Polymeric Composite of Magnetite Iron Oxide Nanoparticles and Their Application in Biomedicine: A Review
Abstract
:1. Introduction
2. Iron Oxide Nanoparticles (IONPs) and Superparamagnetic Iron Oxide Nanoparticles (SPIONs)
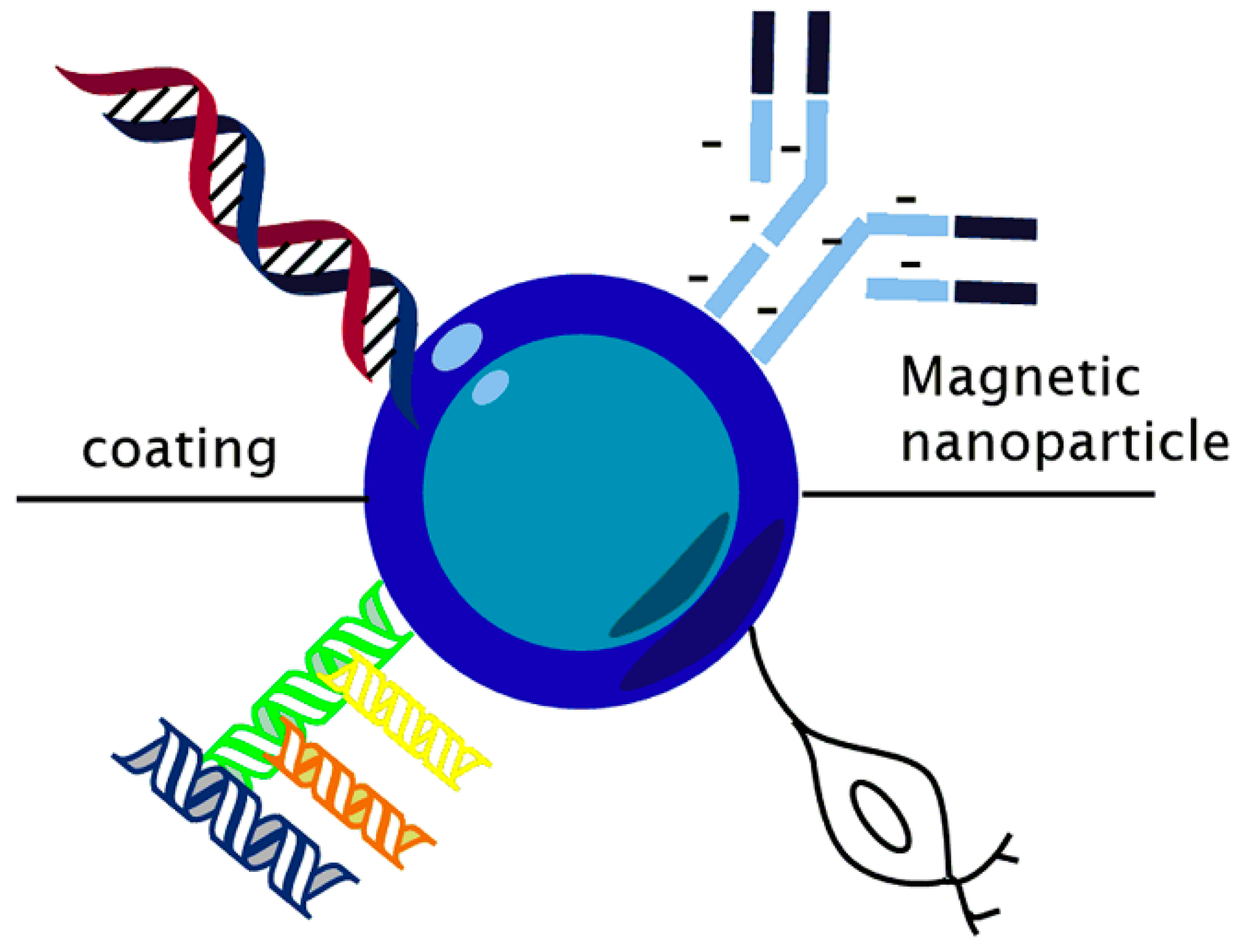
2.1. Funcionalization
2.2. Properties
Superparamagnetims
2.3. Properties Associated with Polymers
3. Methods of Preparation
3.1. Synthesis of Magnetite Iron Oxide Nanoparticles
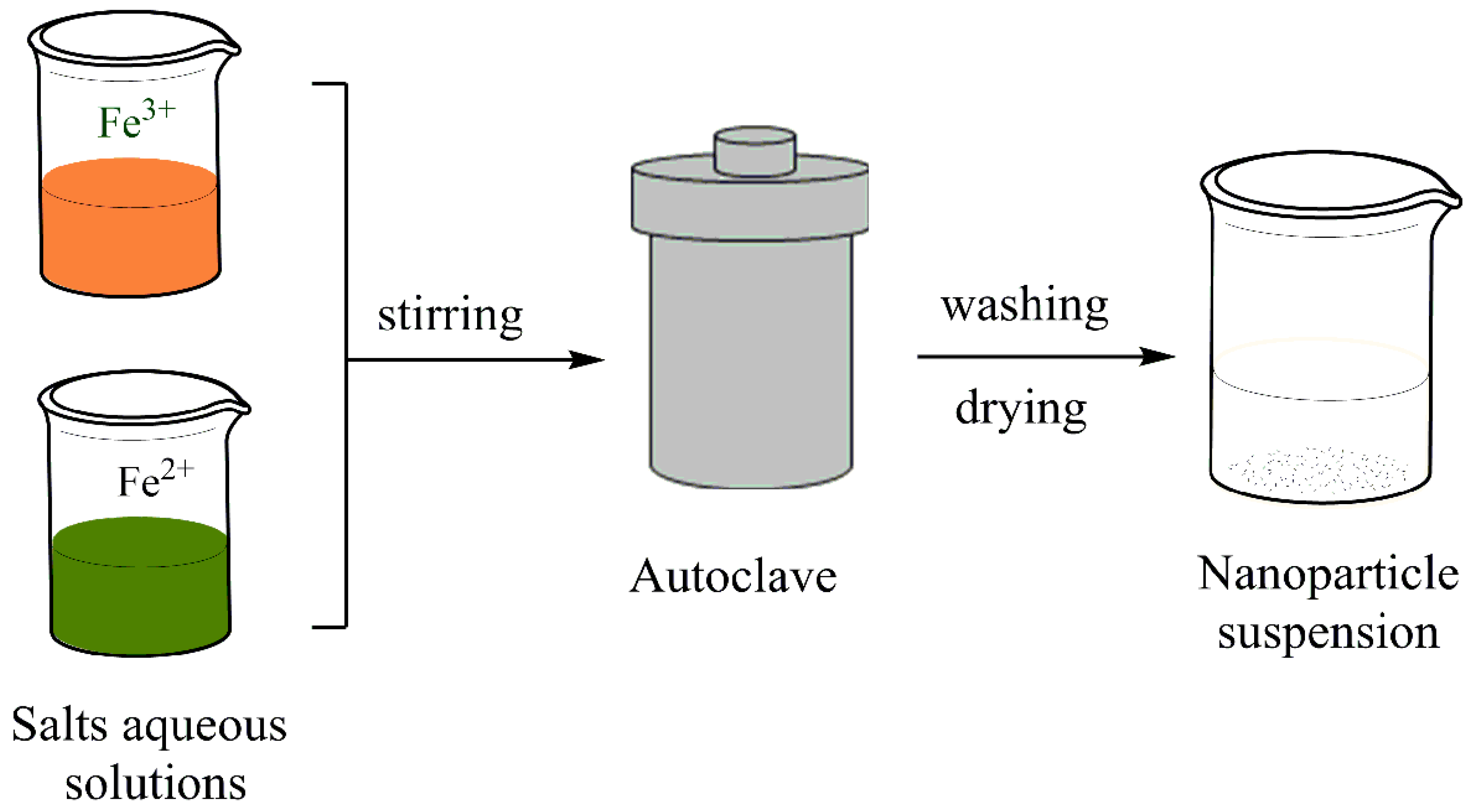
3.1.1. Co-Precipitation
3.1.2. Polyol Method
3.1.3. Hydrothermal Method
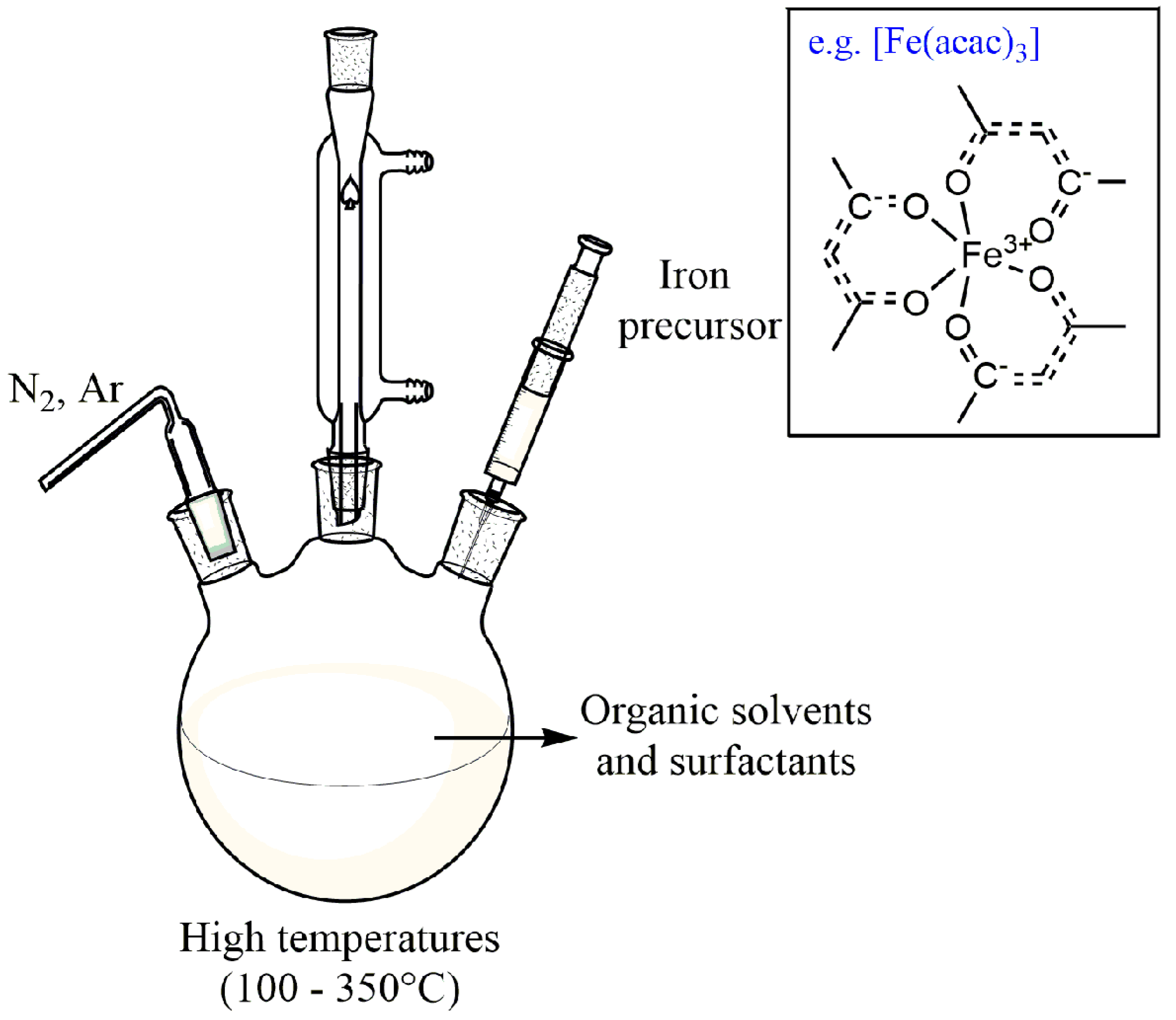
3.1.4. High-Temperature Decomposition of Organic Precursors
3.1.5. Microemulsion (ME)
3.1.6. Sol-Gel Processing
3.2. Fabrication of Hydrogel Magnetite Nanocomposite
3.2.1. Blending Method
3.2.2. In Situ Precipitation
3.2.3. Grafting-Onto Method
4. Characterization
4.1. Structural Analysis
4.1.1. Transmission Electron Microscopy (TEM)
4.1.2. Fourier Transform Infrared Spectroscopy (FTIR)
4.1.3. Small Angle Neutron Scattering (SANS)
4.1.4. X-ray Diffraction (XRD)
4.1.5. Scanning Electron Microscopy (SEM)
4.1.6. Thermogravimetric Analysis (TGA)
4.1.7. Fluorescence Microscopy
4.2. Magnetometric Methods
Magnetic Response Measurements
4.3. Swelling Analysis
4.4. Cytotoxicity Analysis
5. Applications
5.1. In Vivo Applications
5.1.1. Therapeutic Applications: Hyperthermia/Ablation
5.1.2. Drug Release
5.1.3. Diagnostic Applications: Magnetic Resonance Imaging
5.2. In Vitro Applications
Separation and Selection
6. Perspectives and Future Challenges
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acidereli, H.; Karataş, Y.; Burhan, H.; Gülcan, M.; Şen, F. Magnetic Nanoparticles. In Nanoscale Processing, 1st ed.; Thomas, S., Balakrishnan, P., Eds.; Elsevier Inc.: Cham, Switzerland, 2021; pp. 197–236. ISBN 978-0-12-820569-3. [Google Scholar]
- Kim, I.; Yang, H.; Park, C.; Yoon, I.H.; Sihn, Y. Environmental applications of magnetic nanoparticles. In Magnetic Nanoparticle-Based Hybrid Materials, 1st ed.; Ehrmann, A., Nguyen, T.A., Ahmadi, M., Farmani, A., Nguyen-Tri, P., Eds.; Elsevier Ltd.: Cham, Switzerland, 2021; pp. 529–545. ISBN 978-0-12-823688-8. [Google Scholar]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Pardo, S.; Bucio, E. Interaction between Filler and Polymeric Matrix in Nanocomposites: Magnetic Approach and Applications. Polymers 2021, 13, 2998. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, X.; Zhang, Y. Hydrogeles and hydrogel composites for 3D and 4D printing applications. In 3D and 4D Printing of Polymer Nanocomposite Materials, 1st ed.; Sadasivuni, K., Deshmukh, K., Al-Maadeed, M., Eds.; Elsevier Inc.: Cham, Switzerland, 2020; pp. 427–465. ISBN 978-0-12-816805-9. [Google Scholar]
- Wang, W.; Narain, R.; Zeng, H. Hydrogels. In Polymer Science and Nanotechnology, 1st ed.; Narain, R., Ed.; Elsevier Inc.: Cham, Switzerland, 2020; pp. 203–244. ISBN 978-0-12-816806-6. [Google Scholar]
- Bustamante-Torres, M.; Pino-Ramos, V.H.; Romero-Fierro, D.; Hidalgo-Bonilla, S.P.; Magaña, H.; Bucio, E. Synthesis and Antimicrobial Properties of Highly Cross-Linked pH-Sensitive Hydrogels through Gamma Radiation. Polymers 2021, 13, 2223. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Síntesis controlada por forma, tamaño y estructura y biocompatibilidad de nanopartículas de óxido de hierro para teranóstica magnética. Theranostics 2018, 8, 3284–3307. [Google Scholar] [CrossRef] [PubMed]
- Lakshmipriya, T.; Gopinath, S. Introduction to nanoparticles and analytical devices. In Nanoparticles in Analytical and Medical Devices, 1st ed.; Gopinath, S., Ed.; Elsevier Inc.: Cham, Switzerland, 2021; pp. 1–29. ISBN 978-0-12-821163-2. [Google Scholar]
- Seabra, A.; Pelegrino, M.; Haddad, P. Antimicrobial Applications of Superparamagnetic Iron Oxide Nanoparticles: Perspectives and Challenges. In Nanostructures for Antimicrobial Therapy, 1st ed.; Ficai, A., Grumezescu., A.M., Eds.; Elsevier Inc.: Cham, Switzerland, 2017; pp. 531–550. ISBN 978-0-323-4152-8. [Google Scholar]
- Ghaffari, M.; Moztarzadeh, F.; Mollazadeh-Bajestani, S. Drug delivery nanosystems for musculoskeletal regeneration. In Nanoengineering in Musculoskeletal Regeneration, 1st ed.; Razavi, M., Ed.; Elsevier Inc.: Cham, Switzerland, 2020; pp. 77–103. ISBN 978-0-12-820262-3. [Google Scholar]
- Ezealigo, U.; Ezealigo, B.; Aisida, S.; Ezema, F. Iron oxide nanoparticles in biological systems: Antibacterial and toxicology perspective. JCIS Open 2021, 4, 100027. [Google Scholar] [CrossRef]
- Deo, K.; Lokhande, G.; Gaharwar, A. Nanostructured Hydrogels for Tissue Engineering and Regenerative Medicine. In Encyclopedia of Tissue Engineering and Regenerative Medicine; Reis, R.L., Ed.; Elsevier Inc.: Cham, Switzerland, 2019; pp. 21–31. ISBN 978-0-12-813700-0. [Google Scholar]
- Romero-Fierro, D.; Camacho-Cruz, L.; Bustamante-Torres, M.; Hidalgo-Bonilla, S.; Bucio, E. Modification of cotton gauzes with poly(acrylic acid) and poly(methacrylic acid) using gamma radiation for drug loading studies. Radiat. Phys. Chem. 2022, 190, 109787. [Google Scholar] [CrossRef]
- Romero-Fierro, D.; Bustamante-Torres, M.; Hidalgo-Bonilla, S.; Bucio, E. Silver Composites as Antimicrobial Materials. In Environmental and Microbial Biotechnology; Inamuddin, A.A.M., Ahamed, M.I., Prasad, R., Eds.; Springer: Singapore, 2020; pp. 127–147. ISBN 978-981-15-7098-8. [Google Scholar] [CrossRef]
- Reddy, K.; Adinaraya, P.; Venkata, C.; Shetti, N.; Babu, B.; Ravindranadh, K.; Venkatakrishnan, M.; Reddy, M.; Soni, S.; Naveen, S. Functionalized magnetic nanoparticles/biopolymer hybrids: Synthesis methods, properties and biomedical applications. In Methods in Microbiology, 1st ed.; Gurtler, V., Ball, A., Soni, S., Eds.; Elsevier Ltd.: Cham, Switzerland, 2019; pp. 227–254. ISBN 978-0-12-814992-8. [Google Scholar]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; Lima, T.M.T.d.; Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [Green Version]
- Vallabani, N.V.; Singh, S. Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics. 3 Biotech 2018, 8, 279. [Google Scholar] [CrossRef] [Green Version]
- Yusoff, A.H.M.; Salimi, M.N. Superparamagnetic nanoparticles for drug delivery. In Applications of Nanocomposite Materials in Drug Delivery, 1st ed.; Inamuddin, D., Asiri, A., Mohammad, A., Eds.; Elsevier Inc.: Cham, Switzerland, 2018; pp. 843–859. ISBN 978-0-12-813741-3. [Google Scholar]
- Saengruengrit, C.; Ritprajak, P.; Wanichwecharungruang, S.; Sharma, A.; Salvan, G.; Zahn, D.; Insin, N. The combined magnetic field and iron oxide-PLGA composite particles: Effective protein antigen delivery and immune stimulation in dendritic cells. J. Colloid Interface Sci. 2018, 520, 101–111. [Google Scholar] [CrossRef]
- Kiliç, G.; Fernández-Bertólez, N.; Costa, C.; Brandão, F.; Teixeira, J.; Pásaro, E.; Laffon, B.; Valdiglesias, V. The Application, Neurotoxicity, and Related Mechanism of Iron Oxide Nanoparticles. In Neurotoxicity of Nanomaterials and Nanomedicine; Jiang, X., Gao, H., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 127–150. ISBN 978-0-12-804598-5. [Google Scholar]
- Guler, E.; Demir, B.; Guler, B.; Demirkol, D.; Timur, S. BiofuNctionalized nanomaterials for targeting cancer cells. In Nanostructures for Cancer Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier Inc.: Cham, Switzerland, 2017; pp. 51–86. ISBN 978-0-323-46144-3. [Google Scholar] [CrossRef]
- Calderón-Bedoya, A.P.; Botta, P.M.; Bercoff, P.G.; Fanovich, M.A. Magnetic iron oxides nanoparticles obtained by mechanochemical reactions from different solid precursors. J. Alloy. Compd. 2021, 860, 157892. [Google Scholar] [CrossRef]
- Calvo-de la Rosa, J.; Segarra, M. Optimization of the Synthesis of Copper Ferrite Nanoparticles by a Polymer-Assisted Sol–Gel Method. ACS Omega 2019, 4, 18289–18298. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.; Pereira, A.M.; Fernandes, C.; Rocha, M.; Mendes, R.; Fernández-García, M.P.; Guedes, A.; Tavares, P.B.; Grenèche, J.M.; Araújo, J.P.; et al. Superparamagnetic MFe2O4 (M = Fe, Co, Mn) Nanoparticles: Tuning the Particle Size and Magnetic Properties through a Novel One-Step Coprecipitation Route. Chem. Mater. 2012, 24, 1496–1504. [Google Scholar] [CrossRef]
- Wallyn, J.; Anton, N.; Vandamme, T.F. Synthesis, Principles, and Properties of Magnetite Nanoparticles for In Vivo Imaging Applications—A Review. Pharmaceutics 2019, 11, 601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; García-Martín, M.L. Magnetic Nanoparticles as MRI Contrast Agents. Top. Curr. Chem. 2020, 378, 40. [Google Scholar] [CrossRef] [PubMed]
- Materón, E.M.; Miyazaki, C.M.; Carr, O.; Joshi, N.; Picciani, P.H.; Dalmaschio, C.J.; Davis, F.; Shimizu, F.M. Magnetic nanoparticles in biomedical applications: A review. Appl. Surf. Sci. Adv. 2021, 6, 100163. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S. Nanostructures in gene delivery. In Advances in Polymeric Nanomaterials for Biomedical Applications, 1st ed.; Bajpai, A., Saini, R., Eds.; Elsevier Inc.: Cham, Switzerland, 2021; pp. 101–135. ISBN 978-0-12-814657-6. [Google Scholar]
- Ansari, S.A.M.K.; Ficiarà, E.; Ruffinatti, F.A.; Stura, I.; Argenziano, M.; Abollino, O.; Cavalli, R.; Guiot, C.; D’Agata, F. Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Functionalization for Biomedical Applications in the Central Nervous System. Materials 2019, 12, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chee, C.; Leo, B.; Lai, C. Superparamagnetic iron oxide nanoparticles for drug delivery. In Applications of Nanocomposite Materials in Drug Delivery, 1st ed.; Inamuddin, D., Asiri, A.M., Mohammad, A., Eds.; Elsevier Inc.: Cham, Switzerland, 2018; pp. 861–903. ISBN 978-0-12-813741-3. [Google Scholar]
- Cortés-Llanos, B.; Ocampo, S.M.; de la Cueva, L.; Calvo, G.F.; Belmonte-Beitia, J.; Pérez, L.; Salas, G.; Ayuso-Sacido, Á. Influence of Coating and Size of Magnetic Nanoparticles on Cellular Uptake for In Vitro MRI. Nanomaterials 2021, 11, 2888. [Google Scholar] [CrossRef]
- Mylkie, K.; Nowak, P.; Rybczynski, P.; Ziegler-Borowska, M. Polymer-Coated Magnetite Nanoparticles for Protein Immobilization. Materials 2021, 14, 248. [Google Scholar] [CrossRef]
- Roca, A.; Gutiérrez, L.; Gavilán, H.; Fortes Brollo, M.; Veintemillas-Verdaguer, S.; Morales, M. Design strategies for shape-controlled magnetic iron oxide nanoparticles. Adv. Drug Deliv. Rev. 2019, 138, 68–104. [Google Scholar] [CrossRef]
- Kumar, R.; Nayak, M.; Sahoo, G.; Pandey, K.; Sarkar, M.; Ansari, Y.; Das, V.N.R.; Topno, R.K.; Bhawna; Madhukar, M.; et al. Iron oxide nanoparticles based antiviral activity of H1N1 influenza A virus. J. Infect. Chemother. 2019, 25, 325–329. [Google Scholar] [CrossRef]
- Torasso, N.; Vergara-Rubio, A.; Rivas-Rojas, P.; Huck-Iriart, C.; Larrañaga, A.; Fernández-Cirelli, A.; Cerveny, S.; Goyanes, S. Enhancing arsenic adsorption via excellent dispersion of iron oxide nanoparticles inside poly(vinyl alcohol) nanofibers. J. Environ. Chem. Eng. 2021, 9, 104664. [Google Scholar] [CrossRef]
- Alhasan, A.; Fardous, R.; Alsudir, S.; Majrashi, M.; Alghamdi, W.; Alsharaeh, E.; Almalik, A. Polymeric Reactor for the Synthesis of Superparamagnetic-Thermal Treatment of Breast Cancer. Mol. Pharm. 2019, 16, 3577–3587. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, J.; Schmucker, D.; Fortner, J.D. Highly stable superparamagnetic iron oxide nanoparticles as functional draw solutes for osmotically driven water transport. NPJ Clean Water 2020, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Piazza, R.; Nunes, E.; Viali, W.; da Silva, S.; Aragón, F.; Coaquira, J.; de Morais, P.; Marques, R.; Jafelicci, M. Magnetic nanohydrogel obtained by miniemulsion polymerization of poly(acrylic acid) grafted onto derivatized dextran. Carbohydr. Polym. 2017, 178, 378–385. [Google Scholar] [CrossRef]
- Ganapathe, L.S.; Mohamed, M.A.; Mohamad Yunus, R.; Berhanuddin, D.D. Magnetite (Fe3O4) Nanoparticles in Biomedical Application: From Synthesis to Surface Functionalisation. Magnetochemistry 2020, 6, 68. [Google Scholar] [CrossRef]
- Prodan, A.M.; Iconaru, S.L.; Chifiriuc, C.M.; Bleotu, C.; Ciobanu, C.S.; Motelica-Heino, M.; Sizaret, S.; Predoi, D. Magnetic Properties and Biological Activity Evaluation of Iron Oxide Nanoparticles. J. Nanomater. 2013, 2013, 5. [Google Scholar] [CrossRef] [Green Version]
- Hufschmid, R.; Landers, J.; Shasha, C.; Salamon, S.; Wende, H.; Krishnan, K.M. Nanoscale Physical and Chemical Structure of Iron Oxide Nanoparticles for Magnetic Particle Imaging. Phys. Status Solidi Appl. Mater. Sci. 2019, 216, 1800544. [Google Scholar] [CrossRef]
- Espinosa, A.; Muñoz-Noval, A.; García-Hernández, M.; Serrano, A.; Jaramillo-Morena, J.; Figuerola, A.; Quarta, A.; Pellegrino, T.; Wilhelm, C.; García, M.A. Magnetic properties of iron oxide nanoparticles prepared by seeded-growth route. J. Nanopart. Res. 2013, 15, 1514. [Google Scholar] [CrossRef]
- Patsula, V.; Moskvin, M.; Dutz, S.; Horák, D. Size-dependent magnetic properties of iron oxide nanoparticles. J. Phys. Chem. Solids 2016, 88, 24–30. [Google Scholar] [CrossRef]
- Eivari, H.A.; Rahdar, A. Some Properties of Iron Oxide Nanoparticles Synthesized in Different Conditions. World Appl. Program. 2013, 3, 52–55. Available online: https://www.researchgate.net/publication/269571662_Some_Properties_of_Iron_Oxide_Nanoparticles_Synthesized_in_Different_Conditions (accessed on 20 January 2022).
- Savliwala, S.; Chiu-Lam, A.; Unni, M.; Rivera-Rodriguez, A.; Fuller, E.; Sen, K.; Threadcraft, M.; Rinaldi, C. Magnetic Nanoparticles. In Nanoparticles for Biomedical Applications, 1st ed.; Chung, E., Leon, L., Rinaldi, C., Eds.; Elsevier Inc.: Cham, Switzerland, 2020; pp. 195–221. ISBN 978-0-12-816662-8. [Google Scholar]
- Datta, P. Magnetic gels. In Polymeric Gels, 1st ed.; Pal, K., Banerjee, I., Eds.; Elsevier Ltd.: Cham, Switzerland, 2018; pp. 441–465. ISBN 978-0-08-102179-8. [Google Scholar]
- Zhang, J.; Hoshino, K. Nanomaterials for molecular sensing. In Molecular Sensors and Nanodevices, 2nd ed.; Zhang, J., Hoshino, K., Eds.; Elsevier Inc.: Cham, Switzerland, 2019; pp. 413–487. ISBN 978-0-12-814862-4. [Google Scholar]
- Yildirim, T.; Pervez, M.; Li, B.; O’Reilly, R. Size-controlled clustering of iron oxide nanoparticles within fluorescent nanogels using LCST-driven self-assembly. J. Mater. Chem. B 2020, 8, 5330–5335. [Google Scholar] [CrossRef] [PubMed]
- Montazer, M.; Harifi, T. Magnetic nanofiniches for textiles. In Nanofiniching of Textile Materials, 1st ed.; Montazer, M., Harifi, T., Eds.; Elsevier Ltd.: Cham, Switzerland, 2018; pp. 225–240. ISBN 978-0-08-101214-7. [Google Scholar]
- Mosayebi, J.; Kiyasatfar, M.; Laurent, S. Synthesis, Functionalization, and Design of Magnetic Nanoparticles for Theranostic Applications. Adv. Healthc. Mater. 2017, 6, 1700306. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. Review: Remotely controlled magneto-regulation of therapeutics from magnetoelastic gel matrices. Biotechnol. Adv. 2020, 44, 107611. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Torres, M.; Arcentales-Vera, B.; Abad-Sojos, S.; Torres-Constante, O.; Ruiz-Rubio, F.; Bucio, E. Lignin-Based Membrane for Dye Removal. In Membrane Based Methods for Dye Containing Wastewater; Muthu, S.S., Khadir, A., Eds.; Springer: Singapore, 2021; pp. 181–213. ISBN 978-981-16-4822-9. [Google Scholar] [CrossRef]
- Behrens, S.; Appel, I. Magnetic nanocomposites. Curr. Opin. Biotechnol. 2016, 39, 89–96. [Google Scholar] [CrossRef]
- Meyer, R.A.; Green, J.J. Biodegradable polymer iron oxide nanocomposites: The future of biocompatible magnetism. Nanomedicine 2015, 10, 3421–3425. [Google Scholar] [CrossRef] [Green Version]
- Ge, S.; Shi, X.; Sun, K.; Li, C.; Uher, C.; Baker, J.; Banaszak-Holl, M.; Orr, B.G. Facile Hydrothermal Synthesis of Iron Oxide Nanoparticles with Tunable Magnetic Properties. J. Phys. Chem. C 2009, 113, 13593–13599. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Ranjan-Patra, C. Green synthesis of iron oxide nanoparticles using plant extracts and its biological application. In Handbook of Greener Synthesis of Nanomaterials and Compounds, 1st ed.; Kharisov, B., Kharissova, O., Eds.; Elsevier Inc.: Cham, Switzerland, 2021; pp. 139–170. ISBN 978-0-12-822446-5. [Google Scholar]
- Sinha, M.K.; Sahu, S.K.; Meshram, P.; Prasad, L.B.; Pandey, B.D. Low temperature hydrothermal synthesis and characterization of iron oxide powders of diverse morphologies from spent pickle liquor. Powder Technol. 2015, 276, 214–221. [Google Scholar] [CrossRef]
- Cruz, I.; Freire, C.; Araújo, J.; Pereira, C.; Pereira, A. Multifunctional Ferrite Nanoparticles: From Current Trends toward the Future. In Magnetic Nanostructured Materials, 1st ed.; Gendy, A., Barandiaran, M., Hadimani, R., Eds.; Elsevier Inc.: Cham, Switzerland, 2018; pp. 59–116. ISBN 978-0-12-813904-2. [Google Scholar]
- Dembski, S.; Schneider, C.; Christ, B.; Retter, M. Core-shell nanoparticles and their use for in vivo diagnostics. In Core-Shell Nanostructures for Drug Delivery and Theranostics, 1st ed.; Focarete, M., Tampieri, A., Eds.; Elsevier Ltd.: Cham, Switzerland, 2018; pp. 119–141. ISBN 978-0-08-102198-9. [Google Scholar]
- Wu, W.; Jiang, C.Z.; Roy, V.A.L. Designed synthesis and surface engineering strategies of magnetic iron oxide nanoparticles for biomedical applications. Nanoscale 2016, 8, 19421–19474. [Google Scholar] [CrossRef]
- Shanmugasundaram, T.; Radhakrishnan, M.; Poongodi, A.; Kadirvelu, K.; Balagurunathan, R. Bio-inspired synthesis of superparamagnetic iron oxide nanoparticles for enhanced in vitro anticancer therapy. MRS Commun. 2018, 8, 604–609. [Google Scholar] [CrossRef]
- Rashid, H.; Mansoor, M.; Haider, B.; Nasir, R.; Abd-Hamid, S.; Abdulrahman, A. Synthesis and characterization of magnetite nano particles with high selectivity using in-situ precipitation method. Sep. Sci. Technol. 2019, 55, 1207–1215. [Google Scholar] [CrossRef] [Green Version]
- Hachani, R.; Lowdell, M.; Birchall, M.; Hervault, A.; Mertz, D.; Begin-Colin, S.; Thanh, N.T. Polyol synthesis, functionalisation, and biocompatibility studies of superparamagnetic iron oxide nanoparticles as potential MRI contrast agents. Nanoscale 2016, 8, 3278–3287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, B.; Mukherjee, D.; Reddy, B. Novel approaches for preparation of nanoparticles. In Nanostructures for Novel Therapy, 1st ed.; Ficai, D., Grumezescu, A., Eds.; Elsevier Inc.: Cham, Switzerland, 2017; pp. 1–36. ISBN 978-0-323-46142-9. [Google Scholar]
- Kotoulas, A.; Dendrinou-Samara, C.; Angelakeris, M.; Kalogirou, O. The Effect of Polyol Composition on the Structural and Magnetic Properties of Magnetite Nanoparticles for Magnetic Particle Hyperthermia. Materials 2019, 12, 2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiévet, F.; Ammar-Merah, S.; Brayner, R.; Chau, F.; Giraud, M.; Mammeri, F.; Peron, J.; Piquemal, J.Y.; Sicard, L.; Viau, G. The polyol process: A unique method for easy access to metal nanoparticles with tailored sizes, shapes and compositions. Chem. Soc. Rev. 2018, 47, 5187–5233. [Google Scholar] [CrossRef] [PubMed]
- Saddique, A.; Ahmad, Z.; Hoskins, C.; Mirza, M.; Naz, A.; Ahmad, J. Hierarchical synthesis of iron oxide nanoparticles by polyol cum Calcination Method and determination of its optical and magnetic behavior. Mater. Chem. Phys. 2020, 249, 122950. [Google Scholar] [CrossRef]
- Tiwari, R.; Dubey, V.; Dhoble, S.J. Emerging Synthesis Techniques for Luminescent Materials; IGI Global: Hershey, PA, USA, 2018; pp. 277–303. ISBN 9781522551713. [Google Scholar]
- Qiu, J.; Li, Y.; Jia, Y. Synthesis methods. In Persistent Phosphors from Fundamentals to Applications, 1st ed.; Qiu, J., Li, Y., Jia, Y., Eds.; Elsevier Ltd.: Cham, Switzerland, 2021; pp. 31–67. ISBN 978-0-12-818637-4. [Google Scholar]
- Feng, S.H.; Li, G.H. Hydrothermal and Solvothermal Syntheses. In Modern Inorganic Synthetic Chemistry, 2nd ed.; Xu, R., Xu, Y., Eds.; Elsevier B.V.: Cham, Switzerland, 2017; pp. 73–104. ISBN 978-0-444-63591-4. [Google Scholar]
- Huang, G.; Lu, C.H.; Yang, H.H. Magnetic Nanomaterials for Magnetic Bioanalysis. In Novel Nanomaterials for Biomedical, Environmental and Energy Applications, 1st ed.; Wang, X., Chen, X., Eds.; Elsevier Inc.: Cham, Switzerland, 2019; pp. 89–109. ISBN 978-0-12-814497-8. [Google Scholar]
- Biehl, P.; Von der Lühe, M.; Dutz, S.; Schacher, F.H. Synthesis, Characterization, and Applications of Magnetic Nanoparticles Featuring Polyzwitterionic Coatings. Polymers 2018, 10, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozel, F.; Kockar, H.; Karaagac, O. Growth of Iron Oxide Nanoparticles by Hydrothermal Process: Effect of Reaction Parameters on the Nanoparticle Size. J. Supercond. Nov. Magn. 2014, 28, 823–829. [Google Scholar] [CrossRef]
- Unni, M.; Uhl, A.M.; Savliwala, S.; Savitzky, B.H.; Dhavalikar, R.; Garraud, N.; Arnold, D.P.; Kourkoutis, L.F.; Andrew, J.S.; Rinaldi, C. Thermal Decomposition Synthesis of Iron Oxide Nanoparticles with Diminished Magnetic Dead Layer by Controlled Addition of Oxygen. ACS Nano 2017, 11, 2284–2303. [Google Scholar] [CrossRef]
- Da Silva, A.K.; Ricci, T.G.; de Toffoli, A.L.; Maciel, E.V.S.; Nazario, C.E.D.; Lancas, F.M. The role of magnetic nanomaterials in miniaturized sample preparation techniques. In Handbook on Miniaturization in Analytical Chemistry, 1st ed.; Hussain, C., Ed.; Elsevier Inc.: Cham, Switzerland, 2020; ISBN 978-0-12-819763-9. [Google Scholar]
- Besenhard, M.; LaGrow, A.; Famiani, S.; Pucciarelli, M.; Lettieri, P.; Thanh, N.; Gavriilidis, A. Continuous production of iron oxide nanoparticles via fast and economical high temperature synthesis. React. Chem. Eng. 2020, 5, 1474–1483. [Google Scholar] [CrossRef]
- Yu, R.; Quirino, J. Ionic liquids in capillary electrophoresis. In Ionic Liquids in Analytical Chemistry: New Insights and Recent Developments, 1st ed.; Carda-Broch, S., Ruiz-Angel, M., Eds.; Elsevier Inc.: Cham, Switzerland, 2022; pp. 235–274. ISBN 978-0-12-823334-4. [Google Scholar]
- Tiwari, P.; Sinha, V.; Kaur, R. Clinical considerations on micro- and nanodrug delivery systems. In Drug Delivery Trends, 1st ed.; Shegokar, R., Ed.; Elsevier Inc.: Cham, Switzerland, 2020; pp. 77–101. ISBN 978-0-12-8178870-6. [Google Scholar]
- Das, A.; Natarajan, K.; Tiwari, S.; Ganguli, A. Nanostructures synthesized by the reverse microemulsion method and their magnetic properties. Mater. Res. Express 2020, 7, 104001. [Google Scholar] [CrossRef]
- Drmota, A.; Drofenik, M.; Koselj, J.; Žnidaršič, A. Microemulsion Method for Synthesis of Magnetic Oxide Nanoparticles. In Microemulsions—An Introduction to Properties and Applications, 1st ed.; Najjar, R., Ed.; Intech Open: London, UK, 2012; 264p, ISBN 978-953-51-0247-2. [Google Scholar]
- Marciello, M.; Luengo, Y.; Morales, M.P. Iron Oxide Nanoparticles for Cancer Diagnosis and Therapy. In Nanoarchitectonics for Smart Delivery and Drug Targeting, 1st ed.; Holban, A., Grumezescu, A., Eds.; Elsevier Inc.: Cham, Switzerland, 2016; pp. 667–694. ISBN 978-0-323-47347-7. [Google Scholar]
- Ita, K. Microemulsions. In Transdermal Drug Delivery, 1st ed.; Ita, K., Ed.; Elsevier Inc.: Cham, Switzerland, 2020; pp. 97–122. ISBN 978-0-12-822550-9. [Google Scholar]
- Lakshmanan, R.; Okoli, C.; Boutonnet, M.; Järås, S.; Rajarao, G. Microemulsion prepared magnetic nanoparticles for phosphate removal: Time efficient studies. J. Environ. Chem. Eng. 2014, 2, 185–189. [Google Scholar] [CrossRef]
- Salvador, M.; Gutiérrez, G.; Noriega, S.; Moyano, A.; Blanco-López, M.C.; Matos, M. Microemulsion Synthesis of Superparamagnetic Nanoparticles for Bioapplications. Int. J. Mol. Sci. 2021, 22, 427. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Soylak, M. Functionalized nanomaterials for sample preparation methods. In Handbook of Nanomaterials in Analytical Chemistry, 1st ed.; Mustansar, C., Ed.; Elsevier Inc.: Cham, Switzerland, 2020; pp. 375–413. ISBN 978-0-12-816699-4. [Google Scholar]
- Jampílek, J.; Králová, K. Preparation of nanocomposites from agricultural waste and their versatile applications. In Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystems; Elsalam, K.A., Ed.; Elsevier Inc.: Cham, Switzerland, 2020; pp. 51–98. ISBN 978-0-12-821354-4. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Scotti, R.; Di, B.; Redaelli, M. Synthesis and Characterization of Morphology-Controlled TiO2 Nanocrystals: Opportunities and Challenges for their Application in Photocatalytic Materials. In Morphological, Compositional, and Shape Control of Materials for Catalysis, 1st ed.; Fornasiero, P., Cargnello, M., Eds.; Elsevier B.V.: Cham, Switzerland, 2017; pp. 477–540. ISBN 978-0-12-805090-3. [Google Scholar]
- Zhang, J.; Wu, W.; Meng, F.; Ding, H.; Dong, J. Sol–gel-based chemical synthesis of NdFeB hard magnetic nanoparticles. Mod. Phys. Lett. B 2018, 32, 1840070. [Google Scholar] [CrossRef]
- Farag, R.K.; Labena, A.; Fakhry, S.H.; Safwat, G.; Diab, A.; Atta, A.M. Antimicrobial Activity of Hybrids Terpolymers Based on Magnetite Hydrogel Nanocomposites. Materials 2019, 12, 3604. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Jang, T.S.; Pan, H.M.; Jung, H.D.; Sia, M.W.; Xie, S.; Hang, Y.; Chong, S.; Wang, D.; Song, J. 3D Freeform Printing of Nanocomposite Hydrogels through in situ Precipitation in Reactive Viscous Fluid. Int. J. Bioprint. 2020, 6, 258. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Margel, S. Design of Magnetic Hydrogels for Hyperthermia and Drug Delivery. Polymers 2021, 13, 4259. [Google Scholar] [CrossRef]
- Frachini, E.; Petri, D. Magneto-responsive hydrogels: Preparation, characterization, biotechnological and environmental applications. J. Braz. Chem. Soc. 2019, 30, 2010–2028. [Google Scholar] [CrossRef]
- Moja, T.N.; Mishra, A.K.; Mishra, S.B. Nano Size Magnetite Particles Layered with the Blend of Conductive Polymer and Superadsorbent Hydrogel: A Core–Shell Based Nanocomposite for Trivalent Arsenide Uptake form Aqueous Solution. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2131–2142. [Google Scholar] [CrossRef]
- Shi, L.; Zeng, Y.; Zhao, Y.; Yang, B.; Ossipov, D.; Tai, C.W.; Dai, J.; Xu, C. Biocompatible Injectable Magnetic Hydrogel Formed by Dynamic Coordination Network. ACS Appl. Mater. Interfaces 2019, 11, 46233–46240. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, J. Micro-/nanostructured polymer blends containing block copolymers. In Recent Developments in Polymer Macro, Micro and Nano Blends, 1st ed.; Visakh, P.M., Markovic, G., Pasquini, D., Eds.; Elsevier Ltd.: Cham, Switzerland, 2017; pp. 131–161. ISBN 978-0-08-100408-1. [Google Scholar]
- Gang, F.; Jiang, L.; Xiao, Y.; Zhang, J.; Sun, X. Multi-functional magnetic hydrogel: Design strategies and applications. Nano Sel. 2021, 2, 2291–2307. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Xue, Y.; Wang, J.; Li, X.; Wu, X.; Qin, Y.; Chen, W. Sequential in-situ route to synthesize novel composite hydrogels with excellent mechanical, conductive, and magnetic responsive properties. Mater. Des. 2020, 193, 108759. [Google Scholar] [CrossRef]
- Freire, T.M.; Dutra, L.; Queiroz, D.; Ricardo, N.; Barreto, K.; Denardin, J.; Wurm, F.; Sousa, C.P.; Correia, A.N.; Lima-Neto, P.; et al. Fast ultrasound assisted synthesis of chitosan-based magnetite nanocomposites as a modified electrode sensor. Carbohydr. Polym. 2016, 151, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Khairuddin, N.; Siddique, M. Fabrication of acrylic hydrogel incorporated with magnetic nanoparticles and its physical and thermal properties. In Proceedings of the 13th International Engineering Research Conference (13th Eureca 2019), Selangor Darul Ehsan, Malaysia, 27 November 2019. [Google Scholar] [CrossRef]
- Demirci, U.; Khademhosseini, A. Gels Handbook: Fundamentals, Properties, and Applications; World Scientific: Singapore, 2016; pp. 149–187. ISBN 9814656119. [Google Scholar]
- Jiang, Z.; Dou, G. Preparation and Characterization of Chitosan Grafting Hydrogel for Mine-Fire Fighting. ACS Omega 2020, 5, 2303–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Liu, J.; Cui, X.; Wang, X.; Zhang, L.; Tang, P. Recent Advances on Magnetic Sensitive Hydrogels in Tissue Engineering. Front. Chem. 2020, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Tanasa, E.; Zaharia, C.; Radu, I.C.; Surdu, V.A.; Vasile, B.S.; Damian, C.M.; Andronescu, E. Novel nanocomposites based on functionalized magnetic nanoparticles and polyacrylamide: Preparation and complex characterization. Nanomaterials 2019, 9, 1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Nian, G.; Liang, X.; Wu, L.; Yin, T.; Lu, H.; Qu, S.; Yang, W. Adhesive Tough Magnetic Hydrogels with High Fe3O4 Content. ACS Appl. Mater. Interfaces 2019, 11, 10292–10300. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Wani, A.M.; Shah, M.A.; Devi, H.S.; Bhat, M.Y.; Koka, J.A. Preparation, characterization and antifungal activity of iron oxide nanoparticles. Microb. Pathog. 2018, 115, 287–292. [Google Scholar] [CrossRef]
- Mast, J.; Verleysen, E.; Hodoroaba, V.; Kaegi, R. Characterization of nanomaterials by transmission electron microscopy: Measurement procedures. In Characterization of Nanoparticles, 1st ed.; Hodoroaba, V.D., Unger, W.E.S., Shard, A.G., Eds.; Elsevier Inc.: Cham, Switzerland, 2019; pp. 29–48. ISBN 978-0-12-814182-3. [Google Scholar]
- Miyazaki, T.; Iwanaga, A.; Shirosaki, Y.; Kawashita, M. In Situ Synthesis of Magnetic Iron Oxide Nanoparticles in Chitosan Hydrogels as a reaction field: Effect of cross-linking density. Colloids Surf. B Biointerfaces 2019, 179, 334–339. [Google Scholar] [CrossRef]
- Singh, N.; Riyajuddin, S.; Ghosh, K.; Mehta, S.; Dan, A. Chitosan-Graphene Oxide Hydrogels with Embedded Magnetic Iron Oxide Nanoparticles for Dye Removal. ACS Appl. Nano Mater. 2019, 2, 7379–7392. [Google Scholar] [CrossRef]
- Abbasi, B.; Iqbal, J.; Mahmood, T.; Qyyum, A.; Kanwal, S. Biofabrication of iron oxide nanoparticles by leaf extract of Rhamnus virgata: Characterization and evaluation of cytotoxic, antimicrobial and antioxidant potentials. Appl. Organomet. Chem. 2019, 33, e4947. [Google Scholar] [CrossRef]
- Lakshminarayanan, S.; Shereen, M.; Niraimathi, K.; Brindha, P.; Arumugam, A. One-pot green synthesis of iron oxide nanoparticles from Bauhinia tomentosa: Characterization and application towards synthesis of 1, 3 diolein. Sci. Rep. 2021, 11, 8643. [Google Scholar] [CrossRef]
- Madubuonu, N.; Aisida, S.; Ahmad, I.; Botha, S.; Zhao, T.; Maaza, M.; Ezema, F. Bio-inspired iron oxide nanoparticles using Psidium guajava aqueous extract for antibacterial activity. Appl. Phys. A 2020, 126, 72. [Google Scholar] [CrossRef]
- Mirza, A.; Kareem, A.; Nami, S.; Khan, M.; Rehman, S.; Bhat, S.; Mohammad, A.; Nishat, N. Biogenic synthesis of iron oxide nanoparticles using Agrewia optiva and Prunus persica phyto species: Characterization, antibacterial and antioxidant activity. J. Photochem. Photobiol. B Biol. 2018, 185, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Aksu Demirezen, D.; Yıldız, Y.; Yılmaz, Ş.; Demirezen-Yılmaz, D. Green synthesis and characterization of iron oxide nanoparticles using Ficus carica (common fig) dried fruit extract. J. Biosci. Bioeng. 2019, 127, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Calandra, P.; Kiselev, M.A. Structural Characterization of Biomaterials by Means of Small Angle X-rays and Neutron Scattering (SAXS and SANS), and Light Scattering Experiments. Molecules 2020, 25, 5624. [Google Scholar] [CrossRef] [PubMed]
- Woodard, L.; Dennis, C.; Borchers, J.; Attaluri, A.; Velarde, E.; Dawidczyk, C.; Searson, P.; Pomper, M.; Ivkov, R. Nanoparticle architecture preserves magnetic properties during coating to enable robust multi-modal functionality. Sci. Rep. 2018, 8, 12706. [Google Scholar] [CrossRef] [Green Version]
- Bersweiler, M.; Bender, P.; Vivas, L.; Albino, M.; Petrecca, M.; Mühlbauer, S.; Erokhin, S.; Berkov, D.; Sangregorio, C.; Michels, A. Size-dependent spatial magnetization profile of manganese-zinc ferrite Mn0.2Zn0.2Fe2.6O4 nanoparticles. Phys. Rev. B 2019, 100, 144434. [Google Scholar] [CrossRef] [Green Version]
- Bender, P.; Wetterskog, E.; Honecker, D.; Fock, J.; Frandsen, C.; Moerland, C.; Bogart, L.L.; Posth, O.; Szczerba, W.; Gavilán, H.; et al. Dipolar-coupled moment correlations in clusters of magnetic nanoparticles. Phys. Rev. B 2018, 98, 144434. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Aziz, W.; Bumajdad, A.; Al Sagheer, F.; Madkour, M. Selective synthesis and characterization of iron oxide nanoparticles via PVA/PVP polymer blend as structure-directing agent. Mater. Chem. Phys. 2020, 249, 122927. [Google Scholar] [CrossRef]
- Konwar, A.; Chowdhury, D.; Dan, A. Chitosan based in situ and ex situ magnetic iron oxide nanoparticles for rapid endotoxin removal from protein solutions. Mater. Chem. Front. 2019, 3, 716–725. [Google Scholar] [CrossRef]
- Akhtar, K.; Ali, S.; Bahadar, S.; Asiri, A.M. Scanning Electron Microscopy: Principle and Applications in Nanomaterials Characterization. In Handbook of Materials Characterization, 1st ed.; Kumar, S., Ed.; Springer: Cham, Switzerland, 2018; pp. 113–145. ISBN 978-3-319-92954-5. [Google Scholar]
- Shekhar, S.; Mukherjee, M.; Sen, A. Effect of Fe2O3 on the swelling, mechanical and thermal behaviour of NIPAM-based terpolymer. Polym. Bull. 2020, 78, 5029–5054. [Google Scholar] [CrossRef]
- Muri, H.I.; Hoang, L.; Hjelme, D.R. Mapping Nanoparticles in Hydrogels: A Comparison of Preparation Methods for Electron Microscopy. Appl. Sci. 2018, 8, 2446. [Google Scholar] [CrossRef] [Green Version]
- Saqib, S.; Munis, M.; Zaman, W.; Ullah, F.; Shah, S.; Ayaz, A.; Farooq, M.; Bahadur, S. Synthesis, characterization and use of iron oxide nano particles for antibacterial activity. Microsc. Res. Tech. 2018, 82, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Kisley, L. Fluorescence microscopy of biophysical protein dynamics in nanoporous hydrogels. J. Appl. Phys. 2019, 126, 081101. [Google Scholar] [CrossRef] [Green Version]
- Pillarisetti, S.; Uthaman, S.; Huh, K.; Koh, Y.; Lee, S.; Park, I. Multimodal Composite Iron Oxide Nanoparticles for Biomedical Applications. Tissue Eng. Regen. Med. 2019, 16, 451–465. [Google Scholar] [CrossRef]
- Peppas, N.; Hoffman, A. Hydrogels. In Biomaterials Science, 4th ed.; Wagner, W., Sakiyama-Elbert, S., Zhang, G., Yaszemski, M., Eds.; Elsevier Inc.: Cham, Switzerland, 2020; pp. 153–166. ISBN 978-0-12-816137-1. [Google Scholar]
- Che Nan, N.; Zainuddin, N.; Ahmad, M. Preparation and swelling study of CMC hydrogel as potential superabsorbent. Pertanika J. Sci. Technol. 2019, 27, 489–498. [Google Scholar]
- Hoshino, K.; Nakajima, T.; Matsuda, T.; Sakai, T.; Gong, J. Network elasticity of a model hydrogel as a function of swelling ratio: From shrinking to extreme swelling states. Soft Matter 2018, 14, 9693–9701. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, S.; Harini, K.; Gajula, G.; Sarmento, B.; Neves-Petersen, M.; Thiagarajan, V. Multifunctional magnetic iron oxide nanoparticles: Diverse synthetic approaches, surface modifications, cytotoxicity towards biomedical and industrial applications. BMC Mater. 2019, 1, 2. [Google Scholar] [CrossRef]
- Amani, A.; Montazer, M.; Mahmoudirad, M. Synthesis of applicable hydrogel corn silk/ZnO nanocomposites on polyester fabric with antimicrobial properties and low cytotoxicity. Int. J. Biol. Macromol. 2019, 123, 1079–1090. [Google Scholar] [CrossRef]
- Al-Shabib, N.; Husain, F.; Ahmed, F.; Khan, R.; Khan, M.; Ansari, F.; Alam, M.; Ahmed, M.; Khan, M.; Baig, M.; et al. Low Temperature Synthesis of Superparamagnetic Iron Oxide (Fe3O4) Nanoparticles and Their ROS Mediated Inhibition of Biofilm Formed by Food-Associated Bacteria. Front. Microbiol. 2018, 9, 2567. [Google Scholar] [CrossRef] [Green Version]
- Dulińska-Litewka, J.; Łazarczyk, A.; Hałubiec, P.; Szafrański, O.; Karnas, K.; Karewicz, A. Superparamagnetic Iron Oxide Nanoparticles—Current and Prospective Medical Applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef] [Green Version]
- Sangaiya, P.; Jayaprakash, R. A Review on Iron Oxide Nanoparticles and Their Biomedical Applications. J. Supercond. Nov. Magn. 2018, 31, 3397–3413. [Google Scholar] [CrossRef]
- Vakili-Ghartavol, R.; Momtazi-Borojeni, A.A.; Vakili-Ghartavol, Z.; Aiyelabegan, H.T.; Jaafari, M.R.; Rezayat, S.M.; Arbadi-Bidgoli, S. Toxicity assessment of superparamagnetic iron oxide nanoparticles in different tissues. Artif. Cells Nanomed. Biotechnol. 2020, 48, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Chee, H.L.; Gan, C.R.R.; Ng, M.; Low, L.; Fernig, D.G.; Bhakoo, K.K.; Paramelle, D. Biocompatible Peptide-Coated Ultrasmall Superparamagnetic Iron Oxide Nanoparticles for In Vivo Contrast-Enhanced Magnetic Resonance Imaging. ACS Nano 2018, 12, 6480–6491. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.K.; Asad, M.; Henrich-Noack, P.; Sokolov, M.; Hintz, W.; Grigartzik, L.; Zhang, E.; Dityatev, A.; Van Wachem, B.; Sabel, B.A. Evaluation of Toxicity and Neural Uptake In Vitro and In Vivo of Superparamagnetic Iron Oxide Nanoparticles. Int. J. Mol. Sci. 2018, 19, 2613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Britos, T.N.; Castro, C.E.; Bertassoli, B.M.; Petri, G.; Fonseca, F.L.A.; Ferreira, F.F.; Haddad, P.S. In vivo evaluation of thiol-functionalized superparamagnetic iron oxide nanoparticles. Mater. Sci. Eng. C 2019, 99, 171–179. [Google Scholar] [CrossRef]
- Hedayatnasab, Z.; Dabbagh, A.; Abnisa, F.; Wan-Daud, W.M. Polycaprolactone-coated superparamagnetic iron oxide nanoparticles for in vitro magnetic hyperthermia therapy of cancer. Eur. Polym. J. 2020, 133, 109789. [Google Scholar] [CrossRef]
- Estelrich, J.; Busquets, M.A. Iron Oxide Nanoparticles in Photothermal Therapy. Molecules 2018, 23, 1567. [Google Scholar] [CrossRef] [Green Version]
- Alphandéry, E. Iron oxide nanoparticles for therapeutic applications. Drug Discov. Today 2020, 25, 141–149. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von-Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Soetaert, F.; Korangath, P.; Serantes, D.; Fiering, S.; Ivkov, R. Cancer therapy with iron oxide nanoparticles: Agents of thermal and immune therapies. Adv. Drug Deliv. Rev. 2020, 163–164, 65–83. [Google Scholar] [CrossRef]
- Szczęch, M.; Szczepanowicz, K. Polymeric Core-Shell Nanoparticles Prepared by Spontaneous Emulsification Solvent Evaporation and Functionalized by the Layer-by-Layer Method. Nanomaterials 2020, 10, 496. [Google Scholar] [CrossRef] [Green Version]
- Vangijzegem, T.; Stanicki, D.; Laurent, S. Magnetic iron oxide nanoparticles for drug delivery: Applications and characteristics. Expert Opin. Drug Deliv. 2019, 16, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Panda, J.; Satapathy, B.S.; Majumder, S.; Sarkar, R.; Mukherjee, B.; Tudu, B. Engineered polymeric iron oxide nanoparticles as potential drug carrier for targeted delivery of docetaxel to breast cancer cells. J. Magn. Magn. Mater. 2019, 485, 165–173. [Google Scholar] [CrossRef]
- Chandra-Mohanta, S.; Saha, A.; Sujatha-Devi, P. PEGylated Iron Oxide Nanoparticles for pH Responsive Drug Delivery Application. Mater. Today Proc. 2018, 5, 9715–9725. [Google Scholar] [CrossRef]
- El-Boubbou, K. Magnetic iron oxide nanoparticles as drug carriers: Preparation, conjugation and delivery. Nanomedicine 2018, 13, 929–952. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.; Halbert, M.V.; Stephen, Z.R.; Zhang, M. Iron Oxide Nanoparticles as T 1 Contrast Agents for Magnetic Resonance Imaging: Fundamentals, Challenges, Applications, and Prospectives. Adv. Mater. 2021, 33, 1906539. [Google Scholar] [CrossRef]
- Stephen, Z.R.; Kievit, F.M.; Zhang, M. Magnetite nanoparticles for medical MR imaging. Mater. Today 2011, 14, 330–338. [Google Scholar] [CrossRef]
- Bao, Y.; Sherwood, J.A.; Sun, Z. Magnetic iron oxide nanoparticles as T1 contrast agents for magnetic resonance imaging. J. Mater. Chem. C 2018, 6, 1280–1290. [Google Scholar] [CrossRef]
- Khmara, I.; Strbak, O.; Zavisova, V.; Koneracka, M.; Kubovcikova, M.; Antal, I.; Kavecansky, V.; Lucanska, D.; Dobrota, D.; Kopcansky, P. Chitosan-stabilized iron oxide nanoparticles for magnetic resonance imaging. J. Magn. Magn. Mater. 2019, 474, 319–325. [Google Scholar] [CrossRef]
- Öztürk-Er, E.; Dalgıç-Bozyiğit, G.; Büyükpınar, Ç.; Bakırdere, S. Magnetic Nanoparticles Based Solid Phase Extraction Methods for the Determination of Trace Elements. Crit. Rev. Anal. Chem. 2020, 50, 1–19. [Google Scholar] [CrossRef]
- Baile, P.; Vidal, L.; Canals, A. A modified zeolite/iron oxide composite as a sorbent for magnetic dispersive solid-phase extraction for the preconcentration of nonsteroidal anti-inflammatory drugs in water and urine samples. J. Chromatogr. A 2019, 1603, 33–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köhler, T.; Feoktystov, A.; Petracic, O.; Kentzinger, E.; Bhatnagar-Schöffmann, T.; Feygenson, M.; Nandakumaran, N.; Landers, J.; Wende, H.; Cervellino, A. Mechanism of magnetization reduction in iron oxide nanoparticles. Nanoscale 2021, 13, 6965–6976. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, J.; Salah-Eddine, L.; Abderrhmane, B.; Alonso-González, M.; Guerrero, A.; Romero, A. Green synthesis and characterization of iron oxide nanoparticles by pheonix dactylifera leaf extract and evaluation of their antioxidant activity. Sustain. Chem. Pharm. 2020, 17, 100280. [Google Scholar] [CrossRef]
- Magro, M.; Baratella, D.; Bonaiuto, E.; de A Roger, J.; Vianello, F. New Perspectives on Biomedical Applications of Iron Oxide Nanoparticles. Curr. Med. Chem. 2018, 25, 540–555. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Hidalgo-Bonilla, S.; Bucio, E. Basics and green solvent parameter for environmental remediation. In Green Sustainable Process for Chemical and Environmental Engineering and Science, 1st ed.; Inamuddin, D., Boddula, R., Asiri, A., Eds.; Elsevier Inc.: Cham, Switzerland, 2021; pp. 219–237. ISBN 978-0-12-821884-6. [Google Scholar]
- Salehipour, M.; Rezaei, S.; Mosafer, J.; Pakdin-Parizi, Z.; Motaharian, A.; Mogharabi-Manzari, M. Recent advances in polymer-coated iron oxide nanoparticles as magnetic resonance imaging contrast agents. J. Nanopart. Res. 2021, 23, 48. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustamante-Torres, M.; Romero-Fierro, D.; Estrella-Nuñez, J.; Arcentales-Vera, B.; Chichande-Proaño, E.; Bucio, E. Polymeric Composite of Magnetite Iron Oxide Nanoparticles and Their Application in Biomedicine: A Review. Polymers 2022, 14, 752. https://doi.org/10.3390/polym14040752
Bustamante-Torres M, Romero-Fierro D, Estrella-Nuñez J, Arcentales-Vera B, Chichande-Proaño E, Bucio E. Polymeric Composite of Magnetite Iron Oxide Nanoparticles and Their Application in Biomedicine: A Review. Polymers. 2022; 14(4):752. https://doi.org/10.3390/polym14040752
Chicago/Turabian StyleBustamante-Torres, Moises, David Romero-Fierro, Jocelyne Estrella-Nuñez, Belén Arcentales-Vera, Estefani Chichande-Proaño, and Emilio Bucio. 2022. "Polymeric Composite of Magnetite Iron Oxide Nanoparticles and Their Application in Biomedicine: A Review" Polymers 14, no. 4: 752. https://doi.org/10.3390/polym14040752
APA StyleBustamante-Torres, M., Romero-Fierro, D., Estrella-Nuñez, J., Arcentales-Vera, B., Chichande-Proaño, E., & Bucio, E. (2022). Polymeric Composite of Magnetite Iron Oxide Nanoparticles and Their Application in Biomedicine: A Review. Polymers, 14(4), 752. https://doi.org/10.3390/polym14040752







