 n |
| 40 | 2.31 ± 0.74 i | 78.10 ± 1.56 b | 1804.11 ± 11.78 d | 3120.00 ± 4.54 f | |
| 60 | 41.70 ± 2.24 a | 2.10 ± 0.89 lm | 875.70 ± 19.52 n | 1510.00 ± 0.40 k | |
| Sample: Treatment Conditions | In Contact with Simulant during Ultrasound Treatment | Overall Migration (mg·dm−2) Food Simulant | |
|---|---|---|---|
| HAc | EtOH | ||
| LLDPE (control) | No | 2.27 ± 0.49 d | 2.04 ± 1.26 d |
| LLDPE UST | No | 4.66 ± 3.49 d | 2.43 ± 0.10 d |
| LLDPE: HAc UST | Yes | 147.73 ± 17.86 b | nd |
| LLDPE: EtOH UST | Yes | nd | 76.29 ± 5.36 c |
| PPAcPVDC (control) | No | 0.68 ± 0.10 d | 1.46 ± 0.10 d |
| PPAcPVDC UST | No | 5.92 ± 0.10 d | 7.77 ± 0.39 d |
| PPAcPVDC: HAc UST | Yes | 253.25 ± 19.48 a | nd |
| PPAcPVDC: EtOH UST | Yes | nd | 89.83 ± 3.60 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ščetar, M.; Daniloski, D.; Tinjić, M.; Kurek, M.; Galić, K. Effect of Ultrasound Treatment on Barrier Changes of Polymers before and after Exposure to Food Simulants. Polymers 2022, 14, 990. https://doi.org/10.3390/polym14050990
Ščetar M, Daniloski D, Tinjić M, Kurek M, Galić K. Effect of Ultrasound Treatment on Barrier Changes of Polymers before and after Exposure to Food Simulants. Polymers. 2022; 14(5):990. https://doi.org/10.3390/polym14050990
Chicago/Turabian StyleŠčetar, Mario, Davor Daniloski, Mirela Tinjić, Mia Kurek, and Kata Galić. 2022. "Effect of Ultrasound Treatment on Barrier Changes of Polymers before and after Exposure to Food Simulants" Polymers 14, no. 5: 990. https://doi.org/10.3390/polym14050990
APA StyleŠčetar, M., Daniloski, D., Tinjić, M., Kurek, M., & Galić, K. (2022). Effect of Ultrasound Treatment on Barrier Changes of Polymers before and after Exposure to Food Simulants. Polymers, 14(5), 990. https://doi.org/10.3390/polym14050990









