PVDF/KNO3 Composite Sub-Microfibers Produced by Solution Blow Spinning as a Hydrophobic Matrix for Fertilizer Delivery System
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sample Characterization
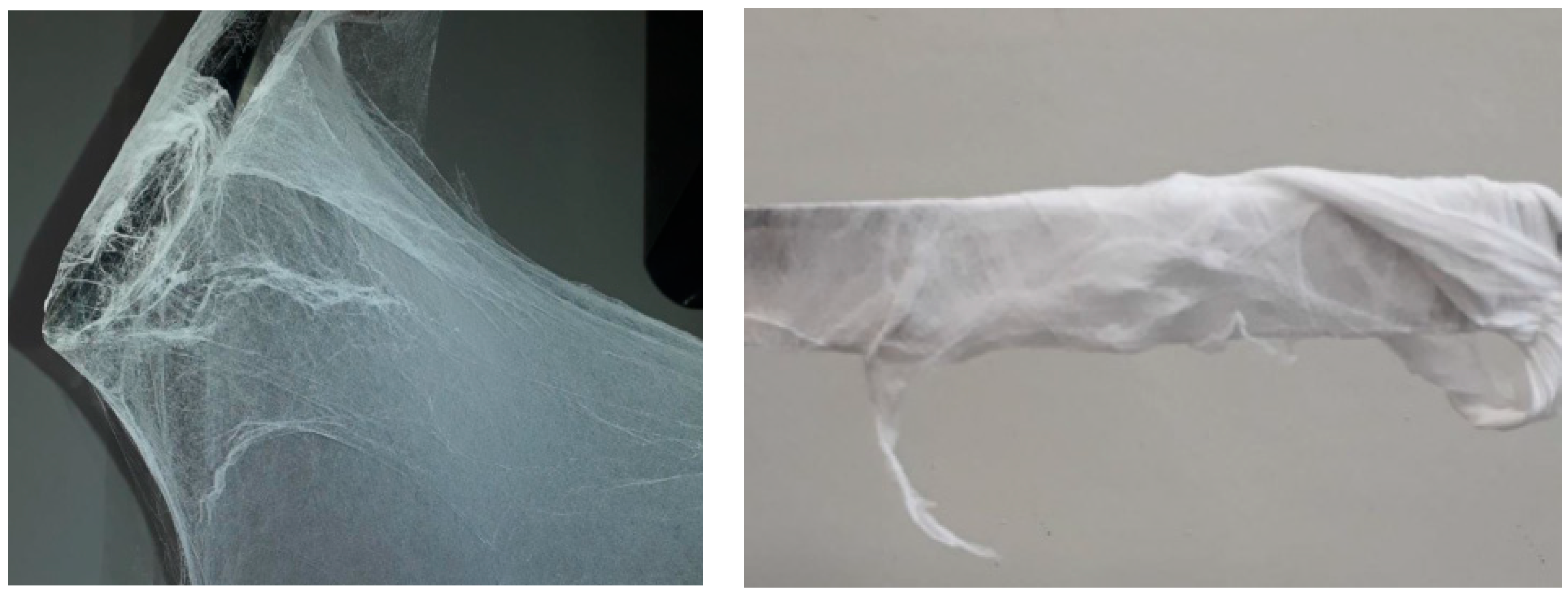
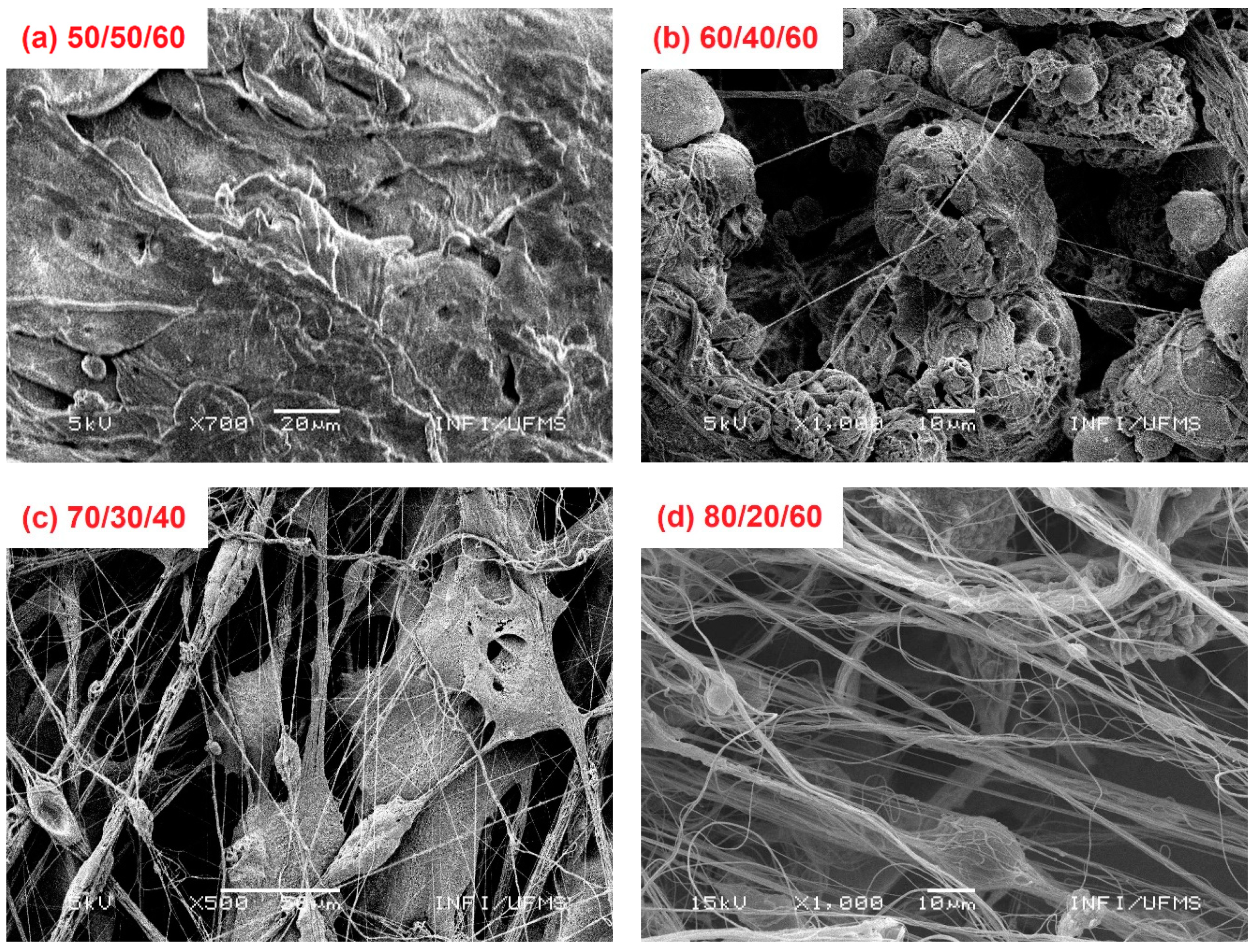
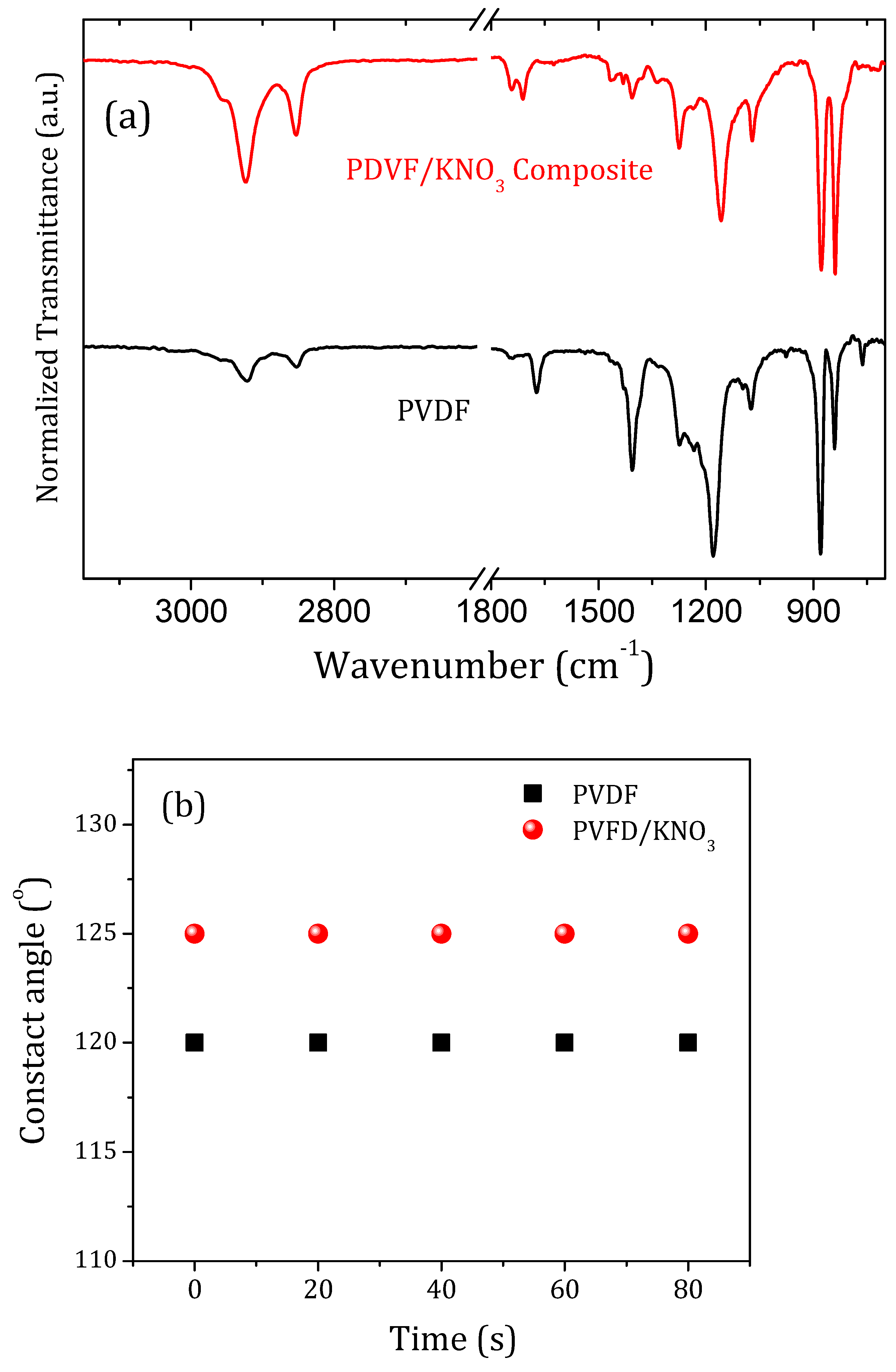
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milani, P.; França, D.; Balieiro, A.G.; Faez, R. Polymers and its applications in agriculture. Polímeros 2017, 27, 256–266. [Google Scholar] [CrossRef]
- Bansal, P. Water-Based Polymeric Nanostructures for Agricultural Applications. Ph.D. Thesis, Philipps-Universitat Marburg, Marbug, Germanny, 2010. [Google Scholar]
- Bisotto-de-Oliveira, R.; Jorge, B.C.; Roggia, I.; Santana, J.; Pereira, C.N. Nanofibers as a vehicle for the synthetic attactant trimedlure to be used for ceratitis capitata wied: (Díptera, tethritidae) capture. J. Res. Updates Polym. Sci. 2014, 3, 40–47. [Google Scholar] [CrossRef]
- Bisotto-de-Oliveira, R.; Morais, R.M.; Roggia, I.; Silva, S.J.N.; Santana, J.; Pereira, C.N. Polymers nanofibers as vehicles for the release of the synthetic sex pheromone of grapholita molesta. Rev. Colomb. Entomol. 2015, 41, 2. [Google Scholar]
- Castenada, L.M.F.; Genro, C.; Roggia, I.; Bender, S.S.; Bender, R.J.; Pereira, C.N. Innovative rice seed coating (Oryza sativa) with polymer nanofibers and microparticles using the electrospinning method. J. Res. Updates Polym. Sci. 2014, 3, 33–39. [Google Scholar]
- Damasceno, R.; Roggia, I.; Pereira, C.; SÁ, E. Rhizobia survival in seeds coated with PVA Electrospun nanofibers. Can. J. Microbiol. 2013, 59, 716–719. [Google Scholar] [CrossRef]
- Gregorio, P.R.; Michavilla, G.; Muller, L.R.; Borges, C.S.; Pomares, M.F.; Sá, E.L.S.; Pereira, C.; Vincent, P.A. Beneficial rhizobacteria immobilized in nanofibers for potential applications as soybean seed bioinoculants. PLoS ONE 2017, 4, e0176930. [Google Scholar] [CrossRef]
- Castro-Enríquez, D.D.; Rodríguez-Félix, F.; Ramírez-Wong, B.; Torres-Chávez, P.I.; Castillo-Ortega, M.M.; Rodríguez-Félix, D.E.; Armenta-Villegas, L.; Ledesma-Osuna, A.I. Preparation, Characterization and Release of Urea from Wheat Gluten Electrospun Membranes. Materials 2012, 5, 2903–2916. [Google Scholar] [CrossRef]
- Kampeerapappun, P.; Phanomkate, N. Slow release fertilizer from core-shell Electrospun fibers. Chiang Mai J. Sci. 2013, 40, 4. [Google Scholar]
- Krishnamoorthy, V.; Rajiv, S. An eco-friendly top-down approach to nutrient incorporated Electrospun seed coating for superior germination potential. J. Adv. App. Sci. Res. 2017, 1, 7. [Google Scholar]
- Palvannan, T.; Saravanakumar, T.; Unnithan, A.R.; Chung, N.J.; Kim, D.H.; Park, S.M. Efficient transformation of phenyl urea herbicide chloroxuron by laccase immobilized on zein polyurethane nanofiber. J. Mol. Catal. B 2014, 99, 3. [Google Scholar] [CrossRef]
- Pule, B.O.; Degni, S.; Torto, N. Electrospun fiber colorimetric probe based on gold nanoparticles for on-site detection of estradiol associated with dairy farming effluents. Afr. J. Online 2015, 41, 27–32. [Google Scholar]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fibers fabrication technique. Biotechnol. Adv. Oxf. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Cena, C.R.; Silva, M.J.; Malmonge, L.F.; Malmonge, J.A. Poly(vinyl pyrrolidone) sub-microfibers produced by solution blow spinning. J. Polym. Res. 2018, 25, 238. [Google Scholar] [CrossRef]
- Cena, C.R.; Larios, G.S.; Bica, M.R.R.; Canassa, T.A.; Freitas, G.Q.; Torsoni, G.B. Desenvolvimento de um sistema Blow-Spinning de baixo custo: Obtenção de microfibras e nanofibras poliméricas e compósitas. Rev. Bras. Física Tecnol. Apl. 2015, 2, 32–44. [Google Scholar] [CrossRef]
- Viana, J.L.; França, T.; Larios, G.S.; Cena, C. Solution Blow Spinning Poly(vinyl alcohol) Sub-microfibers Produced from Different Solvents. Orbital Electron. J. Chem. 2020, 12, 120036. [Google Scholar] [CrossRef]
- Cena, C.R.; Torsoni, G.B.; Zadorosny, L.; Malmonge, L.F.; Carvalho, C.L.; Malmonge, J.A. BSCCO superconductor micro/nanofibers produced by solution blow-spinning technique. Ceram. Int. 2017, 43, 7663–7667. [Google Scholar] [CrossRef][Green Version]
- Cena, C.R.; Behera, A.K.; Behera, B. Structural, dielectric, and electrical properties of lithium niobate microfibers. J. Adv. Ceram. 2016, 5, 84–92. [Google Scholar] [CrossRef]
- Gasparini, T.M.; Bretas, R.E.S.; da Silva, A.B.; Rinaldo, G., Jr. Processing and characterization of oriented electrospun poly(vinylidene fluoride) mats. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 1304–1311. [Google Scholar] [CrossRef]
- Gregorio, R.; Borges, D.S. Effect of crystallization rate on the formation of the polymorphs of solution cast poly(vinylidene fluoride). J. Polym. 2008, 49, 4009–4016. [Google Scholar] [CrossRef]
- Costa, L.M.M.; Bretas, R.E.S.; Filho, R.G. Caracterização de filmes de PVDF-β obtidos por diferentes técnicas. Polímeros 2009, 19, 183–189. [Google Scholar] [CrossRef]
- Jang, W.; Yun, J.; Jeon, K.; Byun, H. PVdF/graphene oxide hybrid membranes via electrospinning for water treatment applications. RSC Adv. 2015, 5, 46711–46717. [Google Scholar] [CrossRef]
- Larios, G.S.; Nogueira, F.S.; Viana, J.L.; Oliveira, R.H.; Ferreira, D.C.; Ilha, V.N.; Canassa, T.A.; Plaça, L.F. Síntese e Caracterização de Sub-microfibras de PVDF/GO via Blow-Spinning. J. Exp. Tech. Instrum. 2018, 1, 1–8. [Google Scholar] [CrossRef]
- Dias, G.C.; Cellet, T.S.P.; Santos, M.C.; Sanches, A.; Malmonge, L.F. PVDF nanofibers obtained by solution blow spinning with use of a commercial airbrush. J. Polym. Res. 2019, 26, 87. [Google Scholar] [CrossRef]
- Messa, L.; Froes, J.D.; Souza, C.; Faez, R. Híbridos de quitosana-argila para encapsulamento e liberação sustentada do fertilizante nitrato de potássio. Química Nova 2016, 39, 1215–1220. [Google Scholar] [CrossRef]
- Kamaz, M.; Sengupta, A.; Gutierrez, A.; Chiao, Y.H.; Wickramasinghe, R. Surface modification of PVDF membranes for treating produced Waters by direct contact membrane distillation. Int. J. Environ. Res. Public Health 2019, 16, 685. [Google Scholar] [CrossRef]
- Kaynak, A.; Mehmood, T.; Dai, X.J.; Magniez, K.; Kouzani, A. Study of Radio Frequency Plasma Treatment of PVDF Film Using Ar, O2 and (Ar + O2) Gases for Improved Polypyrrole Adhesion. Materials 2013, 6, 3482–3493. [Google Scholar] [CrossRef]
- Erbil, H.Y.; Mchale, G.; Newton, M.I. Drop evaporation on solid surfaces: Constant contact angle mode. Langmuir 2002, 18, 2636–2641. [Google Scholar] [CrossRef]
- Arora, G.; Malik, K.; Singh, I. Formulation and evaluation of mucoadhesive matrix tablets of taro gum: Optimization using response surface methodology. Polim. Med. 2011, 41, 23–34. [Google Scholar]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef]
- Permanadewi, I.; Kumoro, A.C.; Wardhani, D.H.; Aryanti, M. Modelling of controlled drug release in gastrointestinal tract simulation. J. Phys. 2019, 1295, 012063. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobral, F.; Silva, M.J.; Canassa, T.; Goncalves, A.-M.; Cena, C. PVDF/KNO3 Composite Sub-Microfibers Produced by Solution Blow Spinning as a Hydrophobic Matrix for Fertilizer Delivery System. Polymers 2022, 14, 1000. https://doi.org/10.3390/polym14051000
Sobral F, Silva MJ, Canassa T, Goncalves A-M, Cena C. PVDF/KNO3 Composite Sub-Microfibers Produced by Solution Blow Spinning as a Hydrophobic Matrix for Fertilizer Delivery System. Polymers. 2022; 14(5):1000. https://doi.org/10.3390/polym14051000
Chicago/Turabian StyleSobral, Fabio, Michael J. Silva, Thalita Canassa, Além-Mar Goncalves, and Cícero Cena. 2022. "PVDF/KNO3 Composite Sub-Microfibers Produced by Solution Blow Spinning as a Hydrophobic Matrix for Fertilizer Delivery System" Polymers 14, no. 5: 1000. https://doi.org/10.3390/polym14051000
APA StyleSobral, F., Silva, M. J., Canassa, T., Goncalves, A.-M., & Cena, C. (2022). PVDF/KNO3 Composite Sub-Microfibers Produced by Solution Blow Spinning as a Hydrophobic Matrix for Fertilizer Delivery System. Polymers, 14(5), 1000. https://doi.org/10.3390/polym14051000






