Durability Performance of Geopolymer Concrete: A Review
Abstract
:1. Introduction
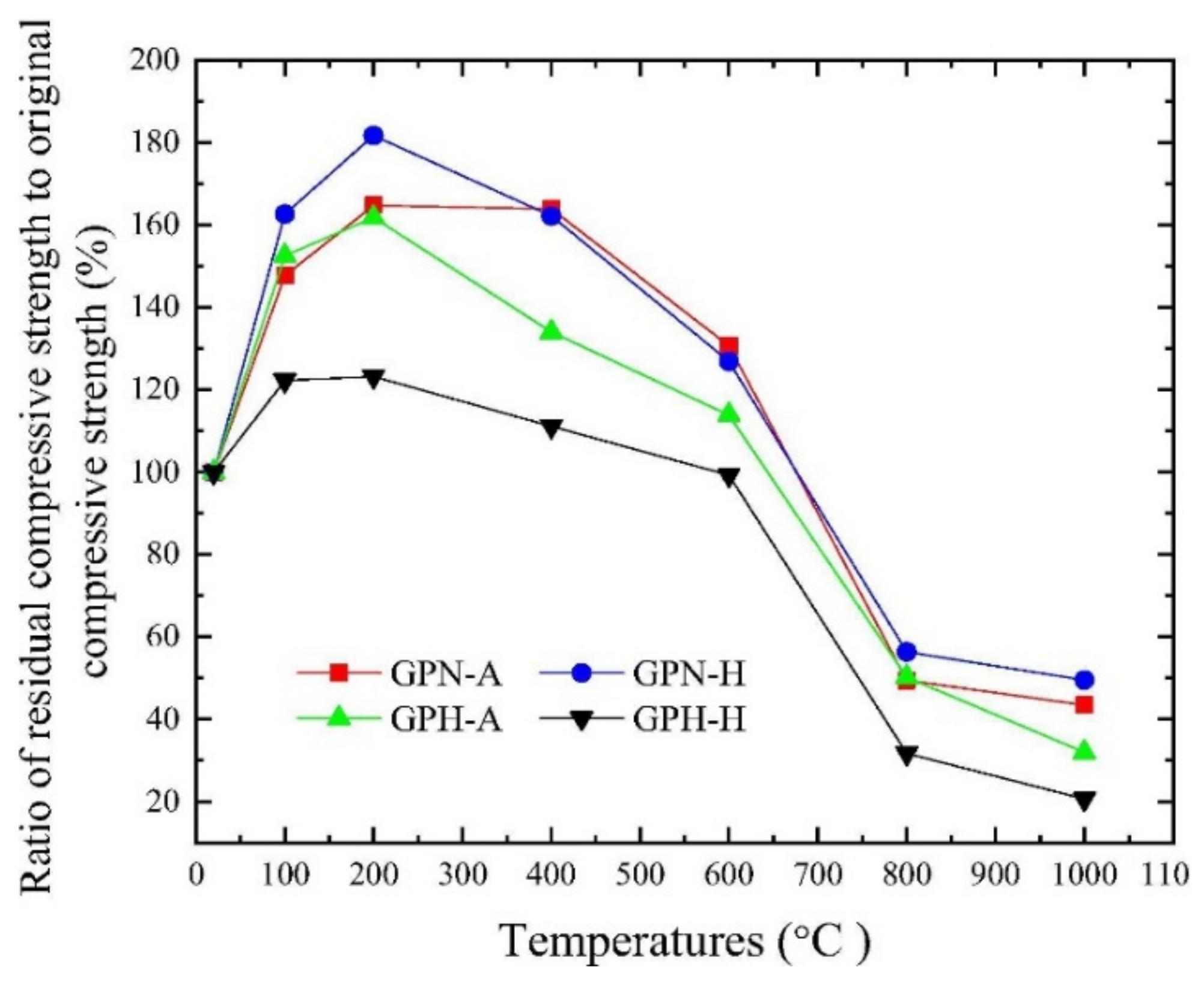
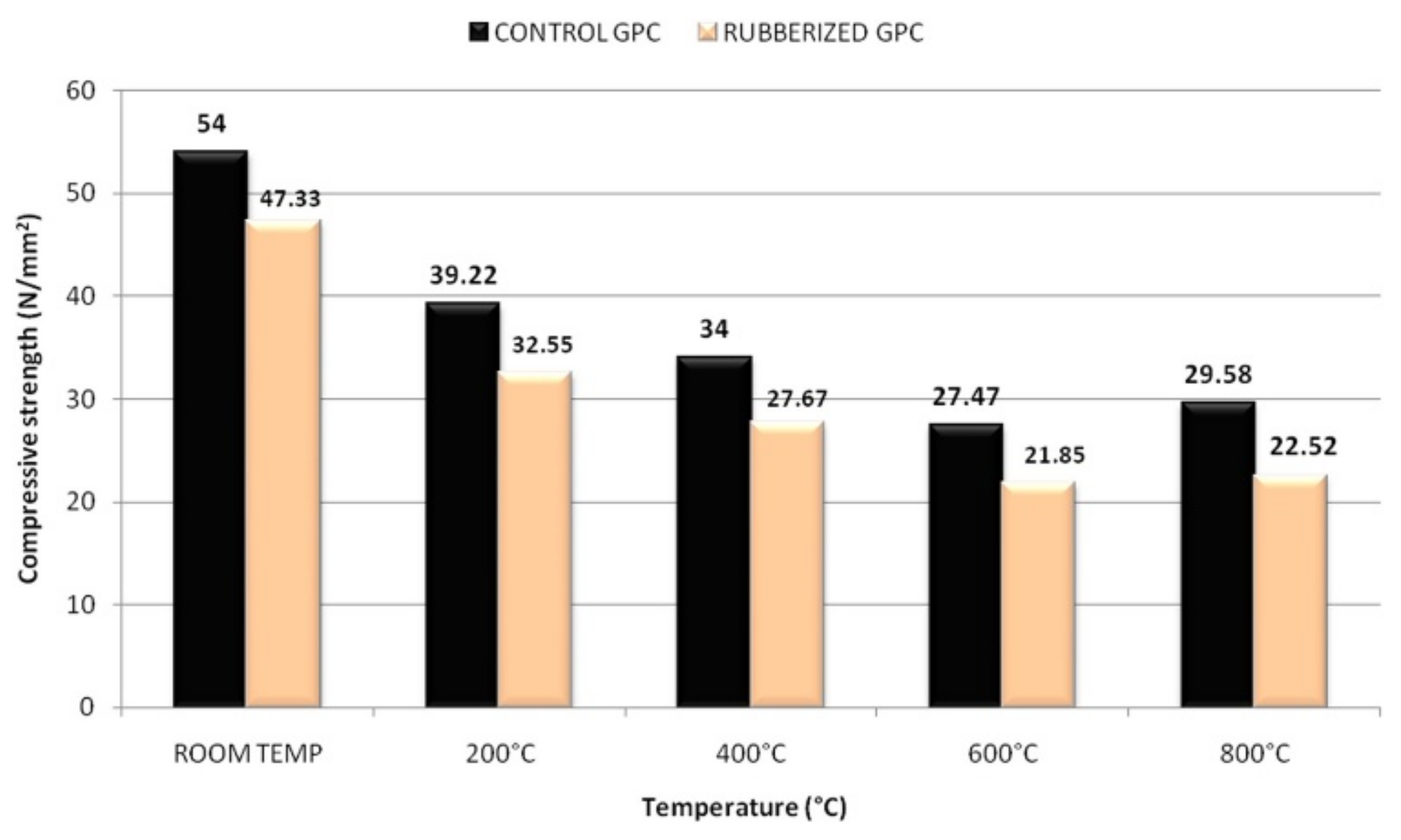
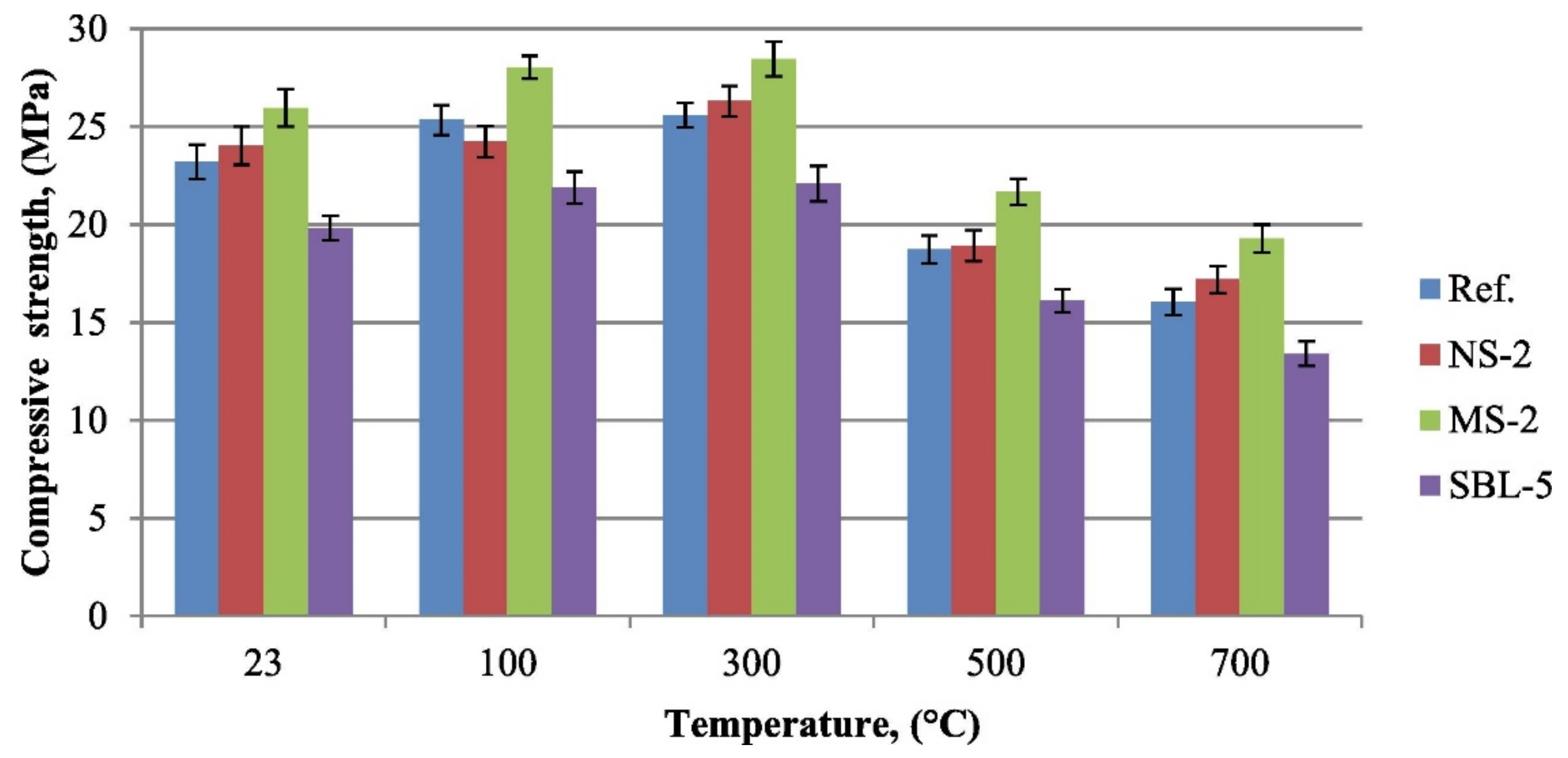
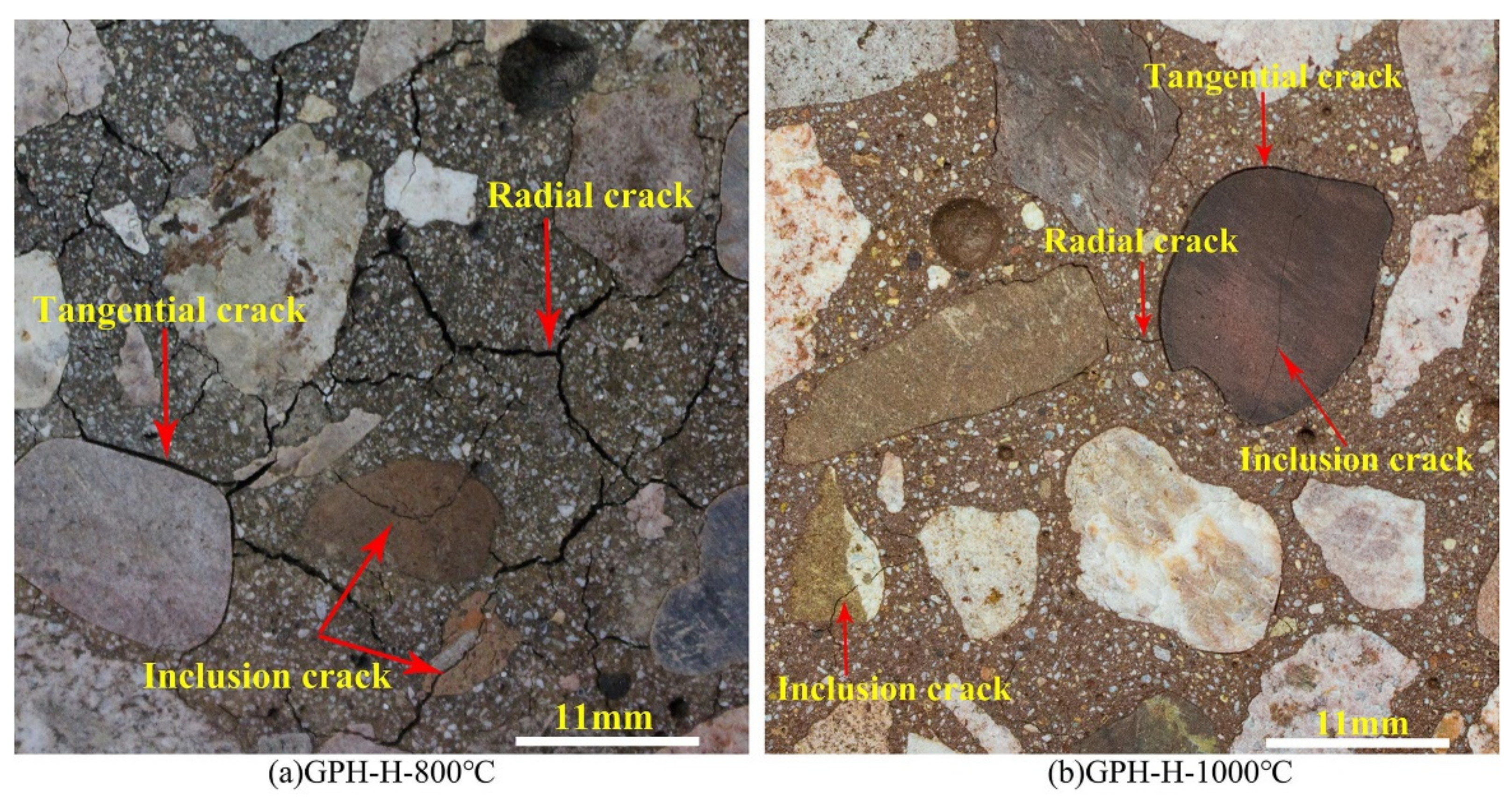
2. Compressive Strength of Geopolymer Concrete at Elevated Temperatures
3. Chloride Ion Penetrability and Corrosion Potential of Geopolymer Concrete
4. Acid Resistance of Geopolymer Concrete
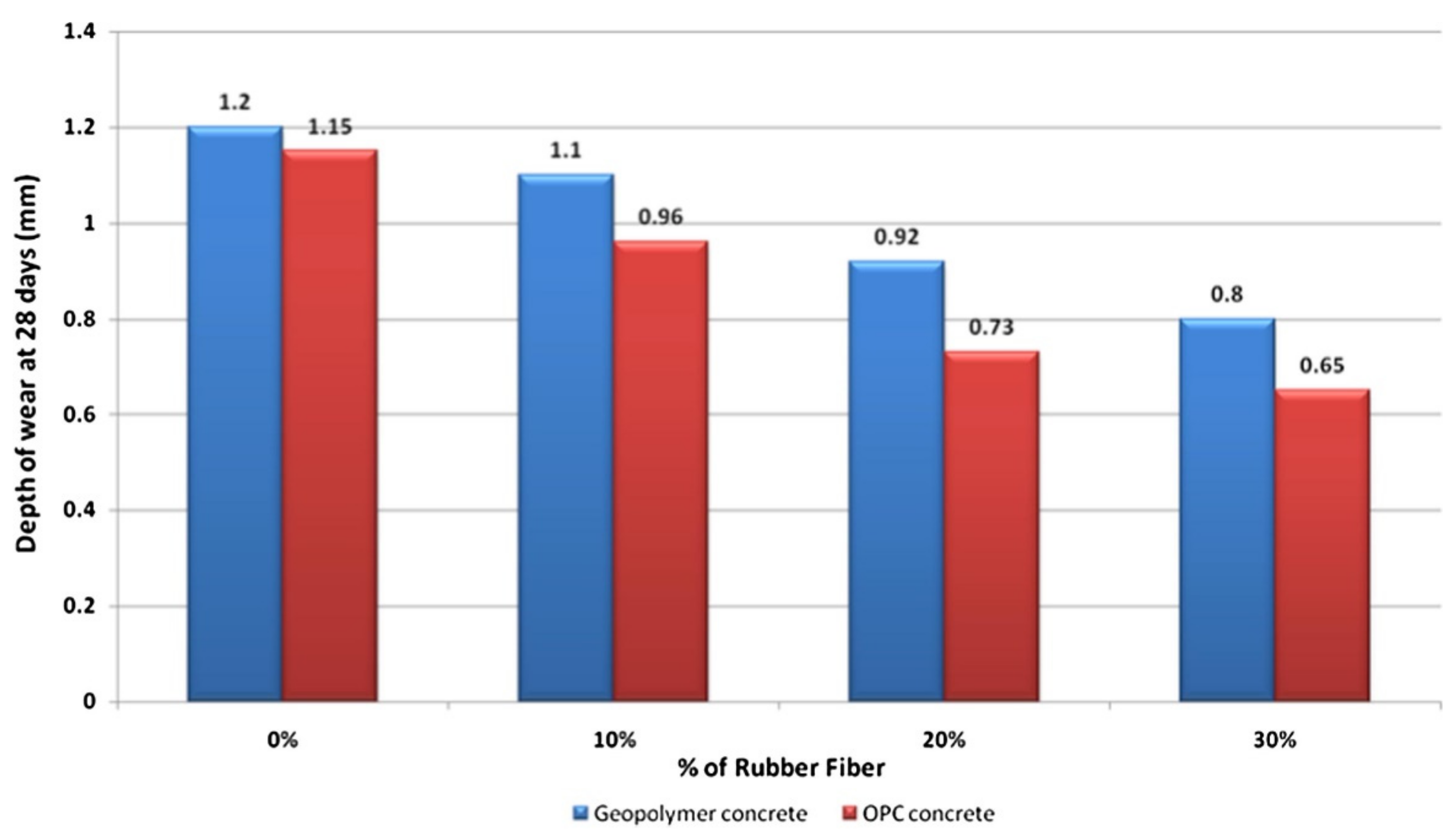
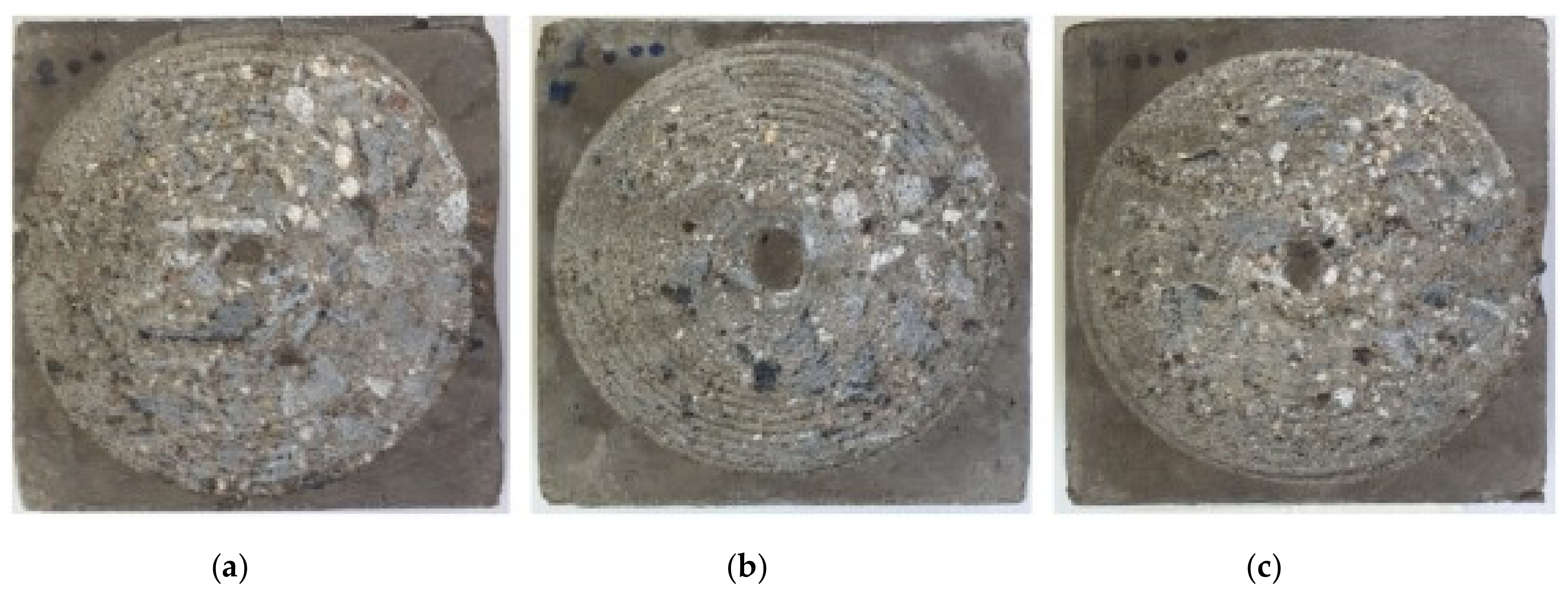
5. Abrasion Resistance of Geopolymer Concrete
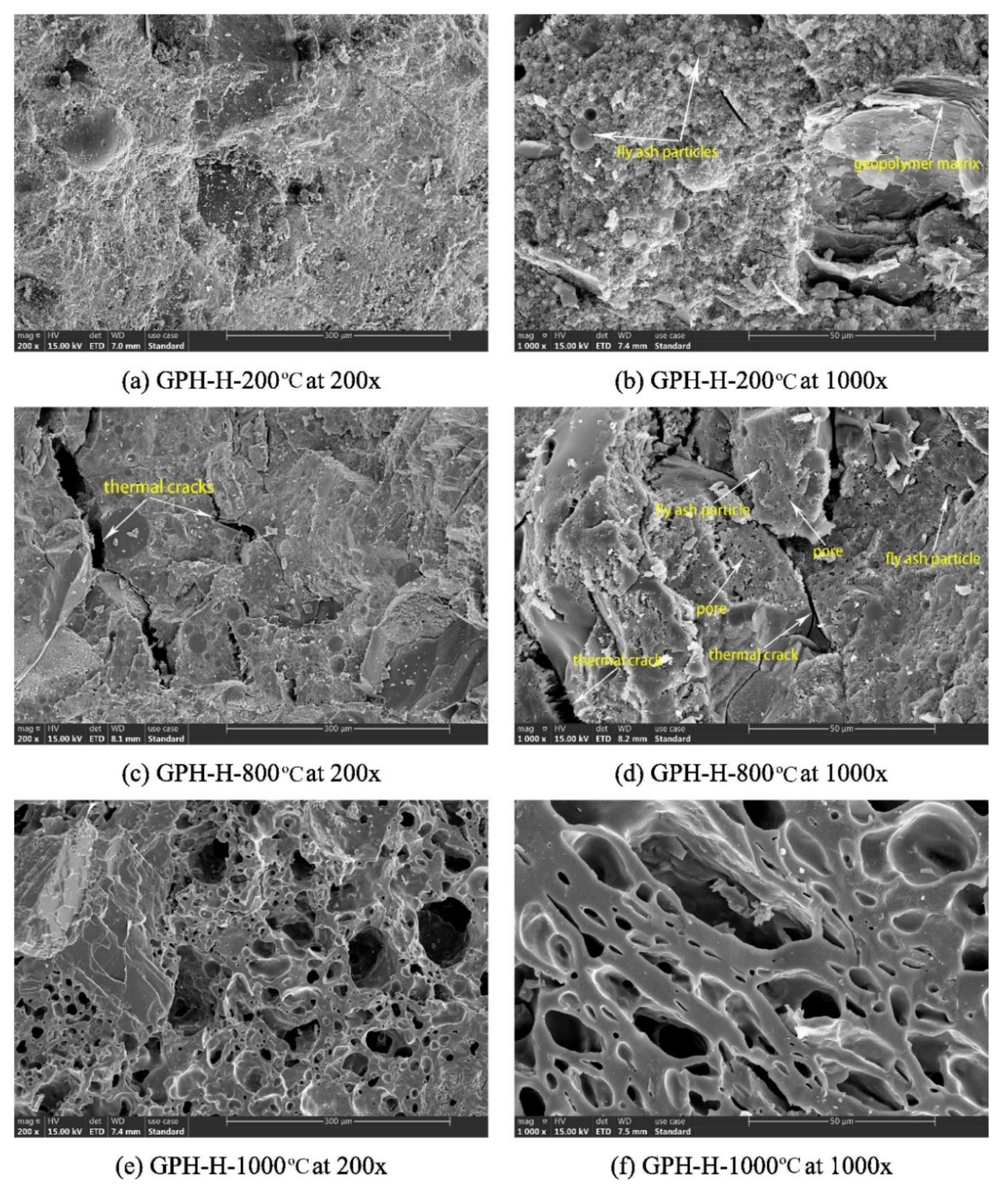
6. Morphological and Chemical Properties of Durable Geopolymer Concrete
7. Research Gap of Geopolymer Concrete
8. Conclusions and Future Recommendations
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shobeiri, V.; Bennet, B.; Xie, T.; Visintin, P. A comprehensive assessment of the global warming potential of geopolymer concrete. J. Clean. Prod. 2021, 297, 126669. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Cristelo, N.; Miranda, T.; Palomo, A. Sustainable alkali activated materials: Precursor and activator derived from industrial wastes. J. Clean. Prod. 2017, 162, 1200–1209. [Google Scholar] [CrossRef]
- Wong, L.S. Microbial cementation of ureolytic bacteria from the genus Bacillus: A review of the bacterial application on cement-based materials for cleaner production. J. Clean. Prod. 2015, 93, 5–17. [Google Scholar] [CrossRef]
- Ting, M.Z.Y.; Wong, K.S.; Rahman, M.E.; Meheron, S.J. Deterioration of marine concrete exposed to wetting-drying action. J. Clean. Prod. 2021, 278, 123383. [Google Scholar] [CrossRef]
- Iqbal, D.M.; Wong, L.S.; Kong, S.Y. Bio-cementation in construction materials: A review. Materials 2021, 14, 2175. [Google Scholar] [CrossRef]
- Aygörmez, Y.; Canpolat, O.; Al-mashhadani, M.M. Assessment of geopolymer composites durability at one year age. J. Build. Eng. 2020, 32, 101453. [Google Scholar] [CrossRef]
- Van Deventer, J.S.J.; Provis, J.L.; Duxson, P. Technical and commercial progress in the adoption of geopolymer cement. Miner. Eng. 2012, 29, 89–104. [Google Scholar] [CrossRef]
- Lodeiro, I.G.; Cristelo, N.; Palomo, A.; Fernández-Jiménez, A. Use of industrial by-products as alkaline cement activators. Constr. Build. Mater. 2020, 253, 119000. [Google Scholar] [CrossRef]
- Assi, L.; Carter, K.; Dever, E.; Anay, R.; Ziehl, P. Sustainable concrete: Building a greener future. J. Clean. Prod. 2018, 198, 1641–1651. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhao, R.; Yuan, Y.; Li, F.; Castel, A.; Xu, T. Ageing coefficient for early age tensile creep of blended slag and low calcium fly ash geopolymer concrete. Constr. Build. Mater. 2020, 262, 119855. [Google Scholar] [CrossRef]
- Zannerni, G.M.; Fattah, K.P.; Al-Tamimi, A.K. Ambient-cured geopolymer concrete with single alkali activator. Sustain. Mater. Technol. 2020, 23, e00131. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; Nicolas, R.S.; Hamdan, S.; van Deventer, J.S.J. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Lodeiro, I.G.; Palomo, A.; Fernández-Jiménez, A. An overview of the chemistry of alkali-activated cement-based binders. In Handbook of Alkali-Activated Cements, Mortars and Concretes, 1st ed.; Pacheco-Torgal, F., Labrincha, J.A., Leonelli, C., Palomo, A., Chindaprasirt, P., Eds.; Elsevier Limited: Amsterdam, The Netherlands, 2015; pp. 19–47. [Google Scholar]
- Provis, J.L.; Yong, S.L.; Duxson, P. Nanostructure/microstructure of metakaolin geopolymers. In Geopolymers: Structures, Processing, Properties and Industrial Applications, 1st ed.; Provis, J.L., Van Deventer, J.S.J., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2009; pp. 72–88. [Google Scholar]
- Xu, H.; Van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef] [Green Version]
- Rahman, S.K.; Al-Ameri, R. A newly developed self-compacting geopolymer concrete under ambient condition. Constr. Build. Mater. 2021, 267, 121822. [Google Scholar] [CrossRef]
- Kastiukas, G.; Ruan, S.; Liang, S.; Zhou, X. Development of precast geopolymer concrete via oven and microwave radiation curing with an environmental assessment. J. Clean. Prod. 2020, 255, 120290. [Google Scholar] [CrossRef]
- Poloju, K.K.; Srinivasu, K. Impact of GGBS and strength ratio on mechanical properties of geopolymer concrete under ambient curing and oven curing. Mater. Today 2021, 42, 962–968. [Google Scholar] [CrossRef]
- Albibah, A.; Alghannam, M.; Abbas, H.; Almusallam, T.; Al-Salloum, Y. Characteristics of metakaolin-based geopolymer concrete for different mix design parameters. J. Mater. Res. Technol. 2021, 10, 84–98. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Yuan, C.; Wang, Q.; Sarker, P.K.; Shi, X. Deterioration of ambient-cured and heat-cured fly ash geopolymer concrete by high temperature exposure and prediction of its residual compressive strength. Constr. Build. Mater. 2020, 262, 120924. [Google Scholar] [CrossRef]
- Chen, K.; Wu, D.; Xia, L.; Cai, Q.; Zhang, Z. Geopolymer concrete durability subjected to aggressive environments—A review of influence factors and comparison with ordinary Portland cement. Constr. Build. Mater. 2021, 279, 122496. [Google Scholar] [CrossRef]
- Wong, L.S.; Oweida, A.F.M.; Kong, S.Y.; Iqbal, D.M.; Regunathan, P. The surface coating mechanism of polluted concrete by Candida ethanolica induced calcium carbonate mineralization. Constr. Build. Mater. 2020, 257, 119482. [Google Scholar] [CrossRef]
- Saranya, P.; Nagarajan, P.; Shashikala, A.P. Behaviour of GGBS-dolomite geopolymer concrete beam-column joints under monotonic loading. Structures 2020, 25, 47–55. [Google Scholar] [CrossRef]
- Meng, Q.; Wu, C.; Hao, H.; Li, J.; Wu, P.; Yang, Y.; Wang, Z. Steel fibre reinforced alkali-activated geopolymer concrete slabs subjected to natural gas explosion in buried utility tunnel. Constr. Build. Mater. 2020, 246, 118447. [Google Scholar] [CrossRef]
- Alrshoudi, F.; Abbas, H.; Abadel, R.; Albidah, A.; Altheeb, A.; Al-Salloum, Y. Compression behavior and modeling of FRP-confined high strength geopolymer concrete. Constr. Build. Mater. 2021, 283, 122759. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Z.; Shao, Z.; Cai, L.; Jin, H.; Zhang, Z.; Qiu, Z.; Rui, X.; Chen, T. Experimental study on durability of fiber reinforced concrete: Effect of cellulose fiber, polyvinyl alcohol fiber and polyolefin fiber. Constr. Build. Mater. 2021, 306, 124867. [Google Scholar] [CrossRef]
- Chithambar Ganesh, A.; Deepak, N.; Deepak, V.; Ajay, S.; Pandian, A.; Karthik. Utilization of PET bottles and plastic granules in geopolymer concrete. Mater. Today 2021, 42, 444–449. [Google Scholar] [CrossRef]
- Alabi, S.A.; Mahachi, J. Chloride ion penetration performance of recycled concrete with different geopolymers. Mater. Today 2021, 38, 762–766. [Google Scholar] [CrossRef]
- Noori, A.S.; Oweed, K.M.; Raouf, R.M.; Abdulrehman, M.A. The relation between destructive and non-destructive tests of geopolymer concrete. Mater. Today 2021, 42, 2125–2133. [Google Scholar] [CrossRef]
- Cárdenas-Pulido, J.; Reyes, J.C.; Carillo, J.; Ramírez, F. Shear behavior of geopolymer concrete panels under diagonal tensile stresses. Eng. Struct. 2020, 212, 110518. [Google Scholar] [CrossRef]
- Rahman, S.S.; Khattak, M.J. Roller compacted geopolymer concrete using recycled concrete aggregate. Constr. Build. Mater. 2021, 283, 122624. [Google Scholar] [CrossRef]
- Khan, I.; Xu, T.; Castel, A.; Gilbert, R.I.; Babaee, M. Roller compacted geopolymer concrete using recycled concrete aggregate. Constr. Build. Mater. 2019, 229, 116840. [Google Scholar] [CrossRef]
- Mesgari, S.; Akbarnezhad, A.; Xiao, J.Z. Recycled geopolymer aggregates as coarse aggregates for Portland cement concrete and geopolymer concrete: Effects on mechanical properties. Constr. Build. Mater. 2020, 236, 117571. [Google Scholar] [CrossRef]
- Mayhoub, O.A.; Nasr, E.A.R.; Ali, Y.; Kohail, M. Properties of slag based geopolymer reactive powder concrete. Ain Shams Eng. J. 2021, 12, 99–105. [Google Scholar] [CrossRef]
- Shi, J.; Liu, B.; Liu, Y.; Wang, E.; He, Z.; Xu, H.; Ren, X. Preparation and characterization of lightweight aggregate foamed geopolymer concretes aerated using hydrogen peroxide. Constr. Build. Mater. 2020, 256, 119442. [Google Scholar] [CrossRef]
- Sáez-Pérez, M.P.; Brümmer, M.; Durán-Suárez, J.A. Effect of the state of conservation of the hemp used in geopolymer and hydraulic lime concretes. Constr. Build. Mater. 2021, 285, 122853. [Google Scholar] [CrossRef]
- Saloni; Singh, A.; Sandhu, V.; Jatin; Parvin. Effects of alccofine and curing conditions on properties of low calcium fly ash-based geopolymer concrete. Mater. Today 2020, 32, 620–625. [Google Scholar] [CrossRef]
- Mathew, G.; Issac, B.M. Effect of molarity of sodium hydroxide on the aluminosilicate content in laterite aggregate of laterised geopolymer concrete. J. Build. Eng. 2020, 32, 101486. [Google Scholar] [CrossRef]
- Jindal, B.B.; Jangra, P.; Garg, A. Effects of ultra fine slag as mineral admixture on the compressive strength, water absorption and permeability of rice husk ash based geopolymer concrete. Mater. Today 2020, 32, 871–877. [Google Scholar]
- Alabi, S.A.; Mahachi, J. Estimating the compressive strength of geopolymer recycled concrete. Mater. Today 2021, 43, 1973–1976. [Google Scholar] [CrossRef]
- Erfanimanesh, A.; Sharbatdar, M.K. Mechanical and microstructural characteristics of geopolymer paste, mortar, and concrete containing local zeolite and slag activated by sodium carbonate. J. Build. Eng. 2020, 32, 101781. [Google Scholar] [CrossRef]
- Bellum, R.R.; Muniraj, K.; Indukuri, C.S.R.; Madduru, S.R.C. Investigation on performance enhancement of fly ash—GGBFS based graphene geopolymer concrete. J. Build. Eng. 2020, 32, 101659. [Google Scholar] [CrossRef]
- Pasupathy, K.; Ramakrishnan, S.; Sanjayan, J. Influence of recycled concrete aggregate on the foam stability of aerated geopolymer concrete. Constr. Build. Mater. 2021, 271, 121850. [Google Scholar] [CrossRef]
- Talha Ghafoor, M.; Khan, Q.S.; Qazi, A.U.; Sheikh, M.N.; Hadi, M.N.S. Influence of alkaline activators on the mechanical properties of fly ash based geopolymer concrete cured at ambient temperature. Constr. Build. Mater. 2021, 273, 121752. [Google Scholar] [CrossRef]
- Ouda, A.S. A preliminary investigation on gamma-ray attenuation of alkali-activated concrete waste based-geopolymer modified with pozzocrete-fly ash. Prog. Nucl. Energy. 2021, 134, 103681. [Google Scholar] [CrossRef]
- Ishak, S.; Lee, H.S.; Singh, J.K.; Ariffin, M.A.M.; Lim, N.H.A.S.; Yang, H.M. Performance of fly ash geopolymer concrete incorporating bamboo ash at elevated temperature. Materials 2019, 12, 3404. [Google Scholar] [CrossRef] [Green Version]
- Luhar, S.; Chaudhary, S.; Luhar, I. Thermal resistance of fly ash based rubberized geopolymer concrete. J. Build. Eng. 2018, 19, 420–428. [Google Scholar] [CrossRef]
- Kantarci, F.; Türkmen, I.; Ekinci, E. Improving elevated temperature performance of geopolymer concrete utilizing nano-silica, micro-silica and styrene-butadiene latex. Constr. Build. Mater. 2021, 286, 122980. [Google Scholar] [CrossRef]
- Rahmadina, A.; Ekaputri, J.J. Mechanical properties of geopolymer concrete exposed to combustion. In Green Infrastructure for Future World, Proceedings of the 6th International Conference of Euro Asia Civil Engineering Forum 2017, Hanyang University; Seoul, Korea, 22–25 August 2017, Park, J.-W., Ayli, H., Hardjasaputra, H., Thayaalan, P., Eds.; EDP Sciences: Seoul, Korea, 2017; Volume 138, pp. 1–10. [Google Scholar]
- Jiang, X.; Zhang, Y.; Xiao, R.; Polaczyk, P.; Zhang, M.; Hu, W.; Bai, Y.; Huang, B. A comparative study on geopolymers synthesized by different classes of fly ash after exposure to elevated temperatures. J. Clean. Prod. 2020, 270, 122500. [Google Scholar] [CrossRef]
- Payakaniti, P.; Chuewangkam, N.; Yensano, R.; Pinitsoontorn, S.; Chindaprasirt, P. Changes in compressive strength, microstructure and magnetic properties of a high-calcium fly ash geopolymer subjected to high temperatures. Constr. Build. Mater. 2020, 265, 120650. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, J.; Zhang, H.; Li, M.; Wu, Y.; Guo, L.; Wang, W.; Duan, P.; Zhang, W.; Zhang, Z. Thermal stability and microstructure of metakaolin-based geopolymer blended with rice husk ash. Appl. Clay Sci. 2020, 196, 105769. [Google Scholar] [CrossRef]
- Bernal, S.A.; Bejarano, J.; Garzón, C.; de Gutiérrez, M.; Delvasto, S.; Rodríguez, E.D. Performance of refractory aluminosilicate particle/fiber-reinforced geopolymer composites. Compos. B Eng. 2012, 43, 1919–1928. [Google Scholar] [CrossRef]
- Lee, W.H.; Wang, J.H.; Ding, Y.C.; Cheng, T.W. A study on the characteristics and microstructures of GGBS/FA based geopolymer paste and concrete. Constr. Build. Mater. 2019, 211, 807–813. [Google Scholar] [CrossRef]
- Mousavinejad, S.H.G.; Sammak, M. Strength and chloride ion penetration resistance of ultra-high-performance fiber reinforced geopolymer concrete. Structures 2021, 32, 1420–1427. [Google Scholar] [CrossRef]
- Ganesan, N.; Abraham, R.; Raj, S.D. Durability characteristics of steel fibre reinforced geopolymer concrete. Constr. Build. Mater. 2015, 93, 471–476. [Google Scholar] [CrossRef]
- ASTM. Standard test method for electrical indication of concrete’s ability to resist chloride ion penetration. In Annual Book of American Society for Testing Materials Standards; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Koushkbaghi, M.; Alipour, P.; Tahmouresi, B.; Mohseni, E.; Saradar, A.; Sarker, P.K. Influence of different monomer ratios and recycled concrete aggregate on mechanical properties and durability of geopolymer concretes. Constr. Build. Mater. 2019, 205, 519–528. [Google Scholar] [CrossRef]
- Mohseni, E.; Kazemi, M.J.; Koushkbaghi, M.; Zehtab, B.; Behforouz, B. Evaluation of mechanical and durability properties of fiber-reinforced lightweight geopolymer composites based on rice husk ash and nanoalumina. Constr. Build. Mater. 2019, 209, 532–540. [Google Scholar] [CrossRef]
- Amorim, N.S., Jr.; Andrade Neto, J.S.; Santana, H.A. Durability and service life analysis of metakaolin-based geopolymer concretes with respect to chloride penetration using chloride migration test and corrosion potential. Constr. Build. Mater. 2021, 287, 122970. [Google Scholar] [CrossRef]
- Okoye, F.N.; Prakash, S.; Singh, N.B. Durability of fly ash based geopolymer concrete in the presence of silica fume. J. Clean. Prod. 2017, 149, 1062–1067. [Google Scholar] [CrossRef]
- Ariffin, M.A.M.; Bhutta, M.A.R.; Hussin, M.W.; Tahir, M.M.; Aziah, N. Sulfuric acid resistance of blended ash geopolymer concrete. Constr. Build. Mater. 2013, 43, 80–86. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. Sulfuric acid resistance of fly ash based geopolymer concrete. Constr. Build. Mater. 2017, 146, 136–143. [Google Scholar] [CrossRef]
- Charkhtab Moghaddam, S.; Madandoust, R.; Jamshidi, M.; Nikbin, I.M. Mechanical properties of fly ash-based geopolymer concrete with crumb rubber and steel fiber under ambient and sulfuric acid conditions. Constr. Build. Mater. 2021, 281, 122571. [Google Scholar] [CrossRef]
- Abhilash, P.; Sashidhar, C.; Ramana Reddy, I.V. Evaluation of performance of geopolymer concrete in acid environment. Int. Res. J. Eng. Technol. 2017, 4, 1433–1438. [Google Scholar]
- Valencia-Saavedra, W.G.; de Gutiérrez, R.M.; Puertas, F. Performance of FA-based geopolymer concretes exposed to acetic and sulfuric acids. Constr. Build. Mater. 2020, 257, 119503. [Google Scholar] [CrossRef]
- Çevik, A.; Alzeebaree, R.; Humur, G.; Niş, A.; Gülşan, M.E. Effect of nano-silica on the chemical durability and mechanical performance of fly ash based geopolymer concrete. Ceramics 2018, 44, 12253–12264. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Nnaemeka, O.F.; Singh, N.B. Durability properties of geopolymer concrete made from fly ash in presence of kaolin. Mater. Today 2020, 29, 781–784. [Google Scholar] [CrossRef]
- Lau, C.K.; Lee, H.; Vimonsatit, V.; Huen, W.Y.; Chindaprasirt, P. Abrasion resistance behaviour of fly ash based geopolymer using nanoindentation and artificial neural network. Constr. Build. Mater. 2019, 212, 635–644. [Google Scholar] [CrossRef]
- Wongsa, A.; Sata, V.; Nuaklong, P.; Chindaprasirt, P. Use of crushed clay brick and pumice aggregates in lightweight geopolymer concrete. Constr. Build. Mater. 2018, 188, 1025–1034. [Google Scholar] [CrossRef]
- Luhar, S.; Chaudhary, S.; Luhar, I. Development of rubberized geopolymer concrete: Strength and durability studies. Constr. Build. Mater. 2019, 204, 740–753. [Google Scholar] [CrossRef]
- Ramujee, K.; Potharaju, M. Abrasion resistance of geopolymer composites. Procedia Mater. Sci. 2014, 6, 1961–1966. [Google Scholar] [CrossRef] [Green Version]
- Naveen Kumar, D.; Ramujee, K. Abrasion resistance of polypropylene fiber reinforced geopolymer concrete. J. Emerg. Technol. 2017, 4, 276–283. [Google Scholar]
- Wongkvanklom, A.; Posi, P.; Kampala, A.; Kaewngao, T.; Chindaprasirt, P. Beneficial utilization of recycled asphaltic concrete aggregate in high calcium fly ash geopolymer concrete. Case Stud. Constr. Mater. 2021, 15, e00615. [Google Scholar] [CrossRef]
- Kathirvel, P.; Sreekumaran, S. Sustainable development of ultra high performance concrete using geopolymer technology. J. Build. Eng. 2021, 39, 102267. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, K.; Wang, S.; Wang, Z.; Yang, Z.; Shumuye, E.D.; Gong, X. Effect of elevated temperature on mechanical properties of high-volume fly ash-based geopolymer concrete, mortar and paste cured at room temperature. Polymers 2021, 13, 1473. [Google Scholar] [CrossRef]
- Le, H.B.; Bui, Q.B.; Tang, L. Geopolymer recycled aggregate concrete: From experiments to empirical models. Materials 2021, 14, 1180. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, W.; Shi, D. Effect of elevated temperature on the properties of geopolymer synthesized from calcined ore-dressing tailing of bauxite and ground-granulated blast furnace slag concrete. Constr. Build. Mater. 2014, 69, 41–48. [Google Scholar] [CrossRef]
- Sontia Metekong, J.V.; Kaze, C.R.; Deutou, J.G.; Venyite, P.; Nana, A.; Kamseu, E.; Melo, U.C.; Tatietse, T.T. Evaluation of performances of volcanic-ash-laterite based blended geopolymer concretes: Mechanical properties and durability. J. Build. Eng. 2021, 34, 101935. [Google Scholar] [CrossRef]
- Thokchom, S. Fly ash geopolymer pastes in sulphuric acid. Int. J. Eng. Innov. Res. 2014, 3, 943–947. [Google Scholar]
- Assi, L.N.; Carter, K.; Deaver, E.; Ziehl, P. Review of availability of source materials for geopolymer/sustainable concrete. J. Clean. Prod. 2020, 263, 121477. [Google Scholar] [CrossRef]
- Ng, C.; Alengaram, J.; Wong, L.S.; Mo, K.H.; Jumaat, M.Z.; Ramesh, S. A review on microstructural study and compressive strength of geopolymer mortar, paste and concrete. Constr. Build. Mater. 2018, 186, 550–576. [Google Scholar] [CrossRef]
- Jindal, B.B.; Sharma, R. The effect of nanomaterials on properties of geopolymers derived from industrial by-products: A state-of-the-art review. Constr. Build. Mater. 2020, 252, 119028. [Google Scholar] [CrossRef]
- Tennakoon, C.; Nicolas, R.S.; Sanjayan, J.G.; Shayan, A. Thermal effects of activators on the setting time and rate of workability loss of geopolymers. Ceramics 2016, 42, 19257–19268. [Google Scholar] [CrossRef]

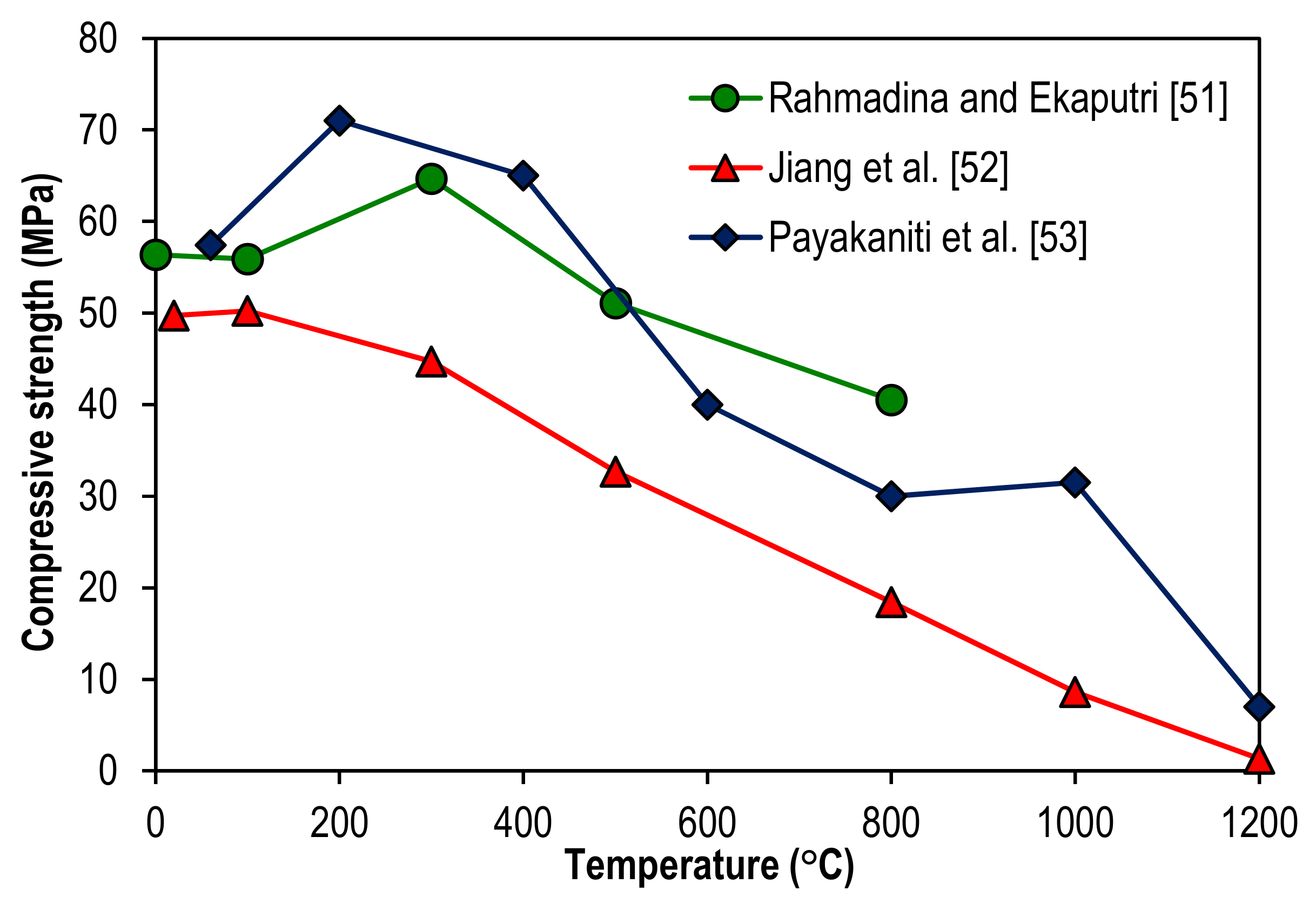


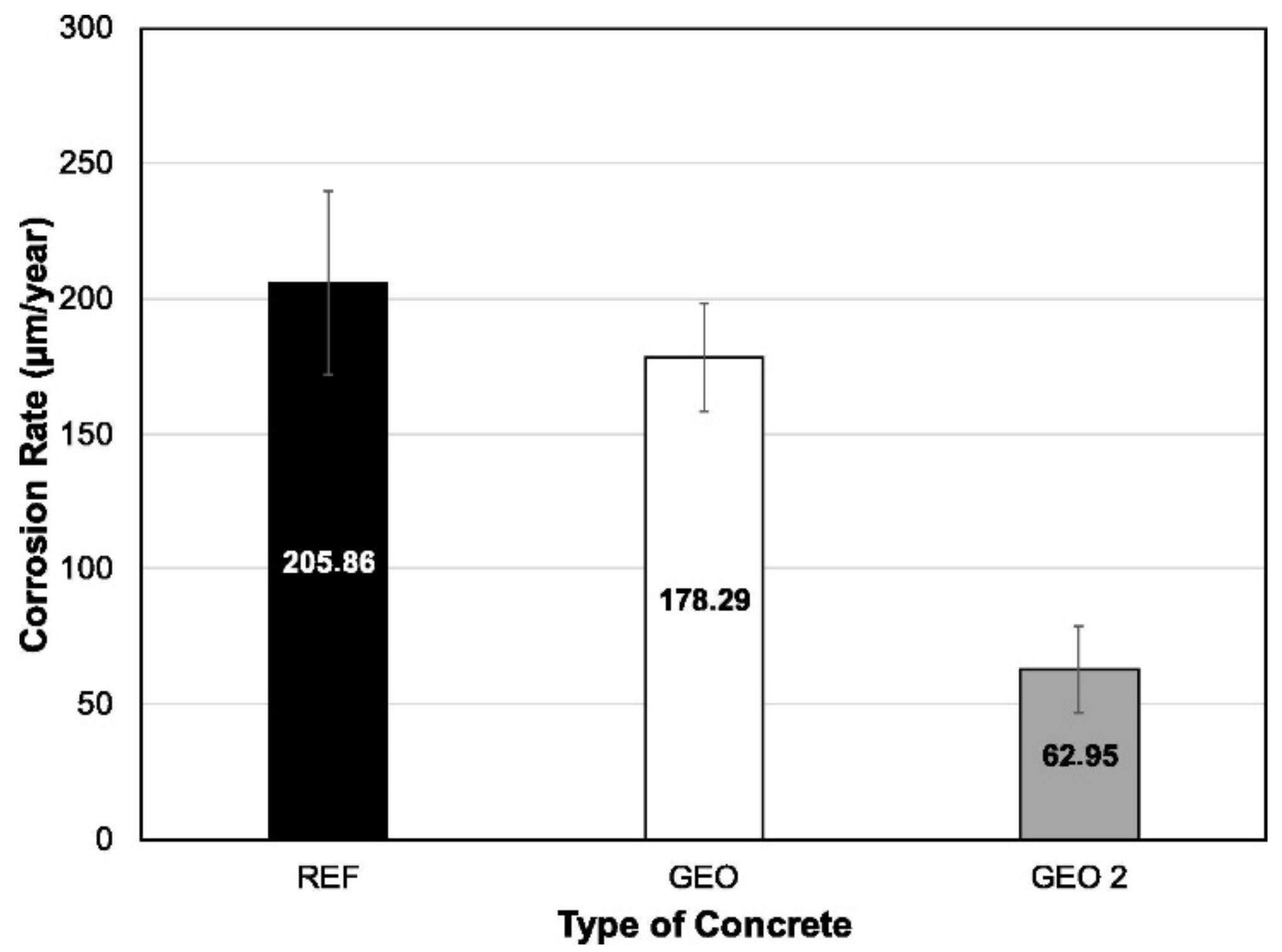
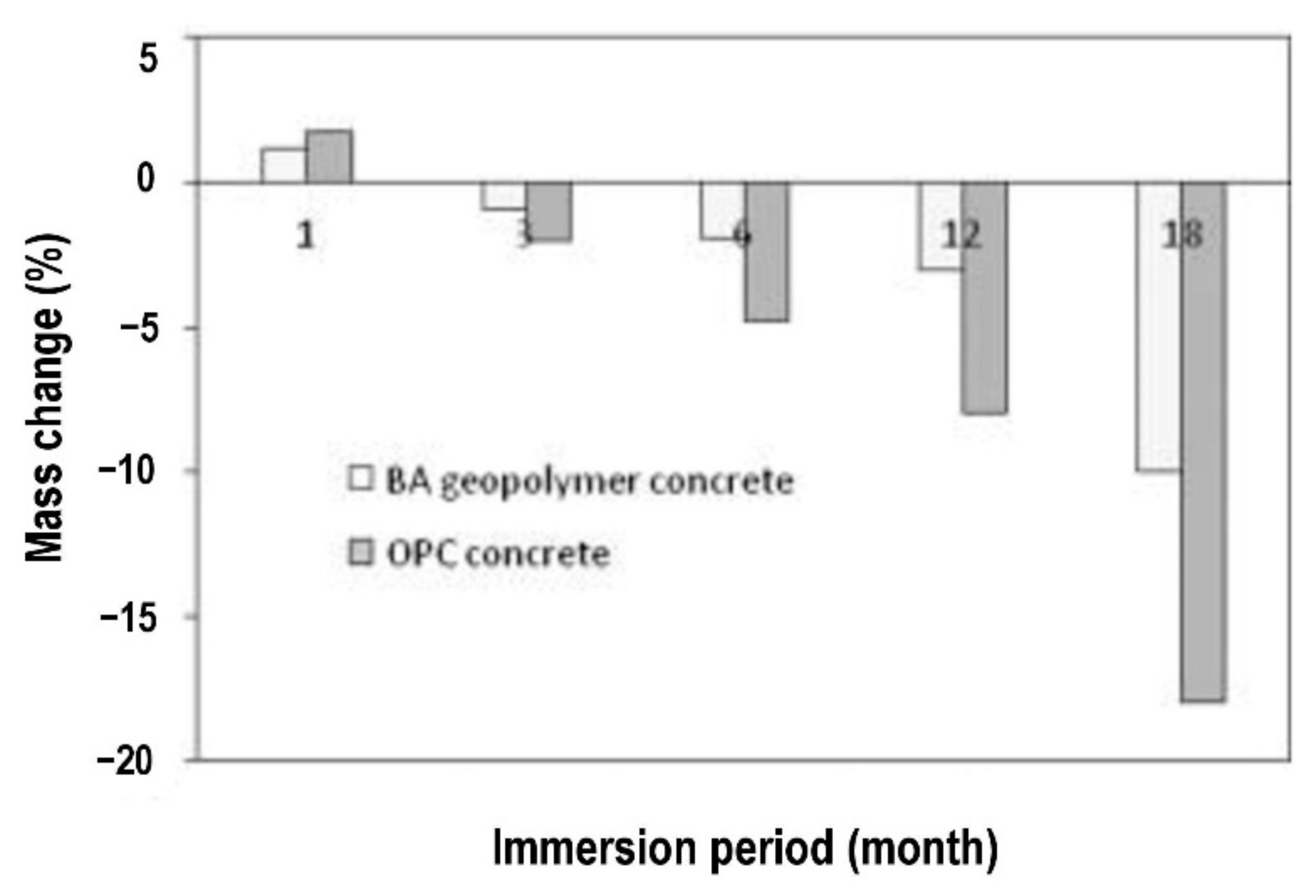
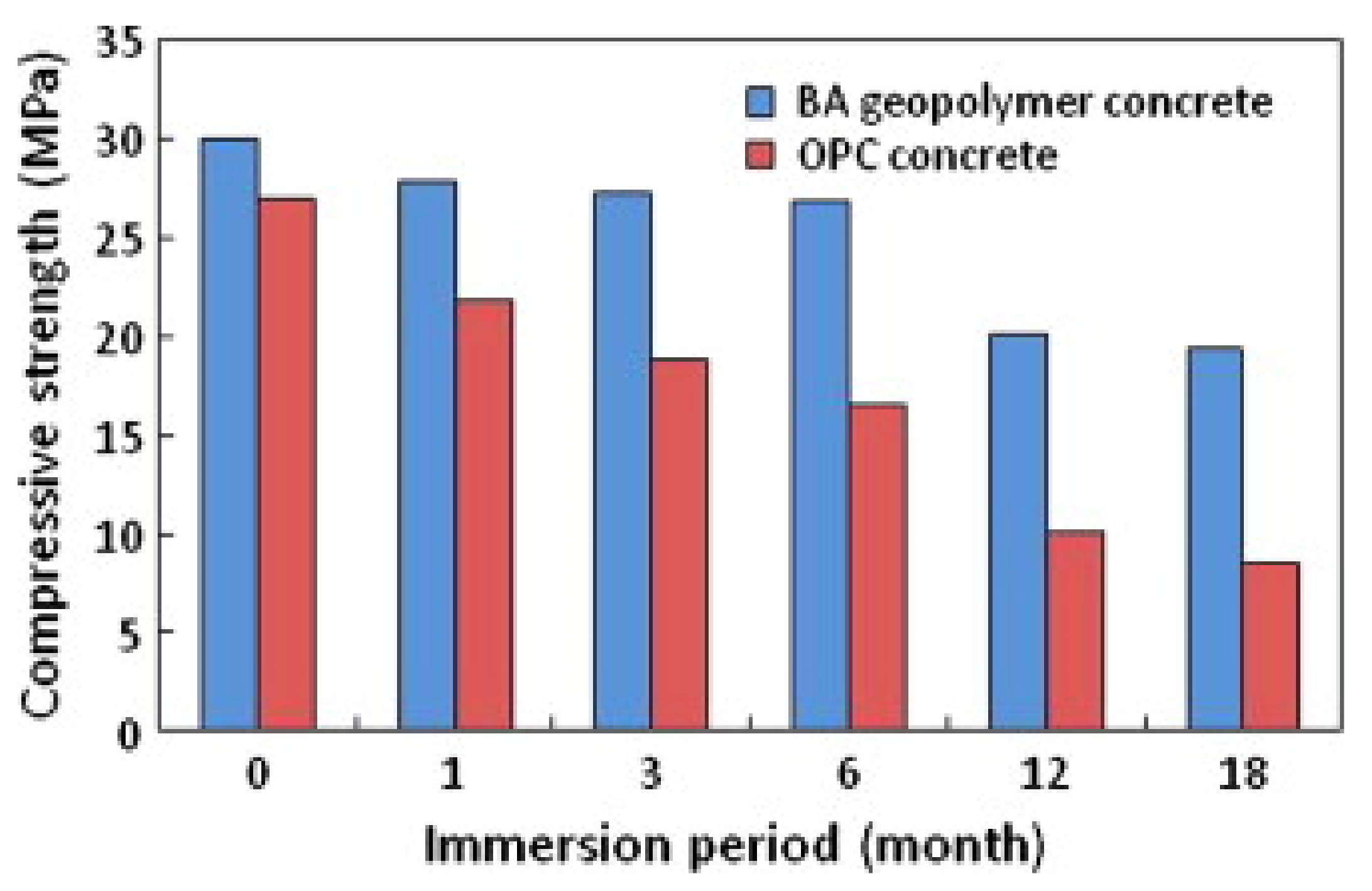



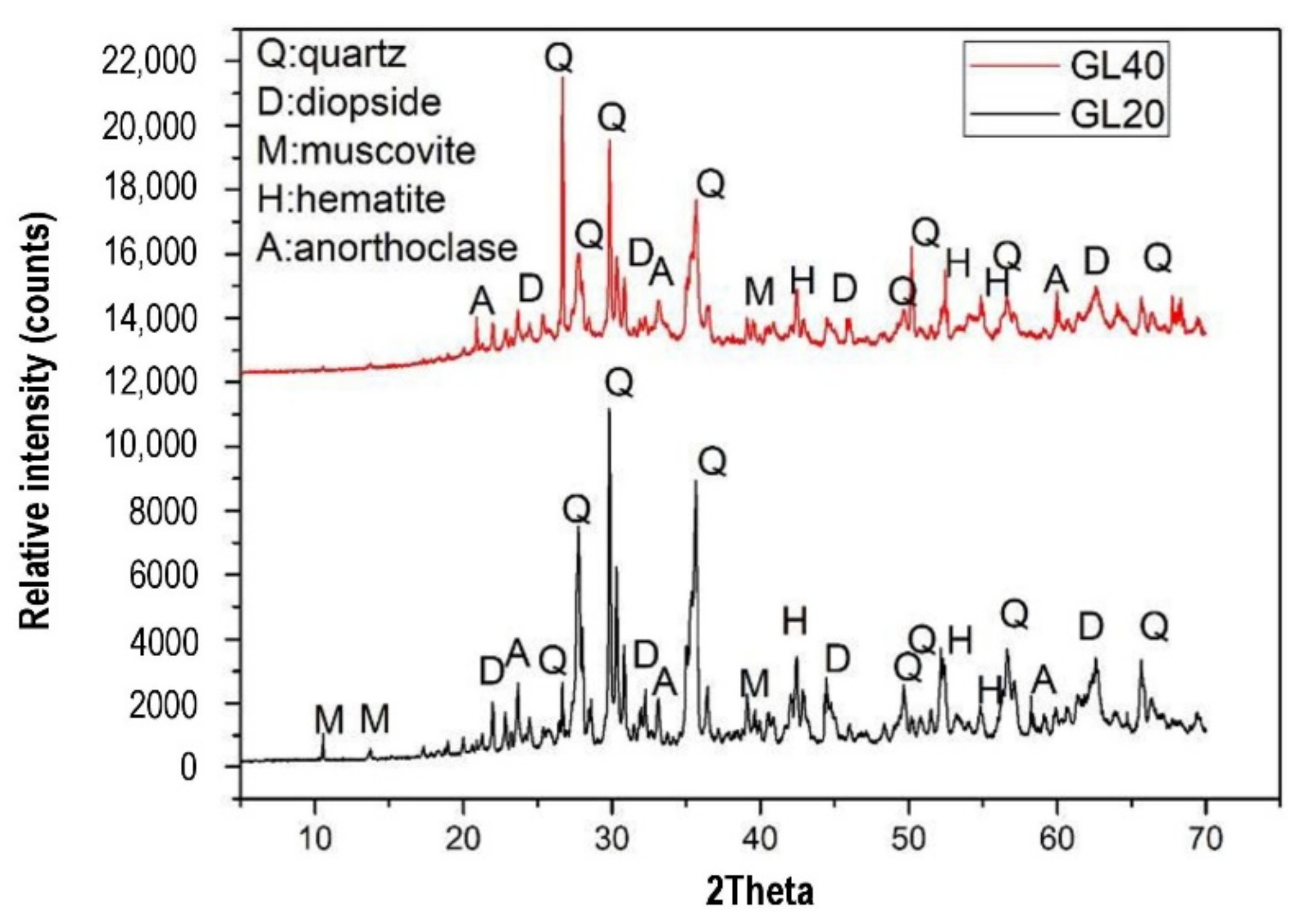
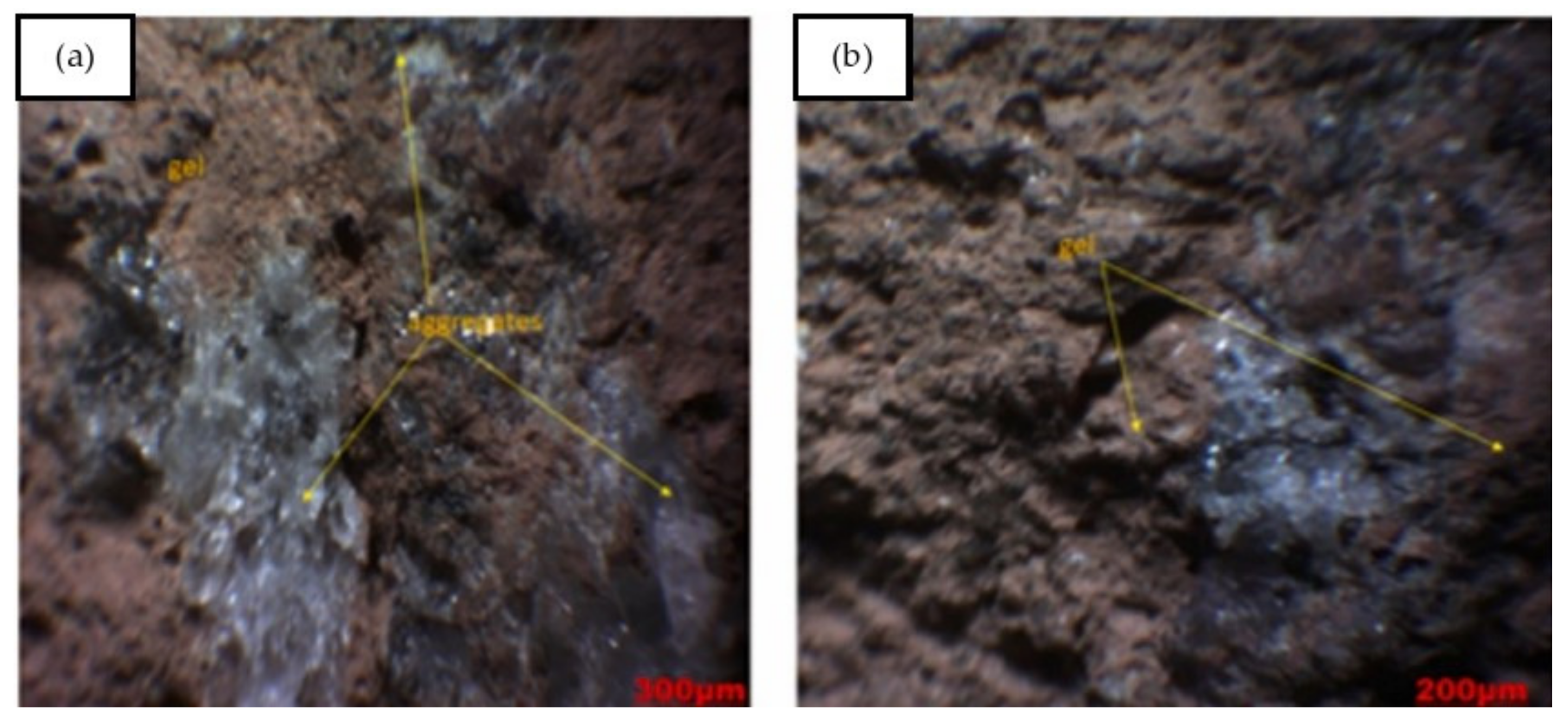
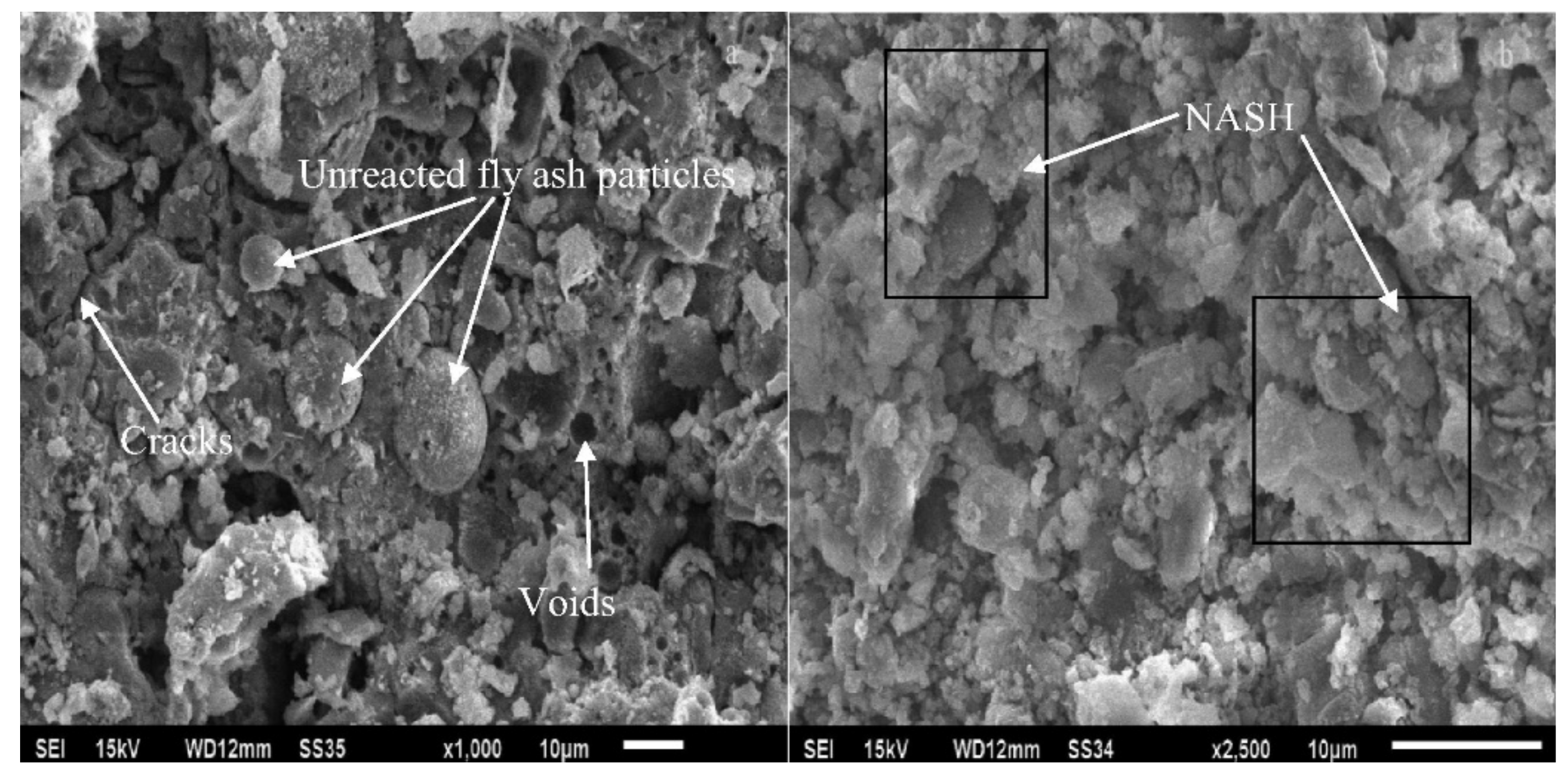
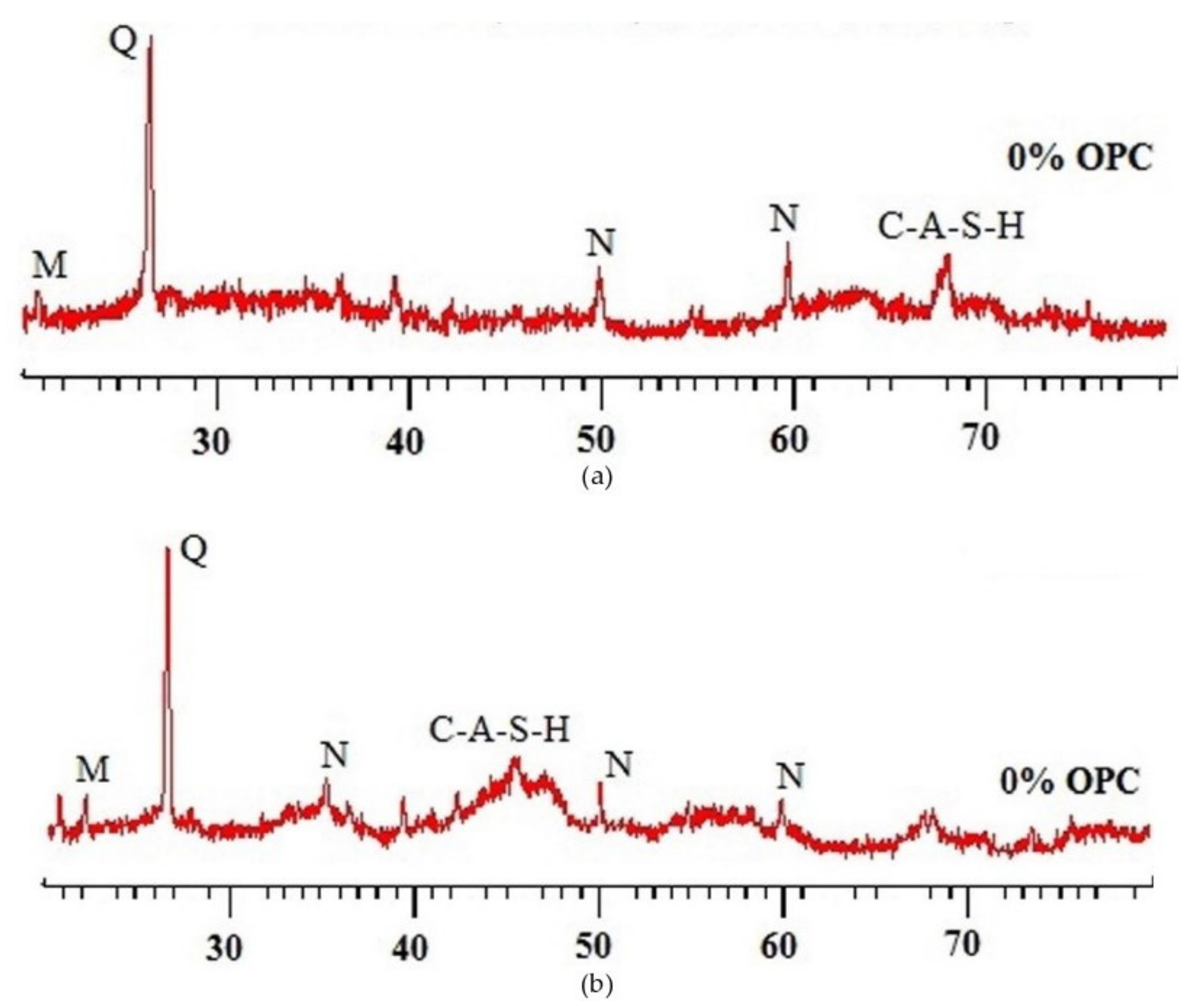
| Published Work | Method of Geopolymerization | Chemical Composition of Geopolymer Specimen Admixture |
|---|---|---|
| Rahmadina and Ekaputri [51] | Alkaline activation by sodium hydroxide and sodium silicate solutions with Class F fly ash as a precursor. | 8 molar concentration of sodium hydroxide, 18.5% sodium oxide, 36.4% silica, and 45.1% water. |
| Jiang et al. [52] | Alkaline activation by sodium hydroxide and sodium silicate solutions with Class F fly ash as a precursor. | 10 molar concentration of sodium hydroxide, 8.3% sodium oxide, 28.7% silica, and 63% water. |
| Payakaniti et al. [53] | Alkaline activation by sodium hydroxide and sodium silicate solutions with high calcium (Class C) lignite fly ash as a precursor. | 10 molar concentration of sodium hydroxide, 12.53% sodium silicate by weight of sodium oxide, 30.24% silica, and 57.23% water. |
| Mix | Steel Fiber (%) | Fly Ash (kg m−3) | Sodium Silicate Solution (kg m−3) | Sodium Hydroxide Solution (kg m−3) | CA (kg m−3) | FA (kg m−3) | Water (kg m−3) | SP (kg m−3) | Cement (kg m−3) |
|---|---|---|---|---|---|---|---|---|---|
| GPC | 0 | 408 | 103 | 41 | 1248 | 600 | 14.5 | 10.2 | 0 |
| SFRGPC1 | 0.25 | 408 | 103 | 41 | 1248 | 600 | 16 | 10.2 | 0 |
| SFRGPC2 | 0.5 | 408 | 103 | 41 | 1248 | 600 | 16 | 14.5 | 0 |
| SFRGPC3 | 0.75 | 408 | 103 | 41 | 1248 | 600 | 18 | 14.5 | 0 |
| SFRGPC4 | 1 | 408 | 103 | 41 | 1248 | 600 | 18 | 16 | 0 |
| CC | 0 | 0 | 0 | 0 | 1266 | 598 | 192 | 0 | 360 |
| SFRC2 | 0.5 | 0 | 0 | 0 | 1266 | 598 | 192 | 4 | 360 |
| Mix | Depth of Chloride Penetration (cm) | Diffusion Coefficient (m2 s−1) | Charge Passed (Coulombs) | Chloride Ion Penetrability as per ASTM |
|---|---|---|---|---|
| GPC | 2.45 | 1.24 × 10−11 | 1321 | Low |
| SFRGPC1 | 2.42 | 1.21 × 10−11 | 1445 | Low |
| SFRGPC2 | 2.39 | 1.18 × 10−11 | 1392 | Low |
| SFRGPC3 | 2.36 | 1.15 × 10−11 | 1566 | Low |
| SFRGPC4 | 2.22 | 1.02 × 10−11 | 1762 | Low |
| CC | 2.49 | 1.28 × 10−11 | 1764 | Low |
| SFRC2 | 2.47 | 1.26 × 10−11 | 1423 | Low |
| Mix ID | Metakaolin | SS | SH | Aggregates | ||
|---|---|---|---|---|---|---|
| Fine Aggregate | NCA | RCA | ||||
| R2 a | 400 | 90 | 45 | 600 | 1250 | 0 |
| R2RCA10 | 400 | 90 | 45 | 600 | 1025 | 115 |
| R2RCA20 | 400 | 90 | 45 | 600 | 910 | 230 |
| R2RCA30 | 400 | 90 | 45 | 600 | 800 | 340 |
| R2.5 | 400 | 115 | 45 | 600 | 1250 | 0 |
| R2.5RCA10 | 400 | 115 | 45 | 600 | 1025 | 115 |
| R2.5RCA20 | 400 | 115 | 45 | 600 | 910 | 230 |
| R2.5RCA30 | 400 | 115 | 45 | 600 | 800 | 340 |
| R3 | 400 | 135 | 45 | 600 | 1250 | 0 |
| R3RCA10 | 400 | 135 | 45 | 600 | 1025 | 115 |
| R3RCA20 | 400 | 135 | 45 | 600 | 910 | 230 |
| R3RCA30 | 400 | 135 | 45 | 600 | 800 | 340 |
| Mix ID | RHA (kg m−3) | NA (kg m−3) | PP (kg m−3) | SS (kg m−3) | SH (kg m−3) | Sand (kg m−3) | CA (kg m−3) | LWA (kg m−3) |
|---|---|---|---|---|---|---|---|---|
| Control | 315 | 40 | 0 | 115 | 45 | 600 | 1250 | 0 |
| PP0.5 | 315 | 40 | 2 | 115 | 45 | 600 | 1250 | 0 |
| PP1 | 315 | 40 | 4 | 115 | 45 | 600 | 1250 | 0 |
| LWA10 | 315 | 40 | 0 | 115 | 45 | 600 | 1125 | 55 |
| PP0.5-LWA10 | 315 | 40 | 2 | 115 | 45 | 600 | 1125 | 55 |
| PP1-LWA10 | 315 | 40 | 4 | 115 | 45 | 600 | 1125 | 55 |
| LWA20 | 315 | 40 | 0 | 115 | 45 | 600 | 1000 | 110 |
| PP0.5-LWA20 | 315 | 40 | 2 | 115 | 45 | 600 | 1000 | 110 |
| PP1-LWA20 | 315 | 40 | 4 | 115 | 45 | 600 | 1000 | 110 |
| Mix | Material Consumption (kg m−3 of Concrete) | ||||||
|---|---|---|---|---|---|---|---|
| Portland Cement | Metakaolin | Sand | Gravel | Water | Sodium Hydroxide | Sodium Silicate | |
| REF | 402.69 | 0.00 | 736.92 | 954.37 | 241.60 | 0.00 | 0.00 |
| GEO | 0.00 | 340.55 | 623.21 | 807.10 | 0.00 | 170.27 | 323.52 |
| GEO2 | 0.00 | 355.93 | 651.35 | 843.55 | 0.00 | 177.96 | 265.88 |
| Published Work | Key Discovery on Geopolymer Concrete’s Resistance to Sulfuric Acid |
|---|---|
| Abhilash et al. [67] | After submersion in a 2% sulfuric acid solution for 28 days, the geopolymer concrete was tested to have a 33.82 MPa compressive strength, which is 5.72% less than that without the acid exposure. |
| Valencia-Saavedra et al. [68] | After 28-day submersion in a sulfuric acid solution of 1 molar concentration, the geopolymer concrete was tested to have a 34.34 MPa compressive strength, which is 20% less than that without the acid exposure. |
| Çevik et al. [69] | After immersion in a 5% sulfuric acid solution for one month, the geopolymer concrete was tested to have a 39.2 MPa compressive strength, which is 19% less than that without the acid exposure. |
| Published Work | Alkaline Activator | Precursor | Geopolymer Concrete Admixture |
|---|---|---|---|
| Abhilash et al. [67] | Sodium silicate and sodium hydroxide | Fly ash | 409 kg m−3 fly ash, 41 kg m−3 sodium hydroxide, 102 kg m−3 sodium silicate, 549 kg m−3 slag (fine aggregate), 903 kg m−3 crushed stone (coarse aggregate), and 387 kg m−3 coal washery rejects (coarse aggregate). |
| Valencia-Saavedra et al. [68] | Sodium silicate, sodium hydroxide, and granulated blast furnace slag | Fly ash | 320 kg m−3 fly ash, 80 kg m−3 granulated blast furnace slag, 28.55 kg m−3 sodium hydroxide, 158.37 kg m−3 sodium silicate, 972.7 kg m−3 sand, and 704.4 kg m−3 gravel (Liquid/solid ratio = 0.48). |
| Çevik et al. [69] | Sodium silicate and sodium hydroxide | Fly ash and nano-silica | 500 kg m−3 fly ash, 15 kg m−3 nano-silica, 225 kg m−3 sodium hydroxide and sodium silicate, 1150 kg m−3 coarse aggregate, 575 kg m−3 fine aggregate, and 6 kgm−3 superplasticizer. |
| Published Work | Compressive Strength of Geopolymer Concrete without Acid Exposure (MPa) | Compressive Strength of Geopolymer Concrete with Acid Exposure (MPa) | Compressive Strength Reduction (%) |  |
| Abhilash et al. [67] | 35.87 | 33.82 | 5.72 | |
| Valencia-Saavedra et al. [68] | 42.92 | 34.34 | 20 | |
| Çevik et al. [69] | 48.4 | 39.2 | 19 | |
| Average | 14.91 |
| Time (Hours) | Average Depth of Wear (mm) | |
|---|---|---|
| Geopolymer Concrete | OPC Concrete | |
| 12 | 2.8 | 4.5 |
| 24 | 4.4 | 7.2 |
| Concrete Type | Concrete Admixture |
|---|---|
| Geopolymer concrete | 327 kg m−3 fly ash, 54.33 kg m−3 sodium hydroxide (8 M), 108.67 kg m−3 sodium silicate, 672 kg m−3 fine aggregate, 1248 kg m−3 coarse aggregate, and 22 kg m−3 water (Liquid/binder ratio = 0.50). |
| OPC concrete | 327 kg m−3 fly ash, 328 kg m−3 ordinary Portland cement, 2 kg m−3 sulphonated napthalene based superplasticizer (GLENIUM B233), 672 kg m−3 fine aggregate, 1248 kg m−3 coarse aggregate, and 163 kg m−3 water (Liquid/binder ratio = 0.50). |
| Liquid Alkaline/Ash Ratio | Surface Abrasion Weight Loss (g) | ||
|---|---|---|---|
| 00RACA | 20RACA | 40RACA | |
| 0.45 | 2.08 | 2.22 | 2.11 |
| 0.55 | 2.14 | 2.11 | 1.88 |
| 0.65 | 3.16 | 2.27 | 2.06 |
| 0.75 | 4.16 | 3.21 | 3.16 |
| Mix ID | Coarse Aggregate (kg m−3) | Sand (kg m−3) | Fly Ash (kg m−3) | Sodium Hydroxide (kg m−3) | Sodium Silicate (kg m−3) | |
|---|---|---|---|---|---|---|
| RACA | NA | |||||
| 0.45LA—00RACA | 0 | 1090 | 590 | 428 | 96.3 | 96.3 |
| 0.45LA—20RACA | 220 | 875 | 590 | 428 | 96.3 | 96.3 |
| 0.45LA—40RACA | 440 | 655 | 590 | 428 | 96.3 | 96.3 |
| 0.55LA—00RACA | 0 | 1090 | 590 | 428 | 117.7 | 117.7 |
| 0.55LA—20RACA | 220 | 875 | 590 | 428 | 117.7 | 117.7 |
| 0.55LA—40RACA | 440 | 655 | 590 | 428 | 117.7 | 117.7 |
| 0.65LA—00RACA | 0 | 1090 | 590 | 428 | 139.1 | 139.1 |
| 0.65LA—20RACA | 220 | 875 | 590 | 428 | 139.1 | 139.1 |
| 0.65LA—40RACA | 440 | 655 | 590 | 428 | 139.1 | 139.1 |
| 0.75LA—00RACA | 0 | 1090 | 590 | 428 | 160.5 | 160.5 |
| 0.75LA—20RACA | 220 | 875 | 590 | 428 | 160.5 | 160.5 |
| 0.75LA—40RACA | 440 | 655 | 590 | 428 | 160.5 | 160.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, L.S. Durability Performance of Geopolymer Concrete: A Review. Polymers 2022, 14, 868. https://doi.org/10.3390/polym14050868
Wong LS. Durability Performance of Geopolymer Concrete: A Review. Polymers. 2022; 14(5):868. https://doi.org/10.3390/polym14050868
Chicago/Turabian StyleWong, Leong Sing. 2022. "Durability Performance of Geopolymer Concrete: A Review" Polymers 14, no. 5: 868. https://doi.org/10.3390/polym14050868
APA StyleWong, L. S. (2022). Durability Performance of Geopolymer Concrete: A Review. Polymers, 14(5), 868. https://doi.org/10.3390/polym14050868






