Characterization of a Radiofluorogenic Polymer for Low-Energy Electron Beam Penetration Depth Visualization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composition
2.2. Fabrication
2.3. Irradiation Process
2.4. Thermogravimetric Analysis (TGA)
2.5. Differential Scanning Calorimetry (DSC)
2.6. Refractive Index
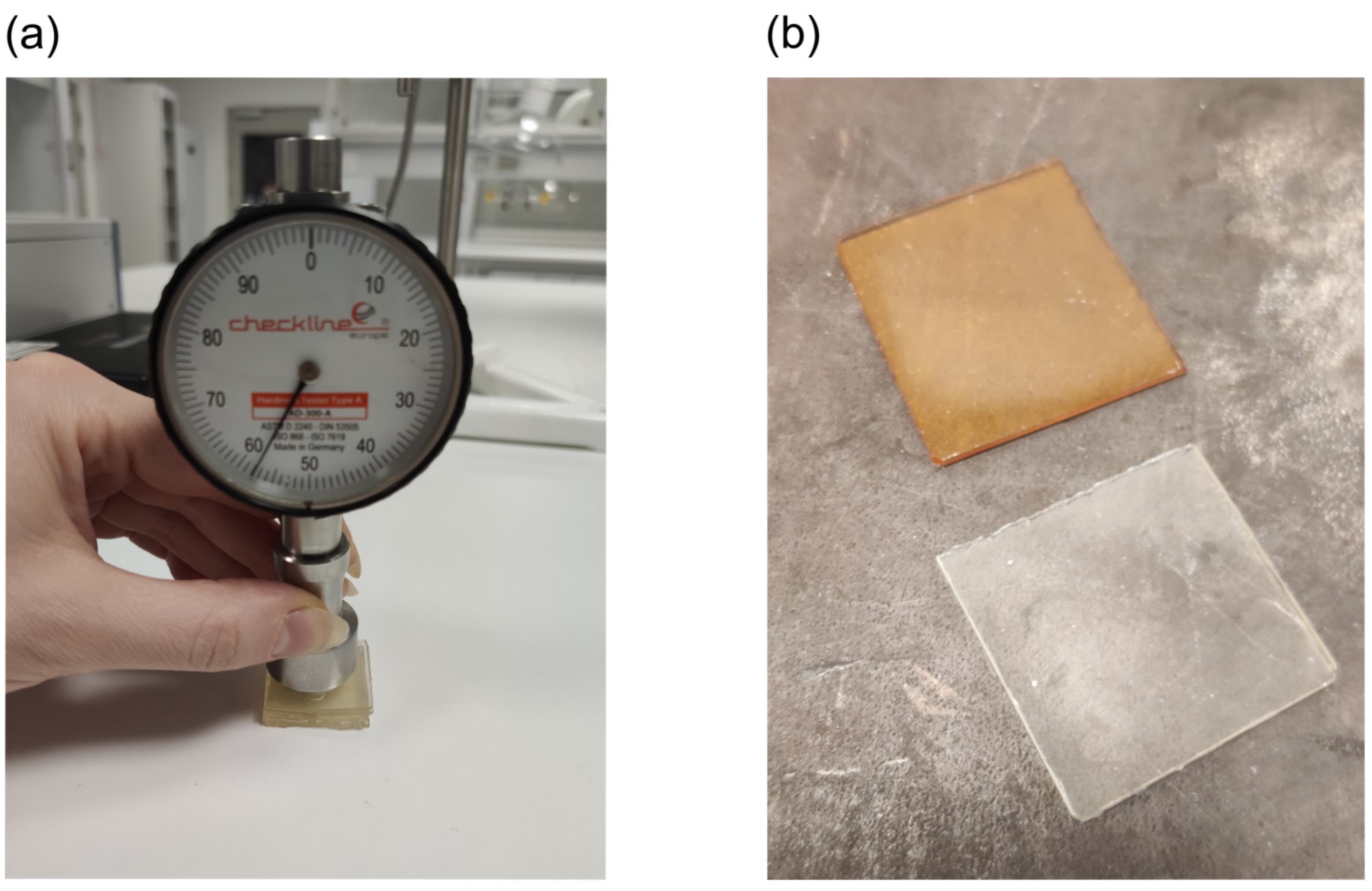
2.7. Hardness
2.8. Rheology
2.9. Water Affinity
2.10. Absorbance and Fluorescence Spectra
3. Results
3.1. Dosimeter Composition
3.1.1. Solubility of the Dye
3.1.2. Host Polymer: PEGDA vs. PPGDA
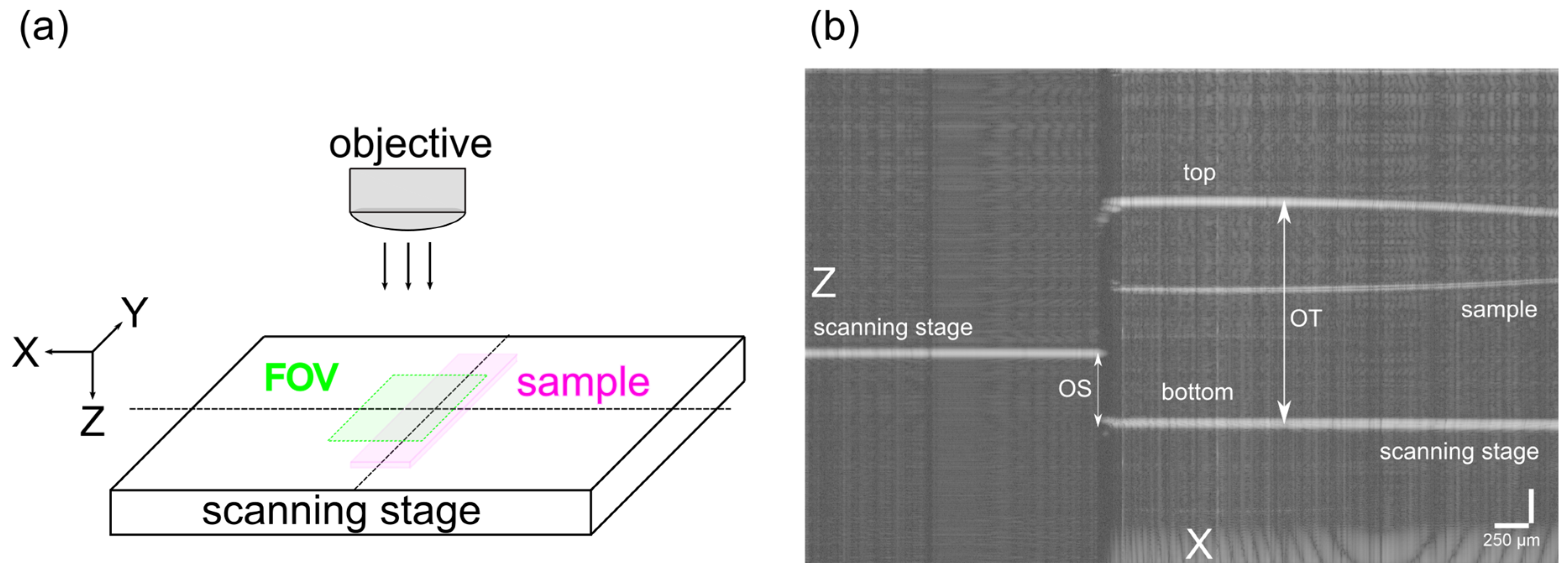
3.2. Sample Preparation: Curing Mold
3.3. Material Characteristics
3.3.1. Thermal Stability
3.3.2. Glass Transition Temperature
3.3.3. Refractive Index
3.3.4. Hardness
3.3.5. Rheology
3.3.6. Water Affinity
3.4. Fluorescence Signal
3.4.1. Fluorescence from the Dye Dissolved in a Liquid and Solid
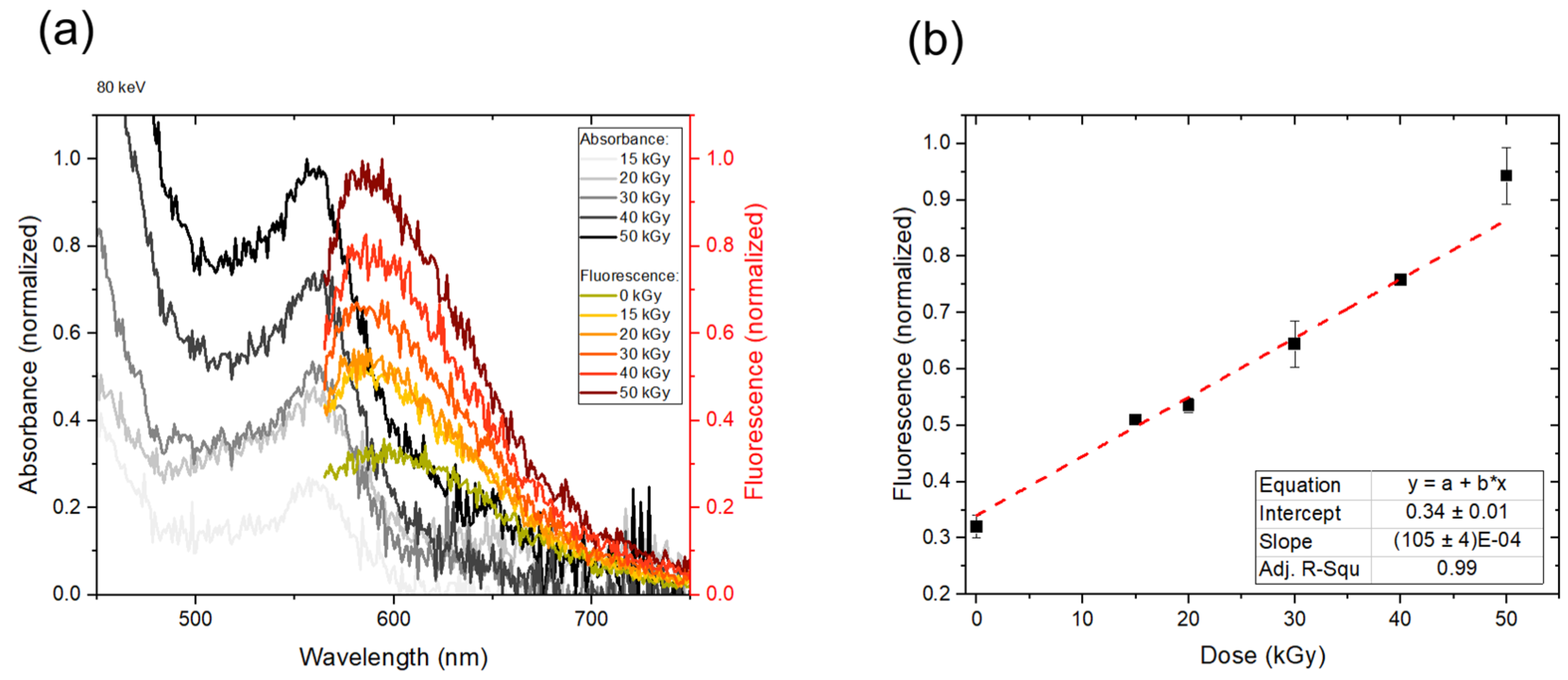
3.4.2. Absorbance and Fluorescence Spectra from an 80 keV E-Beam-Irradiated Polymer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Auslender, V.L.; Berejka, A.J.; Bol, J.L.; Brinston, R.; Bryazgin, A.; Calvo, W.A.P.; Chmielewski, A.; Cleland, M.R.; Cokragan, A.; Ehlermann, D.A.E.; et al. Industrial Radiation with Electron Beams and X-rays; IIA: Shropshire, UK, 2011; Available online: http://iiaglobal.com/uploads/documents/Industrial_Radiation_eBeam_Xray.pdf (accessed on 19 December 2021).
- ISO/ASTM 51818; Standard Practice for Dosimetry in an Electron Beam Facility for Radiation Processing at Energies between 80 and 300 keV. ISO: Geneva, Switzerland, 2020.
- Ando, M.; Uryu, T. Synthesis of polymer materials by low energy electron beam. IV. EB-polymerized urethane-acrylate, -methacrylate and -acrylamide. Int. J. Radiat. Appl. Instrum. Part C. Radiat. Phys. Chem. 1988, 31, 607–614. [Google Scholar] [CrossRef]
- Läuppi, U.V. Electron-Beam Processing for Industrial Inkjet Printing: Cross-Linking and Curing. In Handbook of Industrial Inkjet Printing; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 543–556. Available online: https://onlinelibrary.wiley.com/doi/10.1002/9783527687169.ch31 (accessed on 1 March 2022).
- Scharf, W.; Wieszczycka, W. Electron accelerators for industrial processing—A review. AIP Conf. Proc. 1999, 475, 949–952. [Google Scholar] [CrossRef]
- Wetzel, C.; Schönfelder, J.; Schwarz, W.; Funk, R.H.W. Surface modification of polyurethane and silicone for therapeutic medical technics by means of electron beam. Surf. Coat. Technol. 2010, 205, 1618–1623. [Google Scholar] [CrossRef]
- Gotzmann, G.; Portillo, J.; Wronski, S.; Kohl, Y.; Gorjup, E.; Schuck, H.; Rögner, F.H.; Müller, M.; Chaberny, I.F.; Schönfelder, J.; et al. Low-energy electron-beam treatment as alternative for on-site sterilization of highly functionalized medical products—A feasibility study. Radiat. Phys. Chem. 2018, 150, 9–19. [Google Scholar] [CrossRef]
- Berejka, A.J.; Cleland, M.R.; Walo, M. The evolution of and challenges for industrial radiation processing—2012. Radiat. Phys. Chem. 2014, 94, 141–146. [Google Scholar] [CrossRef]
- Berejka, A.J. Irradiation processing in the ‘90’s: Energy savings and environmental benefits. Radiat. Phys. Chem. 1995, 46, 429–437. [Google Scholar] [CrossRef]
- Felis, K.P.; Avnery, T.; Berejka, A.J. Innovative energy efficient low-voltage electron beam emitters. Radiat. Phys. Chem. 2002, 63, 605–608. [Google Scholar] [CrossRef]
- ISO 11137-3; Sterilization of Health Care Products—Radiation—Part 3: Guidance on Dosimetric Aspects of Development, Validation and Routine Control. ISO: Geneva, Switzerland, 2017.
- Miller, A.; Helt-Hansen, J.; Gondim, O.; Tallentire, A. Guide on the Use of Low Energy Electron Beams for Microbiological Decontamination of Surfaces; Panel on Gamma and Electron Irradiation: London, UK, 2013; pp. 1–34. [Google Scholar]
- ICRU. ICRU Report 80: Dosimetry Systems for Use in Radiation Processing; ICRU: Washington, DC, USA, 2008; Volume 8. [Google Scholar] [CrossRef]
- Helt-Hansen, J.; Miller, A.; Duane, S.; Sharpe, P.; McEwen, M.; Clausen, S. Calorimetry for dose measurement at electron accelerators in the 80-120 keV energy range. Radiat. Phys. Chem. 2005, 74, 354–371. [Google Scholar] [CrossRef]
- Helt-Hansen, J.; Miller, A.; Sharpe, P. Dose response of thin-film dosimeters irradiated with 80-120 keV electrons. Radiat. Phys. Chem. 2005, 74, 341–353. [Google Scholar] [CrossRef]
- Helt-Hansen, J.; Miller, A.; Sharpe, P.; Laurell, B.; Weiss, D.; Pageau, G. Dμ-A new concept in industrial low-energy electron dosimetry. Radiat. Phys. Chem. 2010, 79, 66–74. [Google Scholar] [CrossRef]
- Schuster, C.; Kuntz, F.; Strasser, A.; Härtling, T.; Dornich, K.; Richter, D. 3D relative dose measurement with a μm thin dosimetric layer. Radiat. Phys. Chem. 2021, 180, 109238. [Google Scholar] [CrossRef]
- Bernal-Zamorano, M.R.; Sanders, N.H.; Lindvold, L.R.; Andersen, C.E. Radiochromic and radiofluorogenic 3D solid polymer dosimeter: Initial results for high doses. J. Phys. Conf. Ser. 2017, 847, 012016. [Google Scholar] [CrossRef] [Green Version]
- Bernal-Zamorano, M.R.; Sanders, N.H.; Lindvold, L.; Andersen, C.E. Radiochromic and radiofluorogenic 3D solid polymer dosimeter; effect of the photoinitiator. Radiat. Meas. 2017, 106, 192–195. [Google Scholar] [CrossRef]
- Bernal-Zamorano, M.R.; Sanders, N.H.; Lindvold, L.; Andersen, C.E. Radiochromic and radiofluorogenic solid state polymer dosimeter; a third signal: Electron Paramagnetic Resonance (EPR). Radiat. Phys. Chem. 2019, 161, 72–76. [Google Scholar] [CrossRef]
- Skowyra, M.M.; Lindvold, L.R. Radio-fluorogenic, solid-state polymer dosimeter for recording depth-dose profiles related to radiation processing of surfaces. Radiat. Meas. 2020, 136, 106383. [Google Scholar] [CrossRef]
- Ligon, S.C.; Husár, B.; Wutzel, H.; Holman, R.; Liska, R. Strategies to reduce oxygen inhibition in photoinduced polymerization. Chem. Rev. 2014, 114, 577–589. [Google Scholar] [CrossRef]
- Decker, C.; Moussa, K. Real-Time Kinetic Study of Laser-Induced Polymerization. Macromolecules 1989, 22, 4455–4462. [Google Scholar] [CrossRef]
- ELEGOO Standard Resin. Available online: https://www.elegoo.com/products/elegoo-standard-resin (accessed on 8 October 2021).
- ELEGOO ABS-like Resin. Available online: https://www.elegoo.com/products/elegoo-abs-like-resin (accessed on 8 October 2021).
- Ravve, A. Principles of Polymer Chemistry, 3rd ed.; Springer: Niles, IL, USA, 2012; ISBN 9781461422112. [Google Scholar]
- ASTM E1356-08; Standard Test Method for Assignment of the Glass Transition Temperatures by Differential Scanning Calorimetry. ASTM: West Conshohocken, PA, USA, 2008.
- Wang, X.; Zhang, C.; Zhang, L.; Xue, L.; Tian, J. Simultaneous refractive index and thickness measurements of bio tissue by optical coherence tomography. J. Biomed. Opt. 2002, 7, 628–632. [Google Scholar] [CrossRef]
- Kim, Y.L.; Walsh, J.T.; Goldstick, T.K.; Glucksberg, M.R. Variation of corneal refractive index with hydration. Phys. Med. Biol. 2004, 49, 859–868. [Google Scholar] [CrossRef]
- ASTM D2240-15; Standard Test Method for Rubber Property—Durometer Hardness. ASTM: West Conshohocken, PA, USA, 2021.
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics, 95th ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Miller, A.; McLaughlin, W.L. On a Radiochromic Dye Dosemeter; Risø National Laboratory: Roskilde, Denmark, 1980; pp. 1–34. ISBN 8755007376. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 180, Acetone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Acetone (accessed on 21 June 2021).
- Reichardt, C. Solvent Effects on the Absorption Spectra of Organic Compounds. In Solvents and Solvent Effects in Organic Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; pp. 329–388. ISBN 3527306188. Available online: https://onlinelibrary.wiley.com/doi/10.1002/3527601791.ch6 (accessed on 1 March 2022).
- Oster, G.; Joussot-Dubien, J.; Broyde, B. Photoreduction of Dyes in Rigid Media. I. Triphenylmethane Dyes. J. Am. Chem. Soc. 1959, 81, 1869–1872. [Google Scholar] [CrossRef]
- Valeur, B.; Berberan-Santos, M.-N. Molecular Fluorescence. Principles and Applications, 2nd ed.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2012. [Google Scholar]
- Vogel, M.; Rettig, W. Excited State Dynamics of Triphenylmethane-Dyes used for Investigation of Microviscosity Effects. Ber. Bunsenges. Phys. Chem. 1987, 91, 1241–1247. [Google Scholar] [CrossRef]
- Stevens, M.P. Polymer Chemistry: An Introduction, 3rd ed.; Oxford University Press: New York, NY, USA, 1999; ISBN 978-0-19-512444-6. [Google Scholar]
- Holmes, E.O. The effect of the properties of solvents of various dielectric constants and structures on the photoionization of the leucocarbinols and leucocyanides of malachite green, crystal violet, and sunset orange and related phenomena. J. Phys. Chem. 1966, 70, 1037–1046. [Google Scholar] [CrossRef]
- Malucelli, G.; Gozzelino, G.; Ferrero, F.; Bongiovanni, R.; Priola, A. Synthesis of poly(propylene-glycol-diacrylates) and properties of the photocured networks. J. Appl. Polym. Sci. 1997, 65, 491–497. [Google Scholar] [CrossRef]
- Efimenko, K.; Wallace, W.E.; Genzer, J. Surface modification of Sylgard-184 poly(dimethyl siloxane) networks by ultraviolet and ultraviolet/ozone treatment. J. Colloid Interface Sci. 2002, 254, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Compañ, V.; Lopez, M.L.; Monferrer, L.; Garrido, J.; Riande, E.; San Roman, J. Determination of the glass transition temperature of poly(cyclohexyl acrylate) from oxygen permeability measurements. Polymer 1993, 34, 2971–2974. [Google Scholar] [CrossRef]
- Chartoff, R. Thermal characteristics of thermosets formed by free radical photocuring. J. Therm. Anal. Calorim. 2006, 85, 213–217. [Google Scholar] [CrossRef]
- Fox, T.G.; Flory, P.J. Second-order transition temperatures and related properties of polystyrene. I. Influence of molecular weight. J. Appl. Phys. 1950, 21, 581–591. [Google Scholar] [CrossRef]
- Dalnoki-Veress, K.; Forrest, J.A.; Murray, C.; Gigault, C.; Dutcher, J.R. Molecular weight dependence of reductions in the glass transition temperature of thin, freely standing polymer films. Phys. Rev. E 2001, 63, 031801. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, H.; Hell, S.W. Effect of the specimen refractive index on the imaging of a confocal fluorescence microscope employing high aperture oil immersion lenses. Bioimaging 1995, 3, 39–47. [Google Scholar] [CrossRef]
- Birkhoff, R.D.; Painter, L.R.; Heller, J.M. Optical and dielectric functions of liquid glycerol from gas photoionization measurements. J. Chem. Phys. 1978, 69, 4185–4188. [Google Scholar] [CrossRef]
- Hartmann, P. Refractive Index and Dispersion. In Optical Glass; Society of Photo-Optical Instrumentation Engineers: Bellingham, WA, USA, 2014; pp. 33–76. Available online: http://ebooks.spiedigitallibrary.org/content.aspx?bookid=968§ionid=65642104 (accessed on 1 March 2022).
- Gilman, J.J. Chemistry and Physics of Mechanical Hardness; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; ISBN 9780470446836. [Google Scholar]
- Arsu, N.; Stephen Davidson, R.; Holman, R. Factors affecting the photoyellowing which occurs during the photoinitiated polymerization of acrylates. J. Photochem. Photobiol. A Chem. 1995, 87, 169–175. [Google Scholar] [CrossRef]
- Mezger, T.G. The Rheology Handbook, 2nd ed.; Vincentz Network: Hannover, Germany, 2006; ISBN 3-87870-174-8. [Google Scholar]
- ASTM D4473-03; Standard Test Method for Plastics: Dynamic Mechanical Properties: Cure Behavior. ASTM: West Conshohocken, PA, USA, 2003.
- Koseki, K.; Sakamaki, H.; Jeong, K.M. In situ measurement of shrinkage behavior of photopolymers. J. Photopolym. Sci. Technol. 2013, 26, 567–572. [Google Scholar] [CrossRef] [Green Version]
- Ferry, M.; Ngono-Ravache, Y.; Aymes-Chodur, C.; Clochard, M.C.; Coqueret, X.; Cortella, L.; Pellizzi, E.; Rouif, S.; Esnouf, S. Ionizing Radiation Effects in Polymers. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016; Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780128035818020956 (accessed on 9 September 2020).
- Miller, A.A.; Lawton, E.J.; Balwit, J.S. Eflect of Chemical Structure of Vinyl Polymers on Crosslinking and Degradation by Ionizing Radiation. J. Polym. Sci. 1954, XIV, 503–504. [Google Scholar] [CrossRef]
- Lyons, B.J.; Weir, F.E. The Effect of Radiation on the Mechanical Properties of Polymers. In The Radiation Chemistry of Macromolecules; Elsevier: Amsterdam, The Netherlands, 1973; pp. 281–306. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780122198021500216 (accessed on 1 March 2022).
- Law, K.-Y. Definitions for Hydrophilicity, Hydrophobicity, and Superhydrophobicity: Getting the Basics Right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Clough, R.L. High-energy radiation and polymers: A review of commercial processes and emerging applications. Nucl. Instrum. Methods Phys. Res. Sect. B 2001, 185, 8–33. [Google Scholar] [CrossRef]
- Kasha, M. Characterization of electronic transitions in complex molecules. Discuss. Faraday Soc. 1950, 9, 14–19. [Google Scholar] [CrossRef]
- Harris, D.C. Quantitative Chemical Analysis, 8th ed.; W.H. Freeman and Co.: New York, NY, USA, 2007; ISBN 9781429218153. [Google Scholar]
- Odian, G. Principles of Polymerization, 4th ed.; John Wiley & Sons, Inc: New York, NY, USA, 2004; ISBN 3175723993. [Google Scholar]
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 2001, 26, 605–665. [Google Scholar] [CrossRef]
- Kurdikar, D.L.; Peppas, N.A. A kinetic study of diacrylate photopolymerizations. Polymer 1994, 35, 1004–1011. [Google Scholar] [CrossRef]
- Andrzejewska, E. Free-radical photopolymerization of multifunctional monomers. In Three-Dimensional Microfabrication Using Two-Photon Polymerization; Elsevier: Amsterdam, The Netherlands, 2020; pp. 77–99. ISBN 9780128178270. [Google Scholar]
- Sandwall, P.A.; Bastow, B.P.; Spitz, H.B.; Elson, H.R.; Lamba, M.; Connick, W.B.; Fenichel, H. Radio-fluorogenic gel dosimetry with coumarin. Bioengineering 2018, 5, 53. [Google Scholar] [CrossRef] [Green Version]
- Maeyama, T.; Kato, A.; Mochizuki, A.; Sato, N.; Watanabe, Y. Dose-rate-independent and diffusion-free nanoclay-based radio-fluorogenic gel dosimeter. Sens. Actuators A 2019, 298, 111435. [Google Scholar] [CrossRef]
- Luthjens, L.H.; Yao, T.; Warman, J.M. A Polymer-Gel Eye-Phantom for 3D Fluorescent Imaging of Millimetre Radiation Beams. Polymers 2018, 10, 1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warman, J.M.; de Haas, M.P.; Luthjens, L.H. High-energy radiation monitoring based on radio- fluorogenic co-polymerization. I: Small volume in situ probe. Phys. Med. Biol. 2009, 54, 3185–3200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warman, J.M.; Luthjens, L.H.; de Haas, M.P. High-energy radiation monitoring based on radio-fluorogenic co-polymerization II: Fixed fluorescent images of collimated X-ray beams using an RFCP gel. Phys. Med. Biol. 2011, 56, 1487–1508. [Google Scholar] [CrossRef] [PubMed]







| Nominal Irradiation Dose (kGy) | Voltage (kV) | ||
|---|---|---|---|
| 80 | 120 | 200 | |
| Beam Current (mA) | |||
| 10 | 1.746 | - | - |
| 15 | 2.619 | - | - |
| 20 | 3.492 | - | - |
| 30 | 5.238 | 3.065 | 4.639 |
| 40 | 6.894 | - | - |
| 50 | 8.730 | 5.108 | 7.733 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skowyra, M.M.; Ankjærgaard, C.; Yu, L.; Lindvold, L.R.; Skov, A.L.; Miller, A. Characterization of a Radiofluorogenic Polymer for Low-Energy Electron Beam Penetration Depth Visualization. Polymers 2022, 14, 1015. https://doi.org/10.3390/polym14051015
Skowyra MM, Ankjærgaard C, Yu L, Lindvold LR, Skov AL, Miller A. Characterization of a Radiofluorogenic Polymer for Low-Energy Electron Beam Penetration Depth Visualization. Polymers. 2022; 14(5):1015. https://doi.org/10.3390/polym14051015
Chicago/Turabian StyleSkowyra, Magdalena Maria, Christina Ankjærgaard, Liyun Yu, Lars René Lindvold, Anne Ladegaard Skov, and Arne Miller. 2022. "Characterization of a Radiofluorogenic Polymer for Low-Energy Electron Beam Penetration Depth Visualization" Polymers 14, no. 5: 1015. https://doi.org/10.3390/polym14051015
APA StyleSkowyra, M. M., Ankjærgaard, C., Yu, L., Lindvold, L. R., Skov, A. L., & Miller, A. (2022). Characterization of a Radiofluorogenic Polymer for Low-Energy Electron Beam Penetration Depth Visualization. Polymers, 14(5), 1015. https://doi.org/10.3390/polym14051015






