Poly(vinyl pyridine) and Its Quaternized Derivatives: Understanding Their Solvation and Solid State Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
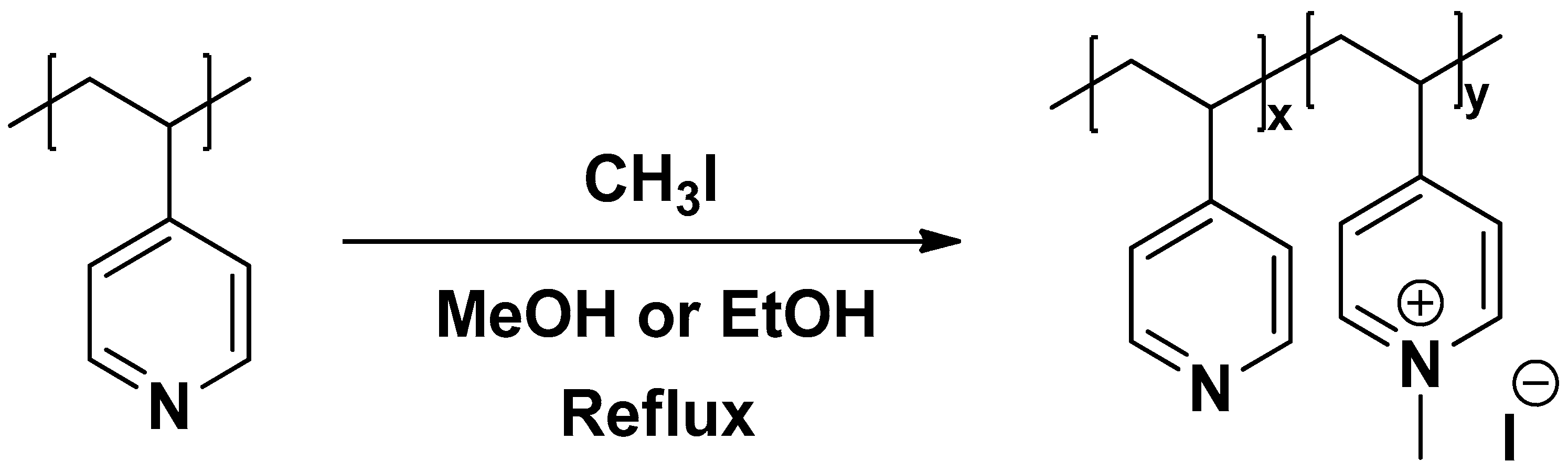
2.2. Synthesis of Poly(N-methyl-4-vinyl pyridinium iodide) Derivatives
2.3. Characterization Methods
2.4. Solvatochromic Properties Study
3. Results
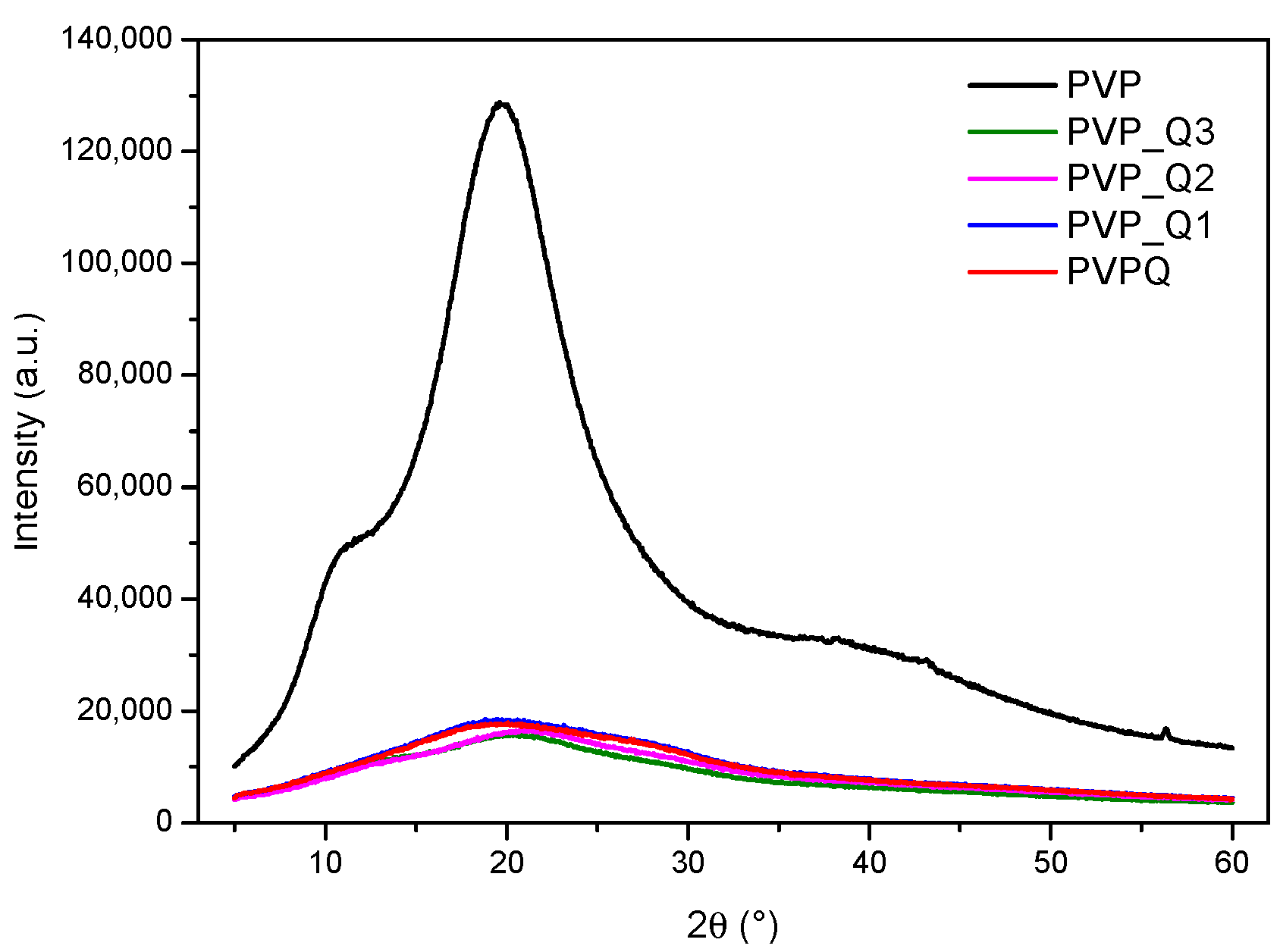
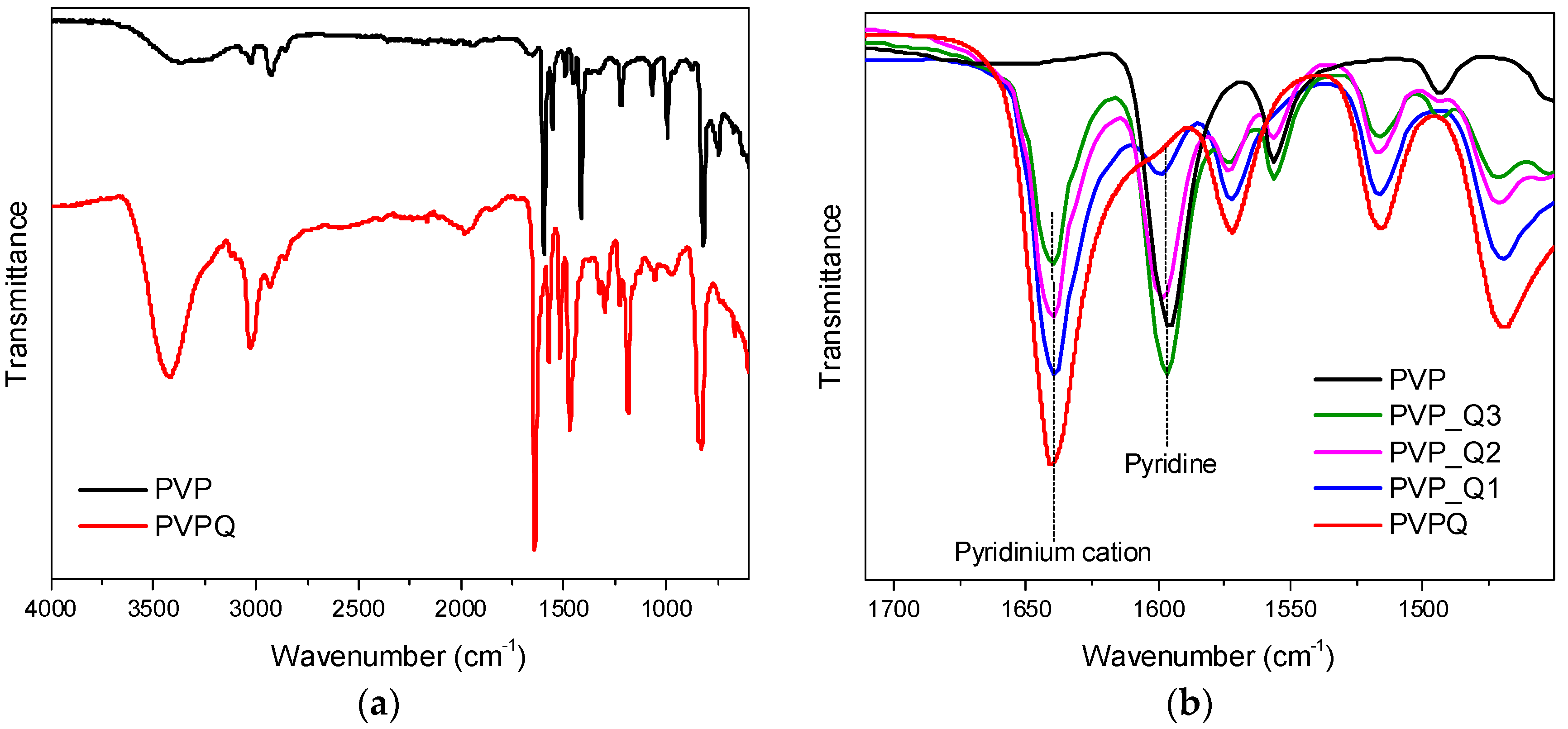
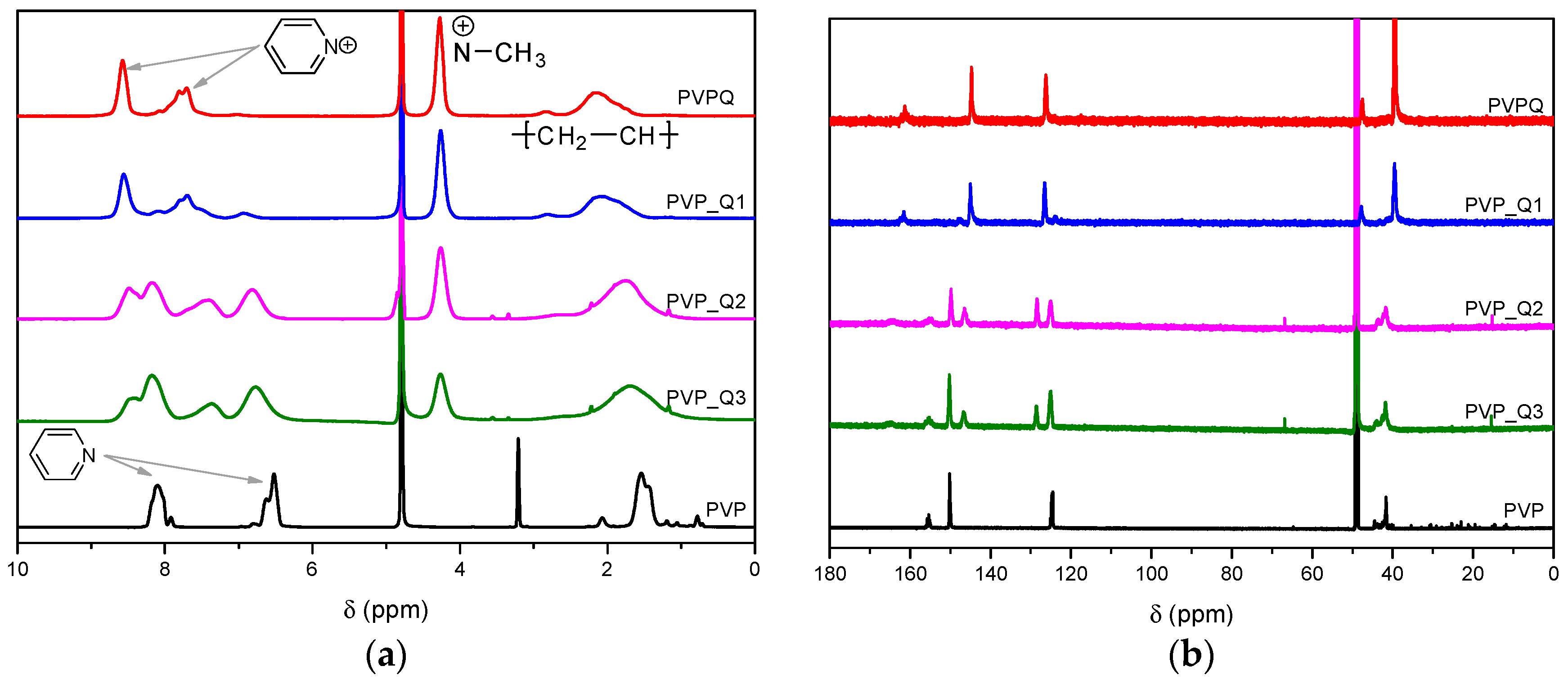
3.1. Polymer Synthesis and Structural Characterization
3.2. Thermal Properties and Stability
3.3. UV-Vis Spectroscopy
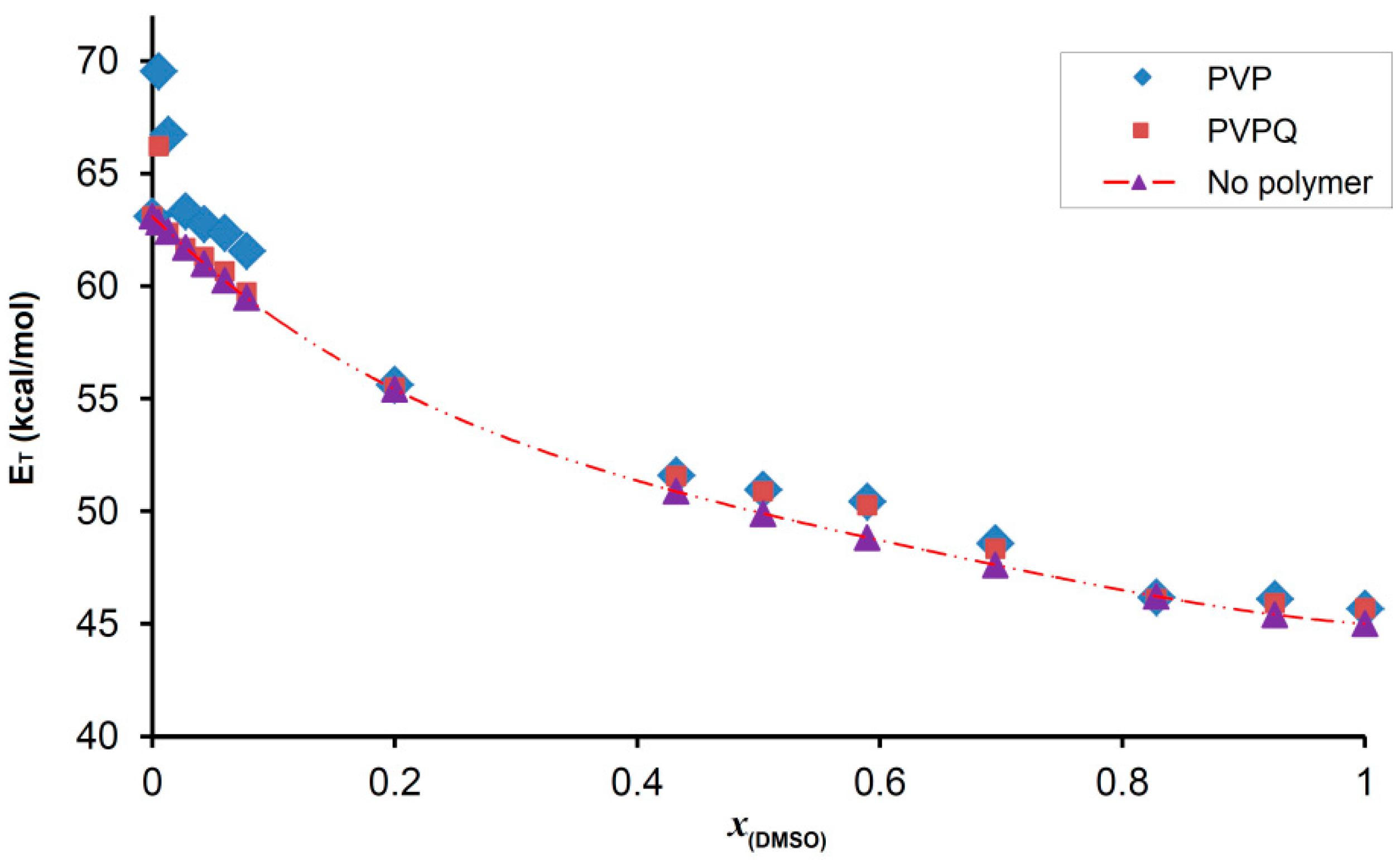
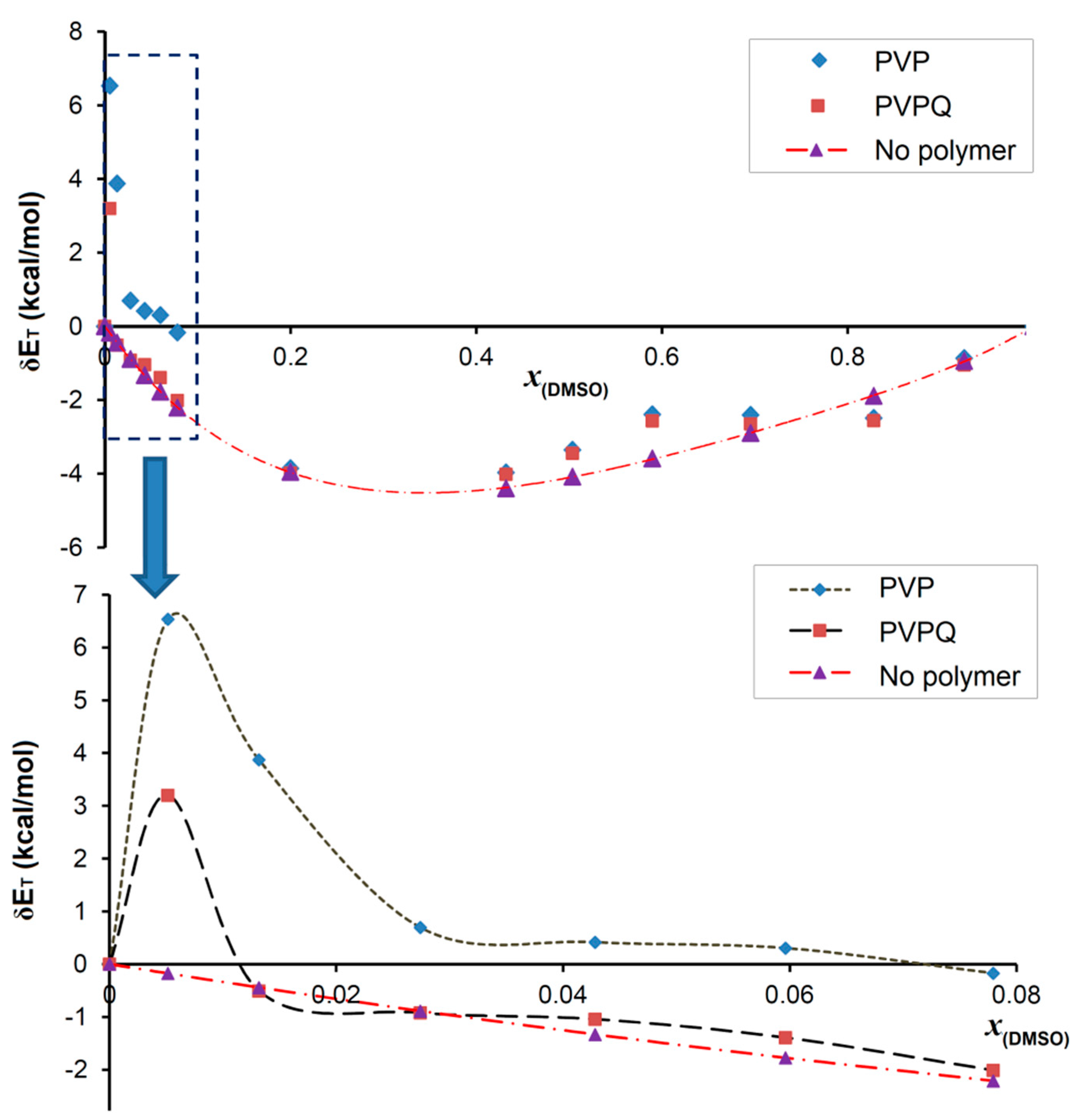
3.4. Solvatochromic Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malavolta, L.; Oliveira, E.; Cilli, E.M.; Nakaie, C.R. Solvation of polymers as model for solvent effect investigation: Proposition of a novel polarity scale. Tetrahedron 2002, 58, 4383–4394. [Google Scholar] [CrossRef]
- Krokhina, L.S.; Kuleznev, V.N.; Lyusova, L.R.; Glagolev, V.A. Effect of solvent on polymer interaction in solution and properties of films obtained. Polym. Sci. USSR 1976, 18, 756–762. [Google Scholar] [CrossRef]
- Wanasingha, N.; Dorishetty, P.; Dutta, N.K.; Choudhury, N.R. Polyelectrolyte gels: Fundamentals, fabrication and applications. Gels 2021, 7, 148. [Google Scholar] [CrossRef] [PubMed]
- Hunkeler, D.; Wandrey, C. Polyelectrolytes: Research, Development, and Applications. Chimia 2001, 55, 223–227. [Google Scholar]
- Vodolazkaya, N.A.; Mchedlov-Petrossyan, N.O.; Bryleva, E.Y.; Biletskaya, S.V.; Scrinner, M.; Kutuzova, L.V.; Ballauff, M. The binding ability and solvation properties of cationic spherical polyelectrolyte brushes as studied using acid-base and solvatochromic indicators. Funct. Mater. 2012, 17, 470–476. [Google Scholar]
- Rabiee, A.; Ershad-Langroudi, A.; Zeynali, M.E. A survey on cationic polyelectrolytes and their applications: Acrylamide derivatives. Rev. Chem. Eng. 2015, 31, 239–261. [Google Scholar] [CrossRef]
- Bucatariu, F.; Teodosiu, C.; Morosanu, I.; Fighir, D.; Ciobanu, R.; Petrila, L.M.; Mihai, M. An overview on composite sorbents based on polyelectrolytes used in advanced wastewater treatment. Polymers 2021, 13, 3963. [Google Scholar] [CrossRef]
- Chen, N.; Lee, Y.M. Anion exchange polyelectrolytes for membranes and ionomers. Prog. Polym. Sci. 2021, 113, 101345. [Google Scholar] [CrossRef]
- Durmaz, E.N.; Sahin, S.; Virga, E.; De Beer, S.; De Smet, L.C.P.M.; De Vos, W.M. Polyelectrolytes as Building Blocks for Next-Generation Membranes with Advanced Functionalities. ACS Appl. Polym. Mater. 2021, 3, 4347–4374. [Google Scholar] [CrossRef]
- Ambade, A.V.; Sandanaraj, B.S.; Klaikherd, A.; Thayumanavan, S. Fluorescent polyelectrolytes as protein sensors. Polym. Int. 2007, 56, 474–481. [Google Scholar] [CrossRef]
- Tamami, B.; Kiasat, A.R. Synthesis and application of quaternized polyvinylpyridine supported dichromate as a new polymeric oxidizing agent. Iran. Polym. J. 1997, 6, 273–279. [Google Scholar]
- Blackmore, I.J.; Gibson, V.C.; Hitchcock, P.B.; Rees, C.W.; Williams, D.J.; White, A.J.P. Pyridine N-alkylation by lithium, magnesium, and zinc alkyl reagents: Synthetic, structural, and mechanistic studies on the bis(imino)pyridine system. J. Am. Chem. Soc. 2005, 127, 6012–6020. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Chen, G.D.; Pan, C.Y.; Luan, B.; Hong, C.Y. Preparation, characterization, and thermal properties of polystyrene-block-quaternized poly(4-vinylpyridine)/montmorillonite nanocomposites. J. Appl. Polym. Sci. 2006, 102, 1950–1958. [Google Scholar] [CrossRef]
- Chernov’yants, M.S.; Burykin, I.V.; Pisanov, R.V.; Shalu, O.A. Synthesis and antimicrobial activity of poly(n-methyl-4-vinylpyridinium triiodide). Pharm. Chem. J. 2010, 44, 61–63. [Google Scholar] [CrossRef]
- Singh, P.K.; Bhattacharya, B.; Nagarale, R.K.; Pandey, S.P.; Kim, K.W.; Rhee, H.W. Ionic liquid doped poly(N-methyl 4-vinylpyridine iodide) solid polymer electrolyte for dye-sensitized solar cell. Synth. Met. 2010, 160, 950–954. [Google Scholar] [CrossRef]
- Borah, K.J.; Dutta, P.; Borah, R. Synthesis, characterization and application of poly(4-vinylpyridine)- supported Brønsted acid as reusable catalyst for acetylation reaction. Bull. Korean Chem. Soc. 2011, 32, 225–228. [Google Scholar] [CrossRef][Green Version]
- Efrati, A.; Tel-Vered, R.; Michaeli, D.; Nechushtai, R.; Willner, I. Cytochrome c-coupled photosystem i and photosystem II (PSI/PSII) photo-bioelectrochemical cells. Energy Environ. Sci. 2013, 6, 2950–2956. [Google Scholar] [CrossRef]
- Xue, Y.; Xiao, H. Antibacterial/antiviral property and mechanism of dual-functional quaternized pyridinium-type copolymer. Polymers 2015, 7, 2290–2303. [Google Scholar] [CrossRef]
- Manouras, T.; Platania, V.; Georgopoulou, A.; Chatzinikolaidou, M.; Vamvakaki, M. Responsive quaternized PDMAEMA copolymers with antimicrobial action. Polymers 2021, 13, 3051. [Google Scholar] [CrossRef]
- Behzadi pour, G.; Nazarpour fard, H.; Fekri aval, L.; Esmaili, P. Polyvinylpyridine-based electrodes: Sensors and electrochemical applications. Ionics 2020, 26, 549–563. [Google Scholar] [CrossRef]
- Raczkowska, J.; Stetsyshyn, Y.; Awsiuk, K.; Zemła, J.; Kostruba, A.; Harhay, K.; Marzec, M.; Bernasik, A.; Lishchynskyi, O.; Ohar, H.; et al. Temperature-responsive properties of poly(4-vinylpyridine) coatings: Influence of temperature on the wettability, morphology, and protein adsorption. RSC Adv. 2016, 6, 87469–87477. [Google Scholar] [CrossRef]
- Malynych, S.; Luzinov, I.; Chumanov, G. Poly(vinyl pyridine) as a universal surface modifier for immobilization of nanoparticles. J. Phys. Chem. B 2002, 106, 1280–1285. [Google Scholar] [CrossRef]
- Hernández-Orta, M.; Pérez, E.; Cruz-Barba, L.E.; Sánchez-Castillo, M.A. Synthesis of bactericidal polymer coatings by sequential plasma-induced polymerization of 4-vinyl pyridine and gas-phase quaternization of poly-4-vinyl pyridine. J. Mater. Sci. 2018, 53, 8766–8785. [Google Scholar] [CrossRef]
- Shin, I.; Lee, K.; Kim, E.; Kim, T.H. Poly(ethylene glycol)-Crosslinked Poly(vinyl pyridine)-based Gel Polymer Electrolytes. Bull. Korean Chem. Soc. 2018, 39, 1058–1065. [Google Scholar] [CrossRef]
- Urakawa, O.; Yasue, A. Glass transition behaviors of poly (vinyl pyridine)/poly (vinyl phenol) revisited. Polym. (Basel) 2019, 11, 1153. [Google Scholar] [CrossRef]
- Abed, Y.; Arrar, Z.; Hammouti, B.; Taleb, M.; Kertit, S.; Mansri, A. Poly(4-vinylpyridine) and poly(4-vinylpyridine poly-3-oxide ethylene) as corrosion inhibitors for Cu60-Zn40 in 0.5 M HNO3. Anti-Corros. Methods Mater. 2001, 48, 304–308. [Google Scholar] [CrossRef]
- Xiao, P.; Dong, T.; Xie, J.; Luo, D.; Yuan, J.; Liu, B. Emergence of white organic light-emitting diodes based on thermally activated delayed fluorescence. Appl. Sci. 2018, 8, 299. [Google Scholar] [CrossRef]
- Panunzi, B.; Diana, R.; Caruso, U. A highly efficient white luminescent zinc (II) based metallopolymer by RGB approach. Polymers 2019, 11, 1712. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.L.; Zhou, X.; Yue, K.; Guo, Z.H. Synthesis and self-assembly of conjugated block copolymers. Polymers 2021, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, R. Mono- and di-quaternized 4,4′-bipyridine derivatives as key building blocks for medium- And environment-responsive compounds and materials. Molecules 2020, 25, 1. [Google Scholar] [CrossRef] [PubMed]
- Inuzuka, K. Electronic Properties of 4-Substituted Pyridines and Their Pyridinium Cations and Dihydropyridyl Radicals in the Ground State. Nippon. Kagaku Kaishi 1977, 1977, 355–361. [Google Scholar] [CrossRef][Green Version]
- Krygowski, T.M.; Szatyłowicz, H.; Zachara, J.E. How H-bonding modifies molecular structure and π-electron delocalization in the ring of pyridine/pyridinium derivatives involved in H-bond complexation. J. Org. Chem. 2005, 70, 8859–8865. [Google Scholar] [CrossRef] [PubMed]
- Bunten, K.A.; Kakkar, A.K. Synthesis of Pyridine/Pyridinium-based Alkynyl Monomers, Oligomers and Polymers: Enhancing Conjugation by Pyridine N-Quaternization. J. Mater. Chem. 1995, 5, 2041–2043. [Google Scholar] [CrossRef]
- Jonforsen, M.; Grigalevicius, S.; Andersson, M.R.; Hjertberg, T. Counter-ion induced solubility of polypyridines. Synth. Met. 1999, 102, 1200–1201. [Google Scholar] [CrossRef]
- Aoki, A.; Rajagopalan, R.; Heller, A. Effect of quaternization on electron diffusion coefficients for redox hydrogels based on poly(4-vinylpyridine). J. Phys. Chem. 1995, 99, 5102–5110. [Google Scholar] [CrossRef]
- Zhou, T.; He, X.; Song, F.; Xie, K. Chitosan Modified by Polymeric Reactive Dyes Containing Quanternary Ammonium Groups as a Novel Anion Exchange Membrane for Alkaline Fuel Cells. Int. J. Electrochem. Sci. 2016, 11, 590–608. [Google Scholar]
- Zhai, L.; Li, H. Polyoxometalate-polymer hybrid materials as proton exchange membranes for fuel cell applications. Molecules 2019, 24, 3425. [Google Scholar] [CrossRef]
- Rumyantsev, A.M.; Pan, A.; Ghosh Roy, S.; De, P.; Kramarenko, E.Y. Polyelectrolyte Gel Swelling and Conductivity vs Counterion Type, Cross-Linking Density, and Solvent Polarity. Macromolecules 2016, 49, 6630–6643. [Google Scholar] [CrossRef]
- Bonardd, S.; Ángel, A.; Norambuena, Á.; Coll, D.; Tundidor-Camba, A.; Ortiz, P.A. Novel polyelectrolytes obtained by direct alkylation and ion replacement of a new aromatic polyamide copolymer bearing pyridinyl pendant groups. Polymers 2021, 13, 1993. [Google Scholar] [CrossRef]
- Gokkaya, D.; Topuzogullari, M.; Arasoglu, T.; Trabzonlu, K.; Ozmen, M.M.; Abdurrahmanoğlu, S. Antibacterial properties of cationic copolymers as a function of pendant alkyl chain length and degree of quaternization. Polym. Int. 2021, 70, 829–836. [Google Scholar] [CrossRef]
- Nagasako, T.; Ogata, T.; Kurihara, S.; Nonaka, T. Synthesis of thermosensitive copolymer beads containing pyridinium groups and their antibacterial activity. J. Appl. Polym. Sci. 2010, 116, 2580–2589. [Google Scholar] [CrossRef]
- Luo, H.; Tang, Q.; Zhong, J.; Lei, Z.; Zhou, J.; Tong, Z. Interplay of Solvation and Size Effects Induced by the Counterions in Ionic Block Copolymers on the Basis of Hofmeister Series. Macromol. Chem. Phys. 2019, 220, 1800508. [Google Scholar] [CrossRef]
- Mondal, P.; Saha, S.K.; Chowdhury, P. Simultaneous polymerization and quaternization of 4-vinyl pyridine. J. Appl. Polym. Sci. 2013, 127, 5045–5050. [Google Scholar] [CrossRef]
- Chovino, C.; Gramain, P. Stereoregularity of poly(4-vinyl-n-alkyl-pyridinium) salts prepared by spontaneous polymerization. Polymer 1999, 40, 4805–4810. [Google Scholar] [CrossRef]
- Jaeger, W.; Bohrisch, J.; Laschewsky, A. Synthetic polymers with quaternary nitrogen atoms-Synthesis and structure of the most used type of cationic polyelectrolytes. Prog. Polym. Sci. 2010, 35, 511–577. [Google Scholar] [CrossRef]
- Laschewsky, A. Recent trends in the synthesis of polyelectrolytes. Curr. Opin. Colloid Interface Sci. 2012, 17, 56–63. [Google Scholar] [CrossRef]
- Bicak, N.; Gazi, M. Quantitative quaternization of poly(4-vinyl pyridine). J. Macromol. Sci.-Pure Appl. Chem. 2003, 40, 585–591. [Google Scholar] [CrossRef]
- Izumrudov, V.A.; Zhiryakova, M.V.; Melik-Nubarov, N.S. Supercharged pyridinium polycations and polyelectrolyte complexes. Eur. Polym. J. 2015, 69, 121–131. [Google Scholar] [CrossRef]
- Boucher, E.A.; Mollett, C.C. Kinetics and Mechanism of the Quaternization of poly(4-vinyl pyridine) with Alkyl and Arylalkyl Bromides in Sulpholane. J. Chem. Soc. Faraday Trans. 1 1982, 78, 75–88. [Google Scholar] [CrossRef]
- Chovino, C.; Gramain, P. Influence of the conformation on chemical modification of polymers: Study of the quaternization of poly(4-vinylpyridine). Macromolecules 1998, 31, 7111–7114. [Google Scholar] [CrossRef]
- Frère, Y.; Gramain, P. Reaction Kinetics of Polymer Substituents. Macromolecular Steric Hindrance Effect in Quaternization of Poly(vinylpyridines). Macromolecules 1992, 25, 3184–3189. [Google Scholar] [CrossRef]
- Velazquez, G.; Herrera-Gómez, A.; Martín-Polo, M.O. Identification of bound water through infrared spectroscopy in methylcellulose. J. Food Eng. 2003, 59, 79–84. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, X.; Zhang, G. Ion-specific effect on dynamics of polyelectrolyte chains. Phys. Chem. Chem. Phys. 2012, 14, 6812–6816. [Google Scholar] [CrossRef] [PubMed]
- Sargsyan, A.; Tonoyan, A.; Davtyan, S.; Schick, C. The amount of immobilized polymer in PMMA SiO2 nanocomposites determined from calorimetric data. Eur. Polym. J. 2007, 43, 3113–3127. [Google Scholar] [CrossRef]
- Wurm, A.; Ismail, M.; Kretzschmar, B.; Pospiech, D.; Schick, C. Retarded Crystallization in Polyamide/Layered Silicates Nanocomposites caused by an Immobilized Interphase. Macromolecules 2010, 43, 1480–1487. [Google Scholar] [CrossRef]
- Klonos, P.; Kulyk, K.; Borysenko, M.V.; Gun’ko, V.M.; Kyritsis, A.; Pissis, P. Effects of Molecular Weight below the Entanglement Threshold on Interfacial Nanoparticles/Polymer Dynamics. Macromolecules 2016, 49, 9457–9473. [Google Scholar] [CrossRef]
- Klonos, P.A.; Patelis, N.; Glynos, E.; Sakellariou, G.; Kyritsis, A. Molecular Dynamics in Polystyrene Single-Chain Nanoparticles. Macromolecules 2019, 52, 9334–9340. [Google Scholar] [CrossRef]
- Pissis, P.; Kyritsis, A. Hydration studies in polymer hydrogels. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 159–175. [Google Scholar] [CrossRef]
- Szkudlarek, M.; Heine, E.; Keul, H.; Beginn, U.; Möller, M. Synthesis, characterization, and antimicrobial properties of peptides mimicking copolymers of maleic anhydride and 4-methyl-1-pentene. Int. J. Mol. Sci. 2018, 19, 2617. [Google Scholar] [CrossRef]
- Aziz, S.B.; Rasheed, M.A.; Ahmed, H.M. Synthesis of polymer nanocomposites based on [methyl cellulose](1-x):(CuS)x (0.02M ≤ x ≤ 0.08 M) with desired optical band gaps. Polymers 2017, 9, 194. [Google Scholar] [CrossRef]
- Nigam, S.; Rutan, S. Applications of Principles and Solvatochromism. Appl. Spectrosc. 2001, 55, 362A. [Google Scholar] [CrossRef]
- Steven Paley, M.; Andrew Mcgill, R.; Howard, S.C.; Wallace, S.E.; Milton Harris, J. Solvatochromism. A New Method for Polymer Characterization. Macromolecules 1990, 23, 4557–4564. [Google Scholar] [CrossRef]
- Deligkiozi, I.; Papadakis, R. Probing Solvation Effects in Binary Solvent Mixtures with the Use of Solvatochromic Dyes. In Dyes and Pigments-Novel Applications and Waste Treatment; Papadakis, R., Ed.; IntechOpen: London, UK, 2021. [Google Scholar]
- Papadakis, R.; Deligkiozi, I.; Nowak, K.E. Study of the preferential solvation effects in binary solvent mixtures with the use of intensely solvatochromic azobenzene involving [2]rotaxane solutes. J. Mol. Liq. 2019, 274, 715–723. [Google Scholar] [CrossRef]
- Papadakis, R. Solute-centric versus indicator-centric solvent polarity parameters in binary solvent mixtures. Determining the contribution of local solvent basicity to the solvatochromism of a pentacyanoferrate(II) dye. J. Mol. Liq. 2017, 241, 211–221. [Google Scholar] [CrossRef]
- Papadakis, R. Preferential Solvation of a Highly Medium Responsive Pentacyanoferrate(II) Complex in Binary Solvent Mixtures: Understanding the Role of Dielectric Enrichment and the Specificity of Solute–Solvent Interactions. J. Phys. Chem. B 2016, 120, 9422–9433. [Google Scholar] [CrossRef] [PubMed]
- Deligkiozi, I.; Voyiatzis, E.; Tsolomitis, A.; Papadakis, R. Synthesis and characterization of new azobenzene-containing bis pentacyanoferrate(II) stoppered push-pull [2]rotaxanes, with α- and β-cyclodextrin. Towards highly medium responsive dyes. Dye. Pigment. 2015, 113, 709–722. [Google Scholar] [CrossRef]
- Pires, P.A.R.; El Seoud, O.A.; Machado, V.G.; De Jesus, J.C.; De Melo, C.E.A.; Buske, J.L.O.; Cardozo, A.P. Understanding Solvation: Comparison of Reichardt’s Solvatochromic Probe and Related Molecular “core” Structures. J. Chem. Eng. Data 2019, 64, 2213–2220. [Google Scholar] [CrossRef]
- Marcus, Y. Solvent Mixtures Properties and Selective Solvation; Marcel Dekker: New York, NY, USA, 2002. [Google Scholar]
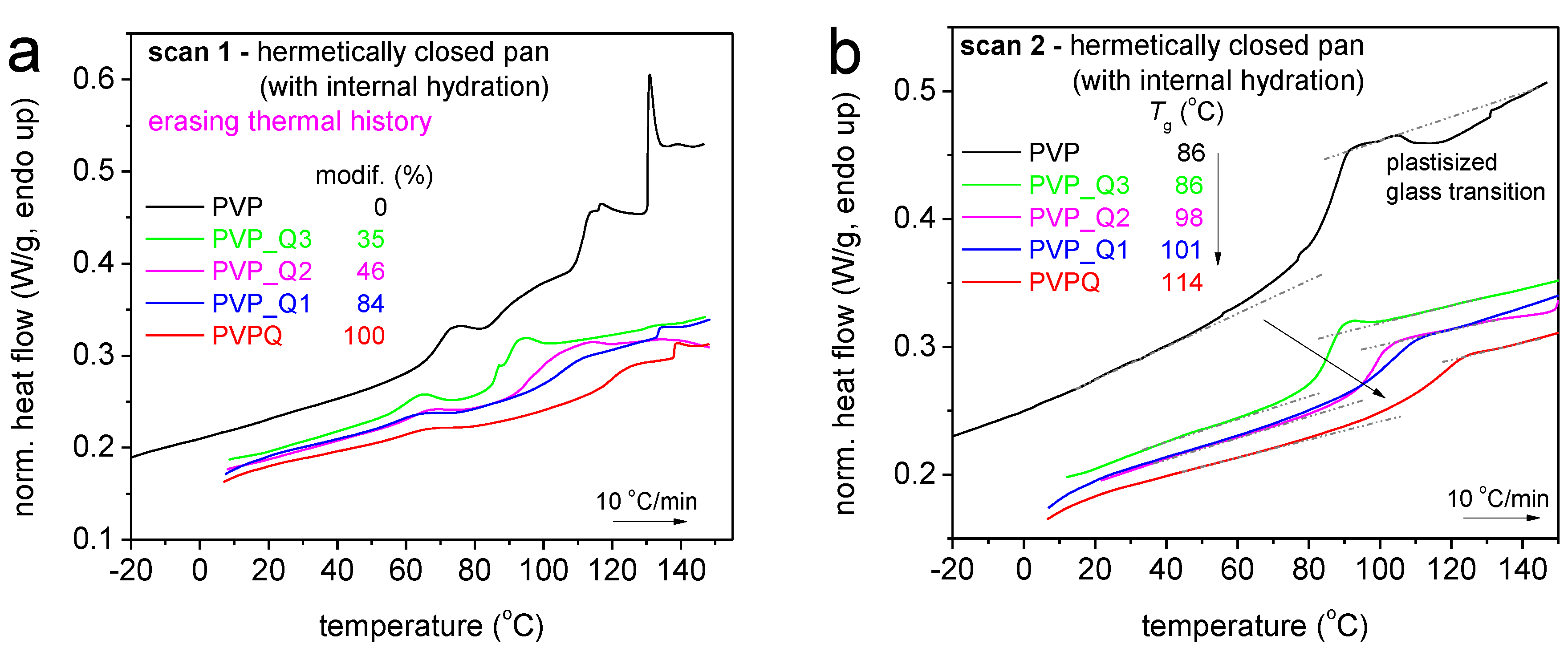
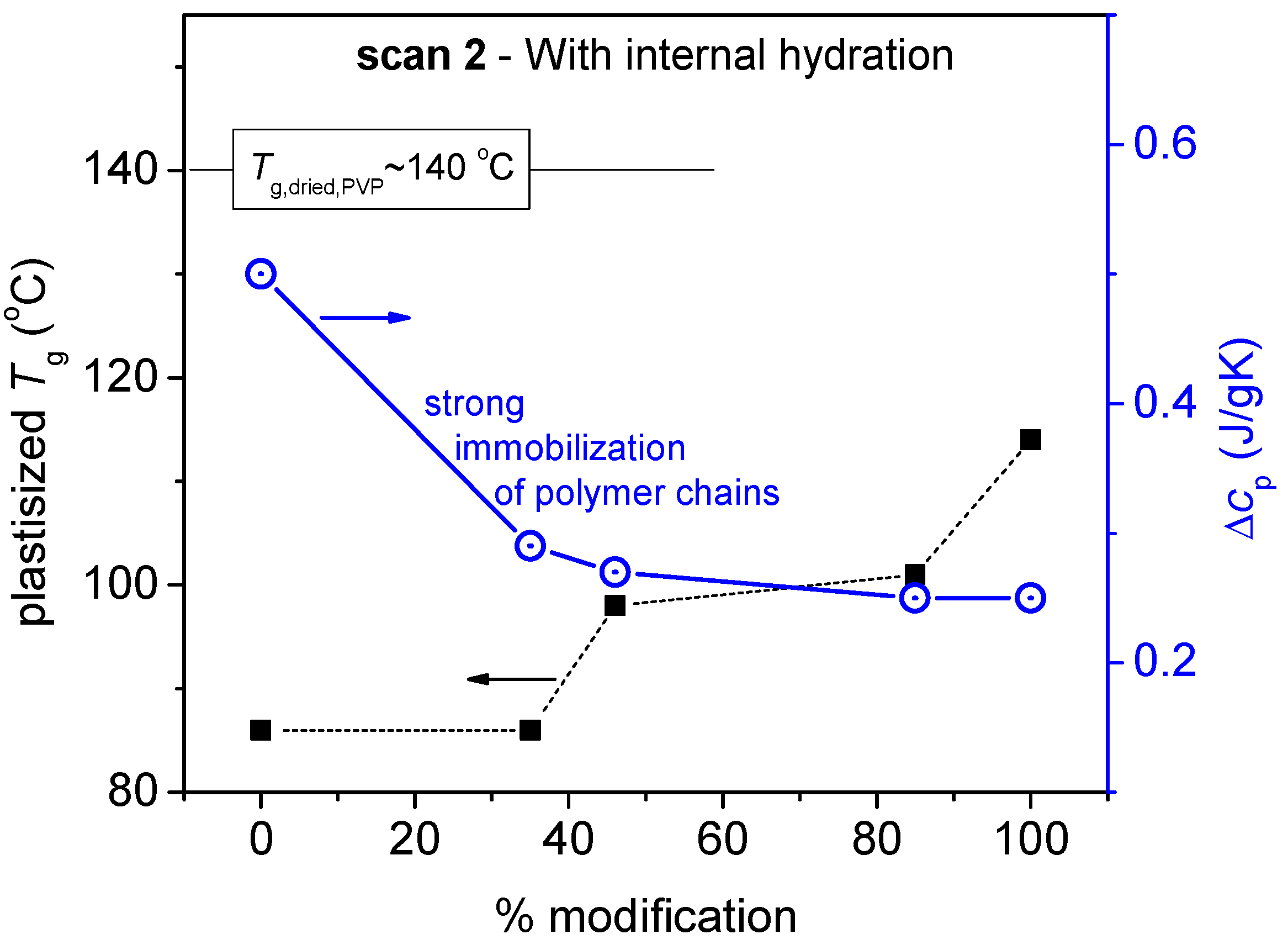
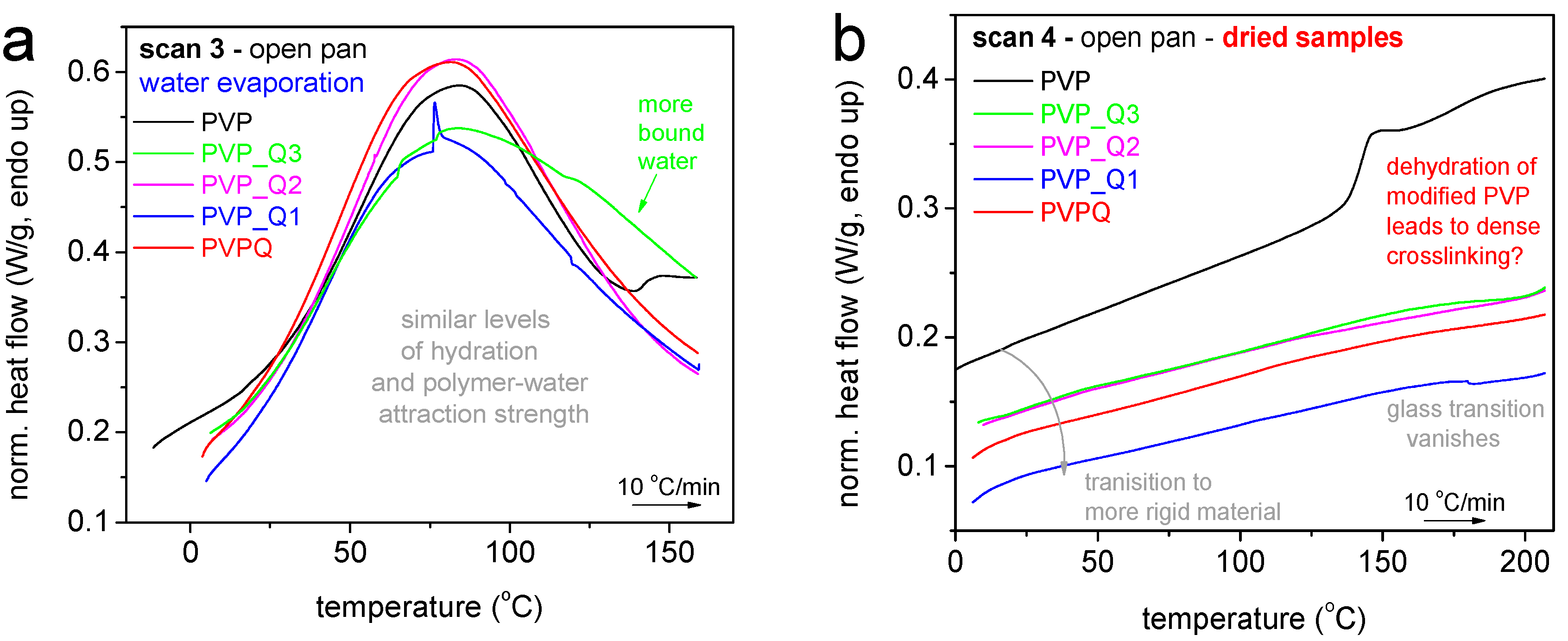
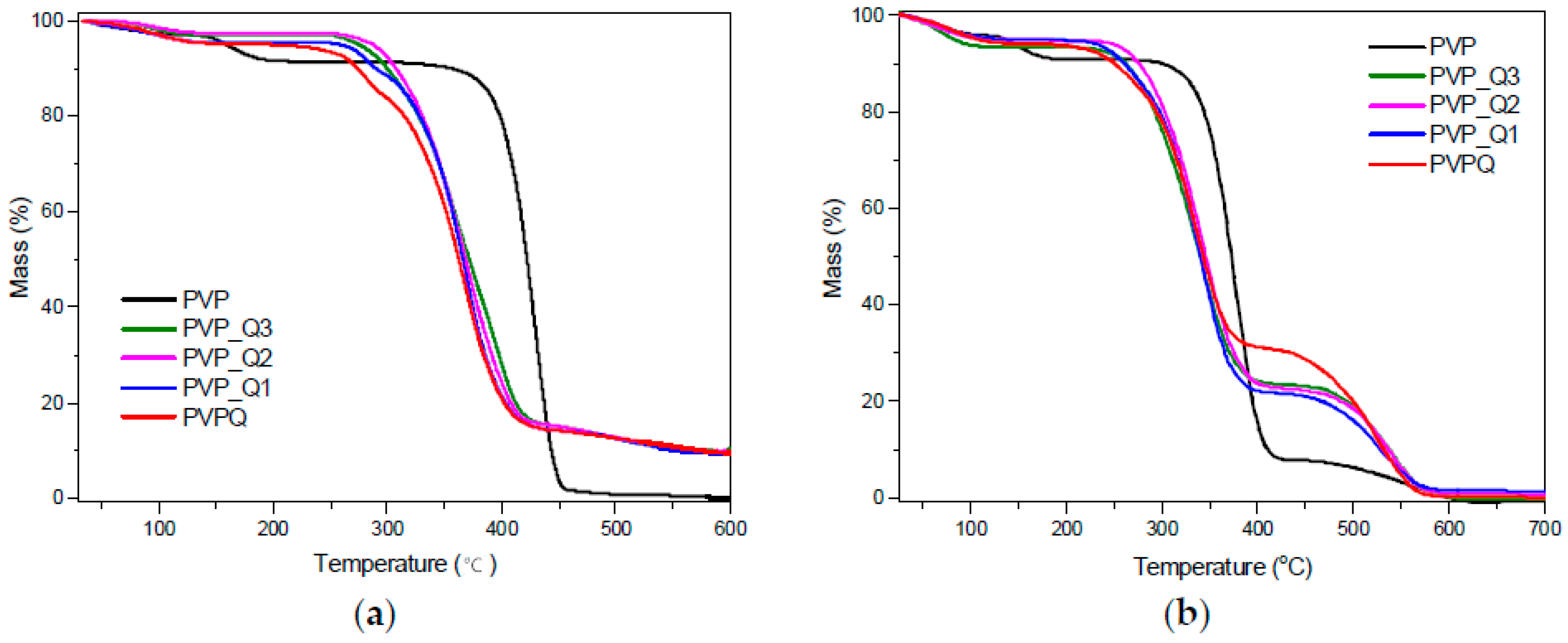
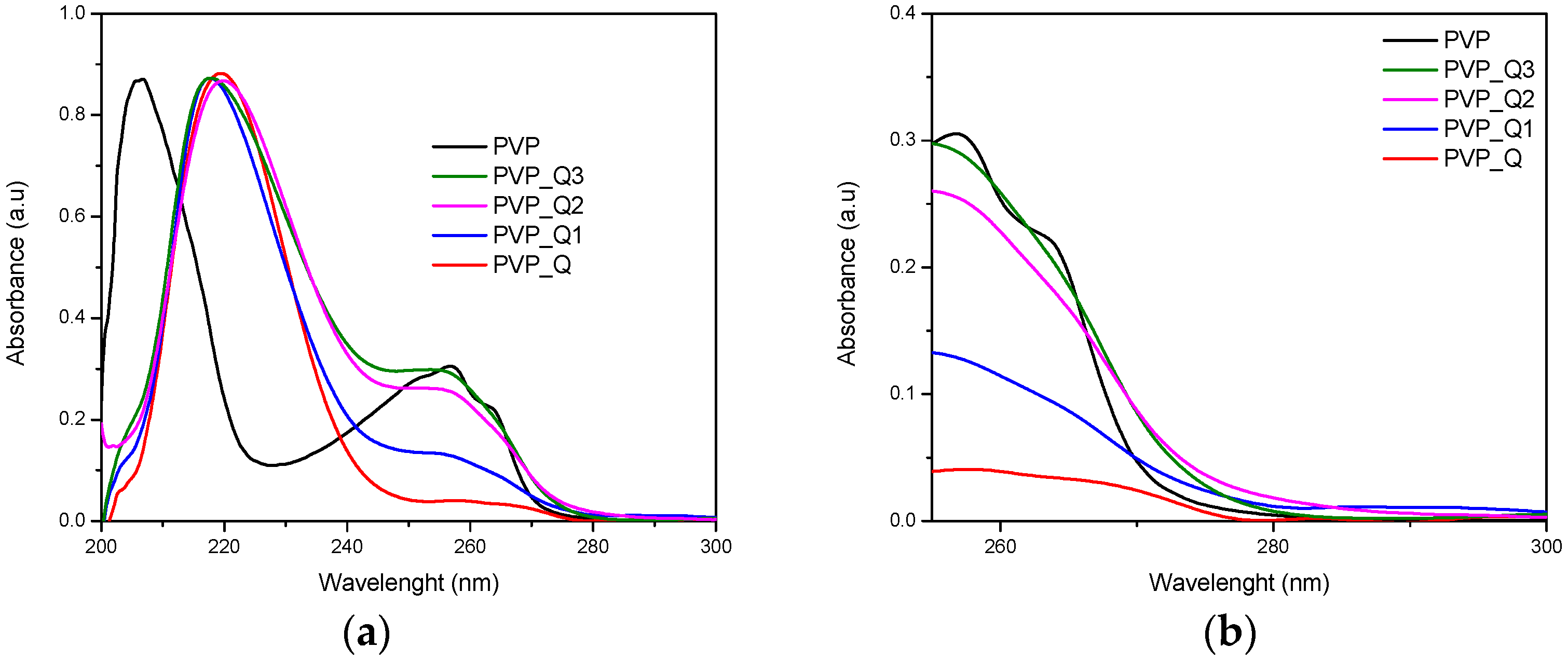
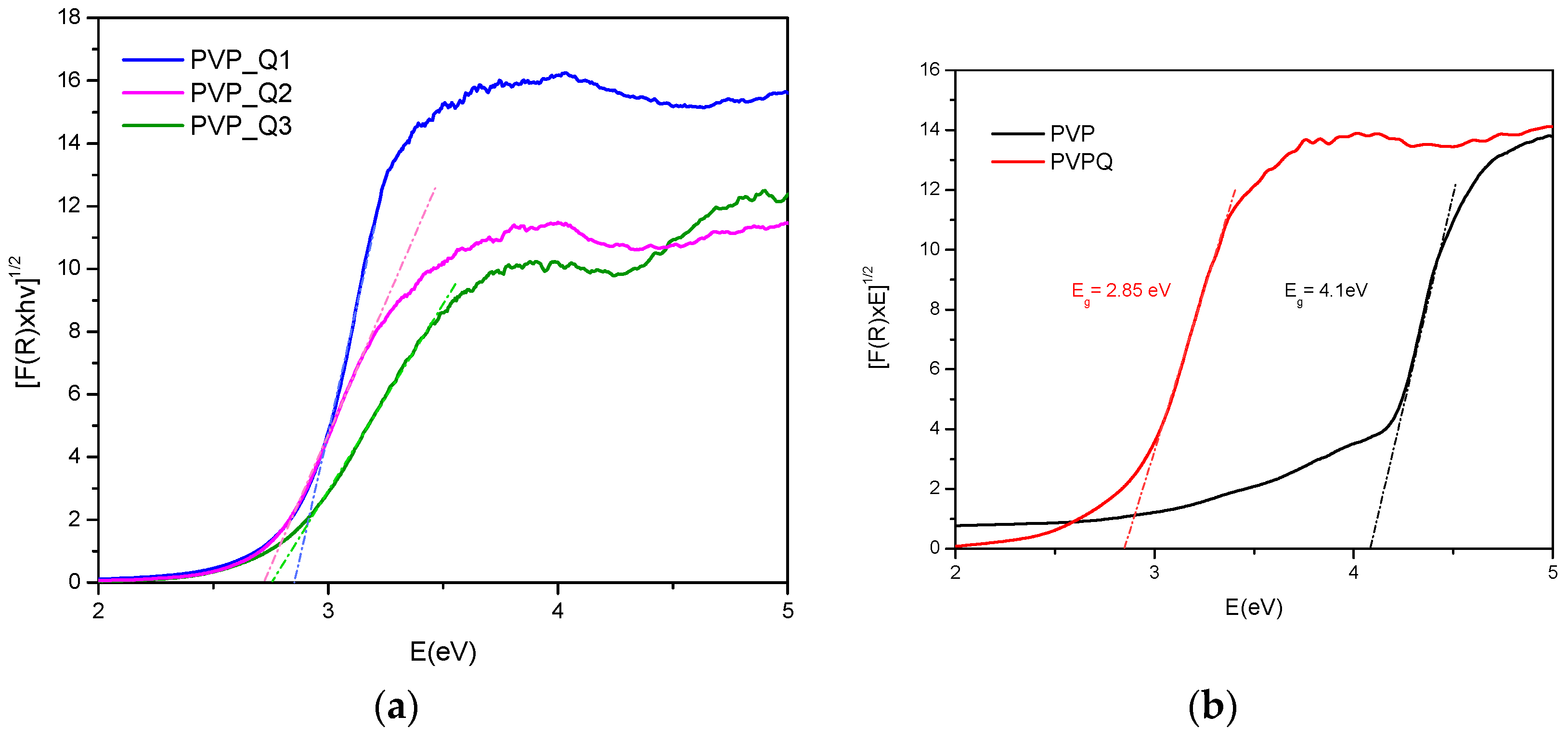
| Sample | Calculated Degree of Quaternization (%) | Tg (°C) |
|---|---|---|
| PVP | 0 | 86 |
| PVP_Q3 | 35 | 86 |
| PVP_Q2 | 46 | 98 |
| PVP_Q1 | 85 | 101 |
| PVPQ | 100 | 114 |
| Sample | Eg (eV)_Indirect |
|---|---|
| PVP | 4.1 |
| PVP_Q3 | 2.83 |
| PVP_Q2 | 2.75 |
| PVP_Q1 | 2.74 |
| PVPQ | 2.85 |
| DMSO: H2O Ratio | PVP (nm) | PVPQ (nm) |
|---|---|---|
| 100:1 | 635 | 634.5 |
| 98:2 | 629 | 631.5 |
| 95:5 | 628 | 629 |
| 90:10 | 597 | 600 |
| 85:15 | 575 | 577 |
| 80:20 | 569 | 570 |
| 75:25 | 562 | 562.5 |
| 50:50 | 515 | 514 |
| 25:75 | 471 | 485.5 |
| 20:80 | 465 | 478 |
| 15:85 | 462 | 473 |
| 10:90 | 458 | 470 |
| 5:95 | 434.5 | 465 |
| 2:98 | 417 | 438 |
| xDMSO | ET (PVP) kcal/mol | ET (PVPQ) kcal/mol | ET (NP *) kcal/mol | δET (PVP) ‡ kcal/mol | δET (PVPQ) ‡ kcal/mol | δET (NP) ‡ kcal/mol |
|---|---|---|---|---|---|---|
| 1.000 | 45.7 | 45.7 | 45.0 | 0.0 | 0.0 | 0.0 |
| 0.926 | 46.1 | 45.9 | 45.4 | −0.9 | −1.0 | −0.9 |
| 0.828 | 46.2 | 46.1 | 46.2 | −2.5 | −2.6 | −1.9 |
| 0.695 | 48.6 | 48.3 | 47.6 | −2.4 | −2.6 | −2.9 |
| 0.590 | 50.4 | 50.3 | 48.8 | −2.4 | −2.6 | −3.6 |
| 0.504 | 51.0 | 50.9 | 49.9 | −3.4 | −3.4 | −4.1 |
| 0.432 | 51.6 | 51.6 | 50.9 | −4.0 | −4.0 | −4.4 |
| 0.200 | 55.6 | 55.5 | 55.4 | −3.9 | −4.0 | −4.0 |
| 0.078 | 61.6 | 59.7 | 59.5 | −0.2 | −2.0 | −2.2 |
| 0.060 | 62.4 | 60.7 | 60.2 | 0.3 | −1.4 | −1.8 |
| 0.043 | 62.8 | 61.3 | 61.0 | 0.4 | −1.0 | −1.3 |
| 0.027 | 63.3 | 61.7 | 61.7 | 0.7 | −0.9 | −0.9 |
| 0.013 | 66.7 | 62.4 | 62.4 | 3.9 | −0.5 | −0.4 |
| 0.005 | 69.5 | 66.2 | 62.8 | 6.5 | 3.2 | −0.2 |
| 0.000 | 63.1 | 63.1 | 63.1 | 0.0 | 0.0 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavronasou, K.; Zamboulis, A.; Klonos, P.; Kyritsis, A.; Bikiaris, D.N.; Papadakis, R.; Deligkiozi, I. Poly(vinyl pyridine) and Its Quaternized Derivatives: Understanding Their Solvation and Solid State Properties. Polymers 2022, 14, 804. https://doi.org/10.3390/polym14040804
Mavronasou K, Zamboulis A, Klonos P, Kyritsis A, Bikiaris DN, Papadakis R, Deligkiozi I. Poly(vinyl pyridine) and Its Quaternized Derivatives: Understanding Their Solvation and Solid State Properties. Polymers. 2022; 14(4):804. https://doi.org/10.3390/polym14040804
Chicago/Turabian StyleMavronasou, Katerina, Alexandra Zamboulis, Panagiotis Klonos, Apostolos Kyritsis, Dimitrios N. Bikiaris, Raffaello Papadakis, and Ioanna Deligkiozi. 2022. "Poly(vinyl pyridine) and Its Quaternized Derivatives: Understanding Their Solvation and Solid State Properties" Polymers 14, no. 4: 804. https://doi.org/10.3390/polym14040804
APA StyleMavronasou, K., Zamboulis, A., Klonos, P., Kyritsis, A., Bikiaris, D. N., Papadakis, R., & Deligkiozi, I. (2022). Poly(vinyl pyridine) and Its Quaternized Derivatives: Understanding Their Solvation and Solid State Properties. Polymers, 14(4), 804. https://doi.org/10.3390/polym14040804









