Effect of Au Plasmonic Material on Poly M-Toluidine for Photoelectrochemical Hydrogen Generation from Sewage Water
Abstract
1. Introduction
2. Experimental Part
2.1. Materials
2.2. Electropolymerization of MT and Preparation of the Electrode
2.3. Characterization Process
3. Results and Discussion
3.1. Electropolymerization of M-Toluidine
3.2. PMT and PMT/Au Analyses
3.3. Electrochemical Hydrogen Generation
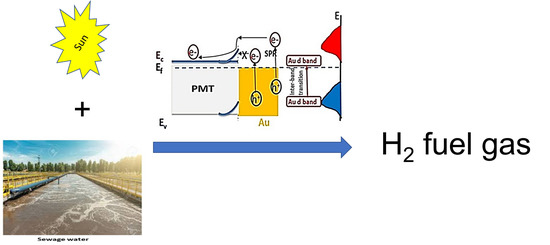
3.4. Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brandt, J.; Salerno, F.; Fuchter, M.J. The added value of small-molecule chirality in technological applications. Nat. Rev. Chem. 2017, 1, 0045. [Google Scholar] [CrossRef]
- Wade, J.; Hilfiker, J.N.; Brandt, J.R.; Liirò-Peluso, L.; Wan, L.; Shi, X.; Salerno, F.; Ryan, S.T.J.; Schöche, S.; Arteaga, O.; et al. Natural optical activity as the origin of the large chiroptical properties in π-conjugated polymer thin films. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Worch, J. Stereochemical enhancement of polymer properties. Nat. Rev. Chem. 2019, 3, 514–535. [Google Scholar] [CrossRef]
- Vøllestad, E.; Strandbakke, R.; Tarach, M.; Catalán-Martínez, D.; Fontaine, M.L.; Beeaff, D.; Clark, D.R.; Serra, J.M.; Norby, T. Mixed proton and electron conducting double perovskite anodes for stable and efficient tubular proton ceramic electrolysers. Nat. Mater. 2019, 18, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Kee, R.; Zhu, H.; Sullivan, N.; Zhu, L.; Bian, L.; Jennings, D.; O’Hayre, R. Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production. Nat. Energy 2019, 4, 230–240. [Google Scholar] [CrossRef]
- Service, R.F. New electrolyzer splits water on the cheap. Science 2020, 367, 80. [Google Scholar] [CrossRef]
- Spitaleri, L.; Nicotra, G.; Zimbone, M.; Contino, A.; Maccarrone, G.; Alberti, A.; Gulino, A. Fast and Efficient Sun Light Photocatalytic Activity of Au_ZnO Core-Shell Nanoparticles Prepared by a One-Pot Synthesis. ACS Omega 2019, 4, 15061–15066. [Google Scholar] [CrossRef]
- Hisatomi, T.; Domen, K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat. Catal. 2019, 2, 387–399. [Google Scholar] [CrossRef]
- Shaban, M.; Rabia, M.; Eldakrory, M.G.; Maree, R.M.; Ahmed, A.M. Efficient photoselectrochemical hydrogen production utilizing of APbI 3 (A = Na, Cs, and Li) perovskites nanorods. Int. J. Energy Res. 2020, 45, 7436. [Google Scholar] [CrossRef]
- Mohamed, F.; Rabia, M.; Shaban, M. Synthesis and characterization of biogenic iron oxides of different nanomorphologies from pomegranate peels for efficient solar hydrogen production. J. Mater. Res. Technol. 2020, 9, 4255–4271. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Zhao, H.; Romanus, H.; El-Hossary, F.M.; EL-Kassem, M.A.; Awad, M.A.; Rabia, M.; Lei, Y. Optical, water splitting and wettability of titanium nitride/titanium oxynitride bilayer films for hydrogen generation and solar cells applications. Mater. Sci. Semicond. Process. 2020, 105, 104704. [Google Scholar] [CrossRef]
- Shaban, M.; Ali, S.; Rabia, M. Design and application of nanoporous graphene oxide film for CO2, H2, and C2H2 gases sensing. J. Mater. Res. Technol. 2019, 8, 4510–4520. [Google Scholar] [CrossRef]
- Almohammedi, A.; Shaban, M.; Mostafa, H.; Rabia, M. Nanoporous TiN/TiO2/Alumina Membrane for Photoelectrochemical Hydrogen Production from Sewage Water. Nanomaterials 2021, 11, 2617. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, A.M.; Rabia, M.; Shaban, M.; Aly, A.H.; Ahmed, A.M. Preparation of hexagonal nanoporous Al2O3/TiO2/TiN as a novel photodetector with high efficiency. Sci. Rep. 2021, 11, 17572. [Google Scholar] [CrossRef]
- Rabia, M.; Shaban, M.; Adel, A.; Abdel-Khaliek, A.A. Effect of plasmonic au nanoparticles on the photoactivity of polyaniline/indium tin oxide electrodes for water splitting. Environ. Prog. Sustain. Energy 2019, 38, 13171. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, D.; Khare, N. Plasmonic Ag nanoparticles decorated Bi 2 S 3 nanorods and nanoflowers: Their comparative assessment for photoelectrochemical water splitting. Int. J. Hydrogen Energy 2019, 44, 3538–3552. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, X.; Zhang, H.; Li, B.; Lu, H.; Ma, T.; Hao, C. Effects of 4-tert-butylpyridine on perovskite formation and performance of solution-processed perovskite solar cells. J. Mater. Chem. A 2015, 3, 22191–22198. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, M.; Liu, J.; Deng, Z.; Li, Y.; Su, B.L. Synergistic promotion of solar-driven H2 generation bythree-dimensionally ordered macroporous structured TiO2-Au-CdS ternary photocatalyst. Appl. Catal. B Environ. 2016, 184, 182–190. [Google Scholar] [CrossRef]
- Shaban, M.; Rabia, M.; El-Sayed, A.M.A.; Ahmed, A.; Sayed, S. Photocatalytic properties of PbS/graphene oxide/polyaniline electrode for hydrogen generation. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Atta, A.; Abdelhamied, M.M.; Essam, D.; Shaban, M.; Alshammari, A.H.; Rabia, M. Structural and physical properties of polyaniline/silver oxide/silver nanocomposite electrode for supercapacitor applications. Int. J. Energy Res. 2021; accepted. [Google Scholar] [CrossRef]
- Sengodan, S.; Choi, S.; Jun, A.; Shin, T.H.; Ju, Y.W.; Jeong, H.Y.; Shin, J.; Irvine, J.T.S.; Kim, G. Layered oxygen-deficient double perovskite as an efficient and stable anode for direct hydrocarbon solid oxide fuel cells. Nat. Mater. 2015, 14, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.H.; Neagu, D.; Miller, D.N.; Irvine, J.T.S. Switching on electrocatalytic activity in solid oxide cells. Nature 2016, 537, 528–531. [Google Scholar] [CrossRef]
- Sayyah, E.-S.M.; Shaban, M.; Rabia, M. A sensor of m -cresol nanopolymer/Pt-electrode film for detection of lead ions by potentiometric methods. Adv. Polym. Technol. 2018, 37, 1296–1304. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Shaban, M.; Rabia, M. A sensor of m-toluidine/m-cresol polymer film for detection of lead ions by potentiometric methods. Sens. Lett. 2016, 14, 522–529. [Google Scholar] [CrossRef]
- Jung, J.W.; Lee, C.L.; Yu, S.; Kim, I.D. Electrospun nanofibers as a platform for advanced secondary batteries: A comprehensive review. J. Mater. Chem. A 2016, 4, 703–750. [Google Scholar] [CrossRef]
- Ognibene, G.; Gangemi, C.M.A.; Spitaleri, L.; Gulino, A.; Purrello, R.; Cicala, G.; Fragalà, M.E. Role of the surface composition of the polyethersulfone–TiiP–H2T4 fibers on lead removal: From electrostatic to coordinative binding. J. Mater. Sci. 2019, 54, 8023–8033. [Google Scholar] [CrossRef]
- Ghosh, S.; Kouamé, N.A.; Ramos, L.; Remita, S.; Dazzi, A.; Deniset-Besseau, A.; Beaunier, P.; Goubard, F.; Aubert, P.H.; Remita, H. Conducting polymer nanostructures for photocatalysis under visible light. Nat. Mater. 2015, 14, 505–511. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, Q. Controlled synthesis and energy applications of one-dimensional conducting polymer nanostructures: An overview. Adv. Energy Mater. 2012, 2, 179–218. [Google Scholar] [CrossRef]
- Modibane, K.D.; Waleng, N.J.; Ramohlola, K.E.; Maponya, T.C.; Monama, G.R.; Makgopa, K.; Hato, M.J. Poly(3-aminobenzoic acid) decorated with cobalt zeolitic benzimidazolate framework for electrochemical production of clean hydrogen. Polymers 2020, 12, 1581. [Google Scholar] [CrossRef]
- Xiao, K.; Tu, B.; Chen, L.; Heil, T.; Wen, L.; Jiang, L.; Antonietti, M. Photo-Driven Ion Transport for a Photodetector Based on an Asymmetric Carbon Nitride Nanotube Membrane. Angew. Chemie Int. Ed. 2019, 58, 12574–12579. [Google Scholar] [CrossRef]
- Belabed, C.; Abdi, A.; Benabdelghani, Z.; Rekhila, G.; Etxeberria, A.; Trari, M. Photoelectrochemical properties of doped polyaniline: Application to hydrogen photoproduction. Int. J. Hydrogen Energy 2013, 38, 6593–6599. [Google Scholar] [CrossRef]
- Mejia, E.; Cherupurakal, N.; Mourad, A.H.I.; Al Hassanieh, S.; Rabia, M. Effect of Processing Techniques on the Microstructure and Mechanical Performance of High-Density Polyethylene. Polymers 2021, 13, 3346. [Google Scholar] [CrossRef] [PubMed]
- Sayyah, S.M.; Shaban, M.; Rabia, M. M-toluidine polymer film coated platinum electrode as a pH sensor by potentiometric methods. Sens. Lett. 2015, 13, 961–966. [Google Scholar] [CrossRef]
- Sayyah, S.M.; El-Deeb, M.M.; Mohamed, A.S. Electrodeposition of poly(3-hydroxyaniline) from acidic aqueous/acetonitrile solution. Int. J. Polym. Mater. Polym. Biomater. 2007, 56, 1079–1097. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Shaban, M.; Rabia, M. Electropolymerization of m -Toluidin on Platinum Electrode from Aqueous Acidic Solution and Character of the Obtained Polymer. Adv. Polym. Technol. 2018, 37, 126–136. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Shaban, M.; Rabia, M. A High-Sensitivity Potentiometric Mercuric Ion Sensor Based on m-Toluidine Films. IEEE Sens. J. 2016, 16, 1541–1548. [Google Scholar] [CrossRef]
- Shaban, M.; Rabia, M.; Fathallah, W.; El-Mawgoud, N.A.; Mahmoud, A.; Hussien, H.; Said, O. Preparation and Characterization of Polyaniline and Ag/Polyaniline Composite Nanoporous Particles and Their Antimicrobial Activities. J. Polym. Environ. 2018, 26, 434–442. [Google Scholar] [CrossRef]
- Schindler, A.; Neumann, G.; Rager, A.; Füglein, E.; Blumm, J.; Denner, T. A novel direct coupling of simultaneous thermal analysis (STA) and Fourier transform-infrared (FT-IR) spectroscopy. J. Therm. Anal. Calorim. 2013, 113, 1091–1102. [Google Scholar] [CrossRef][Green Version]
- Buda, S.; Nawój, M.; Mlynarski, J. Recent Advances in NMR Studies of Carbohydrates. Annu. Rep. NMR Spectrosc. 2016, 89, 185–223. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Rabia, M.; Shaban, M.; Verpoort, F. Heulandite/polyaniline hybrid composite for efficient removal of acidic dye from water; kinetic, equilibrium studies and statistical optimization. Adv. Powder Technol. 2018, 29, 2501–2511. [Google Scholar] [CrossRef]
- Shaban, M.; Abukhadra, M.R.; Rabia, M.; Elkader, Y.A.; Abd El-Halim, M.R. Investigation the adsorption properties of graphene oxide and polyaniline nano/micro structures for efficient removal of toxic Cr(VI) contaminants from aqueous solutions; kinetic and equilibrium studies. Rend. Lincei. Sci. Fis. Nat. 2018, 29, 141. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, N.; Zhang, N.; Song, Y.; Stanislaus, M.S.; Zhao, C.; Yang, Y. Efficient photocatalytic removal of RhB, MO and MB dyes by optimized Ni/NiO/TiO2 composite thin films under solar light irradiation. J. Environ. Chem. Eng. 2018, 6, 2724–2732. [Google Scholar] [CrossRef]
- Contino, A.; Maccarrone, G.; Fragalà, M.E.; Spitaleri, L.; Gulino, A. Conjugated Gold–Porphyrin Monolayers Assembled on Inorganic Surfaces. Chem.–A Eur. J. 2017, 23, 14937–14943. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Thimsen, E.; Le Formal, F.; Grätzel, M.; Warren, S.C. Influence of plasmonic Au nanoparticles on the photoactivity of Fe 2O3 electrodes for water splitting. Nano Lett. 2011, 11, 35–43. [Google Scholar] [CrossRef]
- Peleg, M.; Normand, M.D.; Corradini, M.G. The Arrhenius equation revisited. Crit. Rev. Food Sci. Nutr. 2012, 52, 830–851. [Google Scholar] [CrossRef] [PubMed]
- Rabia, M.; Mohamed, H.S.H.; Shaban, M.; Taha, S. Preparation of polyaniline/PbS core-shell nano/microcomposite and its application for photocatalytic H2 electrogeneration from H2O. Sci. Rep. 2018, 8, 1107. [Google Scholar] [CrossRef]
- Evingür, G.A.; Pekcan, Ö. Optical energy band gap of PAAm-GO composites. Compos. Struct. 2018, 183, 212–215. [Google Scholar] [CrossRef]
- Pan, L.; Liu, Y.; Yao, L.; Ren, D.; Sivula, K.; Grätzel, M.; Hagfeldt, A. Cu2O photocathodes with band-tail states assisted hole transport for standalone solar water splitting. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Helmy, A.; Rabia, M.; Shaban, M.; Ashraf, A.M.; Ahmed, S.; Ahmed, A.M. Graphite/rolled graphene oxide/carbon nanotube photoelectrode for water splitting of exhaust car solution. Int. J. Energy Res. 2020, 44, 7687–7697. [Google Scholar] [CrossRef]
- Eyring, H. The activated complex in chemical reactions. J. Chem. Phys. 1935, 3, 63–71. [Google Scholar] [CrossRef]
- Rabia, M.; Mohamed, S.H.; Zhao, H.; Shaban, M.; Lei, Y.; Ahmed, A.M. TiO2/TiOxNY hollow mushrooms-like nanocomposite photoanode for hydrogen electrogeneration. J. Porous Mater. 2020, 27, 133–139. [Google Scholar] [CrossRef]
- Kolivand, A.; Sharifnia, S. Enhanced photocatalytic hydrogen evolution from water splitting by Z-scheme CdS/BiFeO3 heterojunction without using sacrificial agent. Int. J. Energy Res. 2021, 45, 2739–2752. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, D.; Wen, L.; Xu, R.; Obergfell, M.; Mi, Y.; Zhan, Z.; Nasori, N.; Demsar, J.; Lei, Y. Manipulation of charge transfer and transport in plasmonic-ferroelectric hybrids for photoelectrochemical applications. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.X.; Miao, J.; Wang, H.Y.; Yang, H.; Chen, J.; Liu, B. Electrochemical construction of hierarchically ordered CdSe-sensitized TiO2 nanotube arrays: Towards versatile photoelectrochemical water splitting and photoredox applications. Nanoscale 2014, 6, 6727–6737. [Google Scholar] [CrossRef] [PubMed]
- Taffa, D.H.; Hamm, I.; Dunkel, C.; Sinev, I.; Bahnemann, D.; Wark, M. Electrochemical deposition of Fe2O3 in the presence of organic additives: A route to enhanced photoactivity. RSC Adv. 2015, 5, 103512–103522. [Google Scholar] [CrossRef]
- Sherman, B.D.; Ashford, D.L.; Lapides, A.M.; Sheridan, M.V.; Wee, K.R.; Meyer, T.J. Light-Driven Water Splitting with a Molecular Electroassembly-Based Core/Shell Photoanode. J. Phys. Chem. Lett. 2015, 6, 3213–3217. [Google Scholar] [CrossRef]
- Wei, Y.; Ke, L.; Kong, J.; Liu, H.; Jiao, Z.; Lu, X.; Du, H.; Sun, X.W. Enhanced photoelectrochemical water-splitting effect with a bent ZnO nanorod photoanode decorated with Ag nanoparticles. Nanotechnology 2012, 23, 235401. [Google Scholar] [CrossRef]
- Freeman, E.; Kumar, S.; Thomas, S.R.; Pickering, H.; Fermin, D.J.; Eslava, S. PrFeO3 Photocathodes Prepared Through Spray Pyrolysis. ChemElectroChem 2020, 7, 1365–1372. [Google Scholar] [CrossRef]
- Feng, S.; Ji, W. Advanced Nanoporous Anodic Alumina-Based Optical Sensors for Biomedical Applications. Front. Nanotechnol. 2021, 3, 36. [Google Scholar] [CrossRef]
- Langhammer, C.; Schwind, M.; Kasemo, B.; Zorić, I. Localized Surface Plasmon Resonances in Aluminum Nanodisks. Nano Lett. 2008, 8, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gu, Q.; Hou, L.; Zhang, C.; Lu, Y.; Wang, X.; Long, J. Molecular p–n heterojunction-enhanced visible-light hydrogen evolution over a N-doped TiO2 photocatalyst. Catal. Sci. Technol. 2017, 7, 2039–2049. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Shaban, M.; Aly, A.H.; Ahmed, A.M.; Rabia, M. Preparation and characterization of a high-efficiency photoelectric detector composed of hexagonal Al2O3/TiO2/TiN/Au nanoporous array. Mater. Sci. Semicond. Process. 2022, 139, 106348. [Google Scholar] [CrossRef]









| Band Position (cm−1) | Assignment |
|---|---|
| 3429 | N–H group [35,36,37] |
| 3106 | Aromatic C–H group |
| 2950 | Methyl group stretching [32] |
| 2357 | Adsorbed H2O or CO2 from the atmosphere [38] |
| 1465 and 1407 | C=C benzenoid [32] |
| 1631 | C=C quinoid |
| 1339 | C–N vibrations |
| 1051 | C–H in-plane |
| 595 | C–H out of plane |
| 879 | Aromatic rings para-disubstituted |
| Chemical Shift (ppm) | Assignment and Structure |
|---|---|
| 1.23 | The proton of the methyl group [39] |
| 2.56 | DMSO proton (solvent) [35] |
| 2.3 | Adsorbed H2O |
| 4.07 | Singlet signal for NH proton [35] |
| 6.93 to 7.27 | The protons of the benzene ring [39] |
| Material or Element | Concentration (mg/L) |
|---|---|
| Phenols | 0.015 |
| F− | 1.0 |
| Al3+ | 3.0 |
| NH3 | 5.0 |
| Hg2+ | 0.005 |
| Pb2+ | 0.5 |
| Cd3+ | 0.05 |
| As3+ | 0.05 |
| Cr3+ | 1.0 |
| Cu2+ | 1.5 |
| Ni3+ | 0.1 |
| Fe3+ | 1.5 |
| Mn2+ | 1.0 |
| Zn2+ | 5.0 |
| Ag+ | 0.1 |
| Ba3+ | 2.0 |
| Co2+ | 2.0 |
| Other cations | 0.1 |
| Pesticides | 0.2 |
| CN−1 | 0.1 |
| Industrial washing | 0.5 |
| Coli groups | 4000/100 cm3 |
| Electrode Materials | Electrolyte | IPCE% (390 nm) | Jph (mA cm−2) |
|---|---|---|---|
| BiFeO3 [53] | Na2SO4 | -- | 0.16 |
| Au/Pb(Zr,Ti)O3 [54] | Na2SO4 | -- | 0.06 |
| CdSe/TiO2 nanotube electrode [55] | NaOH | 0.45 | 0.13 |
| Fe2O3/sodium dodecyl sulfonate electrodes [56] | NaOH | 2 | 0.05 |
| SnO2/TiO2 [57] | Na2S2O3 | -- | 0.4 |
| ZnO/Ag [58] | Na2SO4 | 0.5 | 1 |
| PrFeO3 [59] | Na2SO4 | 1.2 | 0.13 |
| ITO/PMT/2 min-Au (present work) | Na2S2O3 | 2.3 | 0.3 |
| ITO/PMT/2 min-Au (present work) | Sewage water | 3.6 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelazeez, A.A.A.; Hadia, N.M.A.; Mourad, A.-H.I.; El-Fatah, G.A.; Shaban, M.; Ahmed, A.M.; Alzaid, M.; Cherupurakal, N.; Rabia, M. Effect of Au Plasmonic Material on Poly M-Toluidine for Photoelectrochemical Hydrogen Generation from Sewage Water. Polymers 2022, 14, 768. https://doi.org/10.3390/polym14040768
Abdelazeez AAA, Hadia NMA, Mourad A-HI, El-Fatah GA, Shaban M, Ahmed AM, Alzaid M, Cherupurakal N, Rabia M. Effect of Au Plasmonic Material on Poly M-Toluidine for Photoelectrochemical Hydrogen Generation from Sewage Water. Polymers. 2022; 14(4):768. https://doi.org/10.3390/polym14040768
Chicago/Turabian StyleAbdelazeez, Ahmed Adel A., N.M.A. Hadia, Abdel-Hamid I. Mourad, Gehad Abd El-Fatah, Mohamed Shaban, Ashour M. Ahmed, Meshal Alzaid, Nizamudeen Cherupurakal, and Mohamed Rabia. 2022. "Effect of Au Plasmonic Material on Poly M-Toluidine for Photoelectrochemical Hydrogen Generation from Sewage Water" Polymers 14, no. 4: 768. https://doi.org/10.3390/polym14040768
APA StyleAbdelazeez, A. A. A., Hadia, N. M. A., Mourad, A.-H. I., El-Fatah, G. A., Shaban, M., Ahmed, A. M., Alzaid, M., Cherupurakal, N., & Rabia, M. (2022). Effect of Au Plasmonic Material on Poly M-Toluidine for Photoelectrochemical Hydrogen Generation from Sewage Water. Polymers, 14(4), 768. https://doi.org/10.3390/polym14040768