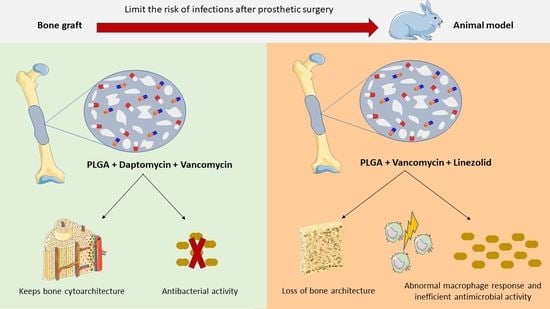
Modification of the Polymer of a Bone Cement with Biodegradable Microspheres of PLGA and Loading with Daptomycin and Vancomycin Improve the Response to Bone Tissue Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Formulations of Bone Cement
2.2. Titanium Rods Coated with Hydroxyapatite Contaminated with S. aureus
2.3. Animal Experimental Model
2.4. Sample Processing: Histological and Immunohistochemical Techniques
2.5. Histological Staging Methods
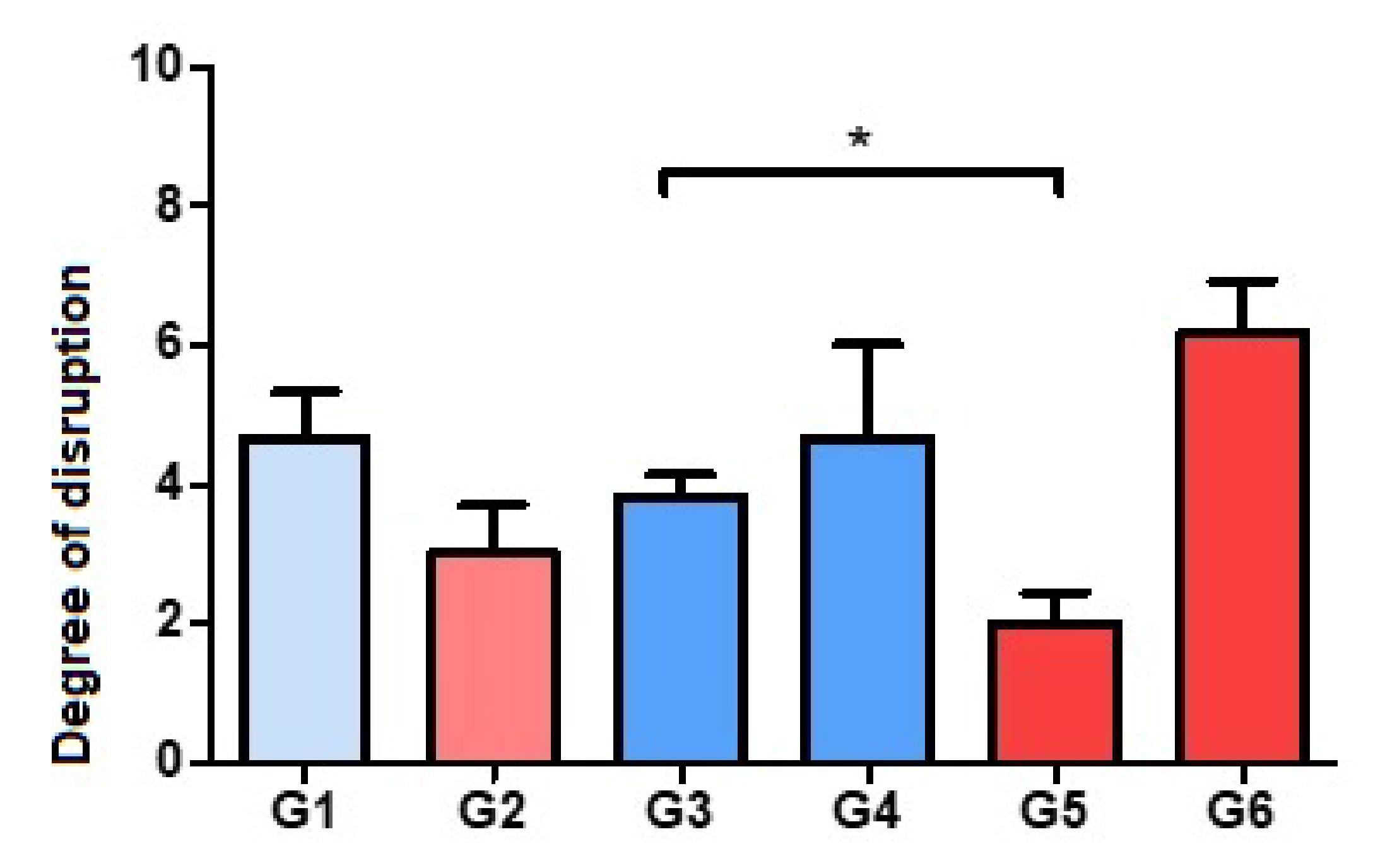
2.5.1. Destructuring of Bone Histoarchitecture
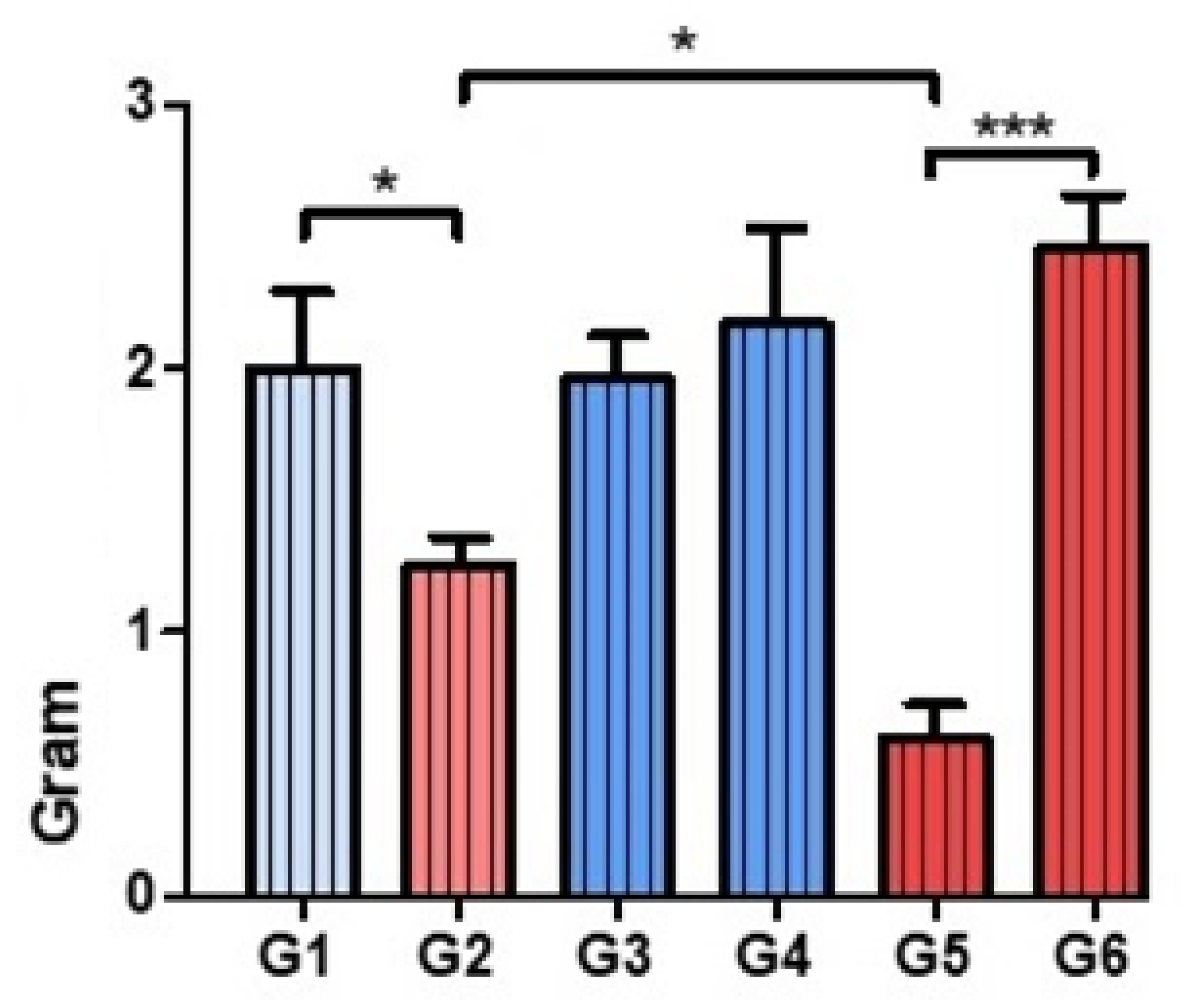
2.5.2. Presence of Bacteria and the Macrophage Response
2.6. Statistical Analysis
3. Results
3.1. Evaluation of Bone Histoarchitecture: Quantitative Analysis of the Degree of Destruction
3.2. Analysis of the Tissue Distribution of Bacteria
3.3. Assessment of the Presence of Macrophages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindeque, B.; Hartman, Z.; Noshchenko, A.; Cruse, P. Infection After Primary Total Hip Arthroplasty. Orthopedics 2014, 37, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-H.; Tsai, S.-W.; Wu, P.-K.; Chen, C.-F.; Wang, H.-Y.; Chen, W.-M. Partial component-retained two-stage reconstruction for chronic infection after uncemented total hip arthroplasty: Results of sixteen cases after five years of follow-up. Int. Orthop. 2017, 41, 2479–2486. [Google Scholar] [CrossRef] [PubMed]
- Yarwood, J.M.; Paquette, K.M.; Tikh, I.B.; Volper, E.M.; Greenberg, E.P. Generation of Virulence Factor Variants in Staphylococcus aureus Biofilms. J. Bacteriol. 2007, 189, 7961–7967. [Google Scholar] [CrossRef]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-joint infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef]
- Cabrita, H.B.; Croci, A.T.; de Camargo, O.P.; de Lima, A.L.L.M. Prospective study of the treatment of infected hip arthroplasties with or without the use of an antibiotic-loaded cement spacer. Clin. Sao Paulo Braz. 2007, 62, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Anagnostakos, K. Therapeutic Use of Antibiotic-loaded Bone Cement in the Treatment of Hip and Knee Joint Infections. J. Bone Jt. Infect. 2017, 2, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef]
- Lamagni, T. Epidemiology and burden of prosthetic joint infections. J. Antimicrob. Chemother. 2014, 69, i5–i10. [Google Scholar] [CrossRef]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the Treatment of Methicillin-Resistant Staphylococcus aureus Infections in Adults and Children: Executive Summary. Clin. Infect. Dis. 2011, 52, 285–292. [Google Scholar] [CrossRef]
- Pillai, S.K.; Wennersten, C.; Venkataraman, L.; Eliopoulos, G.M.; Moellering, J.R.C.; Karchmer, A.W. Development of Reduced Vancomycin Susceptibility in Methicillin-SusceptibleStaphylococcus aureus. Clin. Infect. Dis. 2009, 49, 1169–1174. [Google Scholar] [CrossRef]
- Parra-Ruíz, F.; González-Gómez, A.; Fernández-Gutiérrez, M.; Parra, J.; García-García, J.; Azuara, G.; De la Torre, B.; Buján, J.; Ibarra, B.; Duocastella-Codina, L.; et al. Development of advanced biantibiotic loaded bone cement spacers for arthroplasty associated infections. Int. J. Pharm. 2017, 522, 11–20. [Google Scholar] [CrossRef]
- Hashemian, S.M.; Farhadi, T.; Ganjparvar, M. Linezolid: A review of its properties, function, and use in critical care. Drug Des. Dev. Ther. 2018, 12, 1759–1767. [Google Scholar] [CrossRef]
- Gray, D.A.; Wenzel, M. More Than a Pore: A Current Perspective on the In Vivo Mode of Action of the Lipopeptide Antibiotic Daptomycin. Antibiotics 2020, 9, 17. [Google Scholar] [CrossRef]
- Petty, W.; Spanier, S.; Shuster, J.J.; Silverthorne, C. The influence of skeletal implants on incidence of infection. Experiments in a canine model. J. Bone Jt. Surg. Am. 1985, 67, 1236–1244. [Google Scholar] [CrossRef]
- Smeltzer, M.S.; Thomas, J.R.; Hickmon, S.G.; Skinner, R.A.; Nelson, C.L.; Griffith, D.; Parr, T.R., Jr.; Evans, R.P. Characterization of a rabbit model of staphylococcal osteomyelitis. J. Orthop. Res. Off Publ. Orthop. Res. Soc. 1997, 15, 414–421. [Google Scholar] [CrossRef]
- Ibarra, B.; García-García, J.; Azuara, G.; Vázquez-Lasa, B.; Ortega, M.A.; Asúnsolo, Á.; Román, J.S.; Buján, J.; Honduvilla, N.G.; De La Torre, B. Polylactic-co-glycolic acid microspheres added to fixative cements and its role on bone infected architecture. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2517–2526. [Google Scholar] [CrossRef]
- Azuara, G.; García-García, J.; Ibarra, B.; Parra-Ruiz, F.J.; Asúnsolo, A.; Ortega, M.A.; Vázquez-Lasa, B.; Buján, J.; San Román, J.; de la Torre, B. Experimental study of the application of a new bone cement loaded with broad spectrum antibiotics for the treatment of bone infection. Rev. Espanola Cirugia Ortop. Traumatol. 2019, 63, 95–103. [Google Scholar] [CrossRef]
- Remmele, W.; Schicketanz, K.-H. Immunohistochemical determination of estrogen and progesterone receptor content in human breast cancer: Computer-assisted image analysis (QIC score) vs. subjective grading (IRS). Pathol. Res. Pract. 1993, 189, 862–866. [Google Scholar] [CrossRef]
- Ariza, J.; Cobo, J.; Baraia-Etxaburu, J.; de Benito, N.; Bori, G.; Cabo, J.; Corona, P.; Esteban, J.; Horcajada, J.P.; Lora-Tamayo, J.; et al. Executive summary of management of prosthetic joint infections. Clinical practice guidelines by the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC). Enferm. Infecc. Microbiol. Clin. 2017, 35, 189–195. [Google Scholar] [CrossRef]
- Anagnostakos, K.; Kelm, J.; Grün, S.; Schmitt, E.; Jung, W.; Swoboda, S. Antimicrobial properties and elution kinetics of linezolid-loaded hip spacers in vitro. J. Biomed. Mater Res. B Appl. Biomater. 2008, 87, 173–178. [Google Scholar] [CrossRef]
- Beibei, L.; Yun, C.; Mengli, C.; Nan, B.; Xuhong, Y.; Rui, W. Linezolid versus vancomycin for the treatment of Gram-positive bacterial infections: Meta-analysis of randomised controlled trials. Int. J. Antimicrob. Agents 2010, 35, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kuechle, D.K.; Landon, G.C.; Musher, D.M.; Noble, P.C. Elution of Vancomycin, Daptomycin, and Amikacin From Acrylic Bone Cement. Clin. Orthop. Relat. Res. 1991, 264, 302–308. [Google Scholar] [CrossRef]
- Kaplan, L.; Kurdziel, M.; Baker, K.; Verner, J. Characterization of Daptomycin-loaded Antibiotic Cement. Orthopedics 2012, 35, e503–e509. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eick, S.; Hofpeter, K.; Sculean, A.; Ender, C.; Klimas, S.; Vogt, S.; Nietzsche, S. Activity of Fosfomycin- and Daptomycin-Containing Bone Cement on Selected Bacterial Species Being Associated with Orthopedic Infections. BioMed Res. Int. 2017, 2017, 2318174. [Google Scholar] [CrossRef]
- Stefani, S.; Campanile, F.; Santagati, M.; Mezzatesta, M.L.; Cafiso, V.; Pacini, G. Insights and clinical perspectives of daptomycin resistance in Staphylococcus aureus: A review of the available evidence. Int. J. Antimicrob. Agents 2015, 46, 278–289. [Google Scholar] [CrossRef]
- Grillon, A.; Argemi, X.; Gaudias, J.; Ronde-Ousteau, C.; Boeri, C.; Jenny, J.-Y.; Hansmann, Y.; Lefebvre, N.; Jehl, F. Bone penetration of daptomycin in diabetic patients with bacterial foot infections. Int. J. Infect. Dis. 2019, 85, 127–131. [Google Scholar] [CrossRef]
- Mescola, A.; Ragazzini, G.; Alessandrini, A. Daptomycin Strongly Affects the Phase Behavior of Model Lipid Bilayers. J. Phys. Chem. B 2020, 124, 8562–8571. [Google Scholar] [CrossRef]
- Bue, M.; Tøttrup, M.; Hanberg, P.; Langhoff, O.; Birke-Sørensen, H.; Thillemann, T.M.; Søballe, K. Bone and subcutaneous adipose tissue pharmacokinetics of vancomycin in total knee replacement patients. Acta Orthop. 2018, 89, 95–100. [Google Scholar] [CrossRef]
- Rouse, M.S.; Piper, K.E.; Jacobson, M.; Jacofsky, D.J.; Steckelberg, J.M.; Patel, R. Daptomycin treatment of Staphylococcus aureus experimental chronic osteomyelitis. J. Antimicrob. Chemother. 2006, 57, 301–305. [Google Scholar] [CrossRef]
- Kanazawa, T.; Soejima, T.; Murakami, H.; Inoue, T.; Katouda, M.; Nagata, K.; Wang, J.-W.; Fong, C.-Y.; Su, Y.-S.; Yu, H.-N. An immunohistological study of the integration at the bone-tendon interface after reconstruction of the anterior cruciate ligament in rabbits. J. Bone Jt. Surg. Br. Vol. 2006, 88, 682–687. [Google Scholar] [CrossRef]
- Baos, E.; Candel, F.J.; Merino, P.; Pena, I.; Picazo, J.J. Characterization and monitoring of linezolid-resistant clinical isolates of Staphylococcus epidermidis in an intensive care unit 4 years after an outbreak of infection by cfr-mediated linezolid-resistant Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 2013, 76, 325–329. [Google Scholar] [CrossRef]
- Flamm, R.K.; Mendes, R.; Ross, J.E.; Sader, H.; Jones, R.N. An international activity and spectrum analysis of linezolid: ZAAPS Program results for Diagn. Microbiol. Infect. Dis. 2013, 76, 206–213. [Google Scholar] [CrossRef]
- Sader, H.S.; Flamm, R.K.; Jones, R.N. Antimicrobial activity of daptomycin tested against Gram-positive pathogens collected in Europe, Latin America, and selected countries in the Asia-Pacific Region (2011). Diagn. Microbiol. Infect. Dis. 2013, 75, 417–422. [Google Scholar] [CrossRef]




| Group | Cement | Antibiotic |
|---|---|---|
| 1 | Palacos R | Daptomycin |
| 2 | Palacos R + PLGA | Daptomycin |
| 3 | Palacos R | VancomycinDaptomycin |
| 4 | Palacos R | VancomycinLinezolid |
| 5 | Palacos R+PLGA | VancomycinDaptomycin |
| 6 | Palacos R+PLGA | VancomycinLinezolid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-García, J.; Azuara, G.; Fraile-Martinez, O.; García-Montero, C.; Álvarez-Mon, M.A.; Ruíz-Díez, S.; Álvarez-Mon, M.; Buján, J.; García-Honduvilla, N.; Ortega, M.A.; et al. Modification of the Polymer of a Bone Cement with Biodegradable Microspheres of PLGA and Loading with Daptomycin and Vancomycin Improve the Response to Bone Tissue Infection. Polymers 2022, 14, 888. https://doi.org/10.3390/polym14050888
García-García J, Azuara G, Fraile-Martinez O, García-Montero C, Álvarez-Mon MA, Ruíz-Díez S, Álvarez-Mon M, Buján J, García-Honduvilla N, Ortega MA, et al. Modification of the Polymer of a Bone Cement with Biodegradable Microspheres of PLGA and Loading with Daptomycin and Vancomycin Improve the Response to Bone Tissue Infection. Polymers. 2022; 14(5):888. https://doi.org/10.3390/polym14050888
Chicago/Turabian StyleGarcía-García, Joaquin, Galo Azuara, Oscar Fraile-Martinez, Cielo García-Montero, Miguel Angel Álvarez-Mon, Sara Ruíz-Díez, Melchor Álvarez-Mon, Julia Buján, Natalio García-Honduvilla, Miguel A. Ortega, and et al. 2022. "Modification of the Polymer of a Bone Cement with Biodegradable Microspheres of PLGA and Loading with Daptomycin and Vancomycin Improve the Response to Bone Tissue Infection" Polymers 14, no. 5: 888. https://doi.org/10.3390/polym14050888
APA StyleGarcía-García, J., Azuara, G., Fraile-Martinez, O., García-Montero, C., Álvarez-Mon, M. A., Ruíz-Díez, S., Álvarez-Mon, M., Buján, J., García-Honduvilla, N., Ortega, M. A., & De la Torre, B. (2022). Modification of the Polymer of a Bone Cement with Biodegradable Microspheres of PLGA and Loading with Daptomycin and Vancomycin Improve the Response to Bone Tissue Infection. Polymers, 14(5), 888. https://doi.org/10.3390/polym14050888