Characterization of Fish Gelatin Obtained from Atlantic Cod Skin Using Enzymatic Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gelatin Extraction
2.3. Chemical Composition of Gelatin
2.4. Amino Acid Composition
2.5. Molecular Weight
2.6. Secondary Structure of Gelatin
2.7. Emulsification Capability
2.8. Rheological Tests
- Periodic oscillations with a constant frequency of ω = 6.28 rad/s, and amplitude sweep changes in the range from 0.01% to 1000%.
- Periodic oscillations with a constant amplitude of γ = 1% (corresponding to the domain of linear viscoelasticity), and a frequency sweep in the range from 0.01 to 500 s−1.
- Temperature scanning with a step size of 1 °C/min at a constant frequency of 6.28 rad/s (1 Hz) and constant amplitude of deformation of γ = 1%.
- Time dependence of the elastic modulus at 6 °C, at a constant frequency of ω = 6.28 rad/s and constant amplitude of deformation of γ = 1% (for following the kinetics of gelation); the initial temperature was 25 °C.
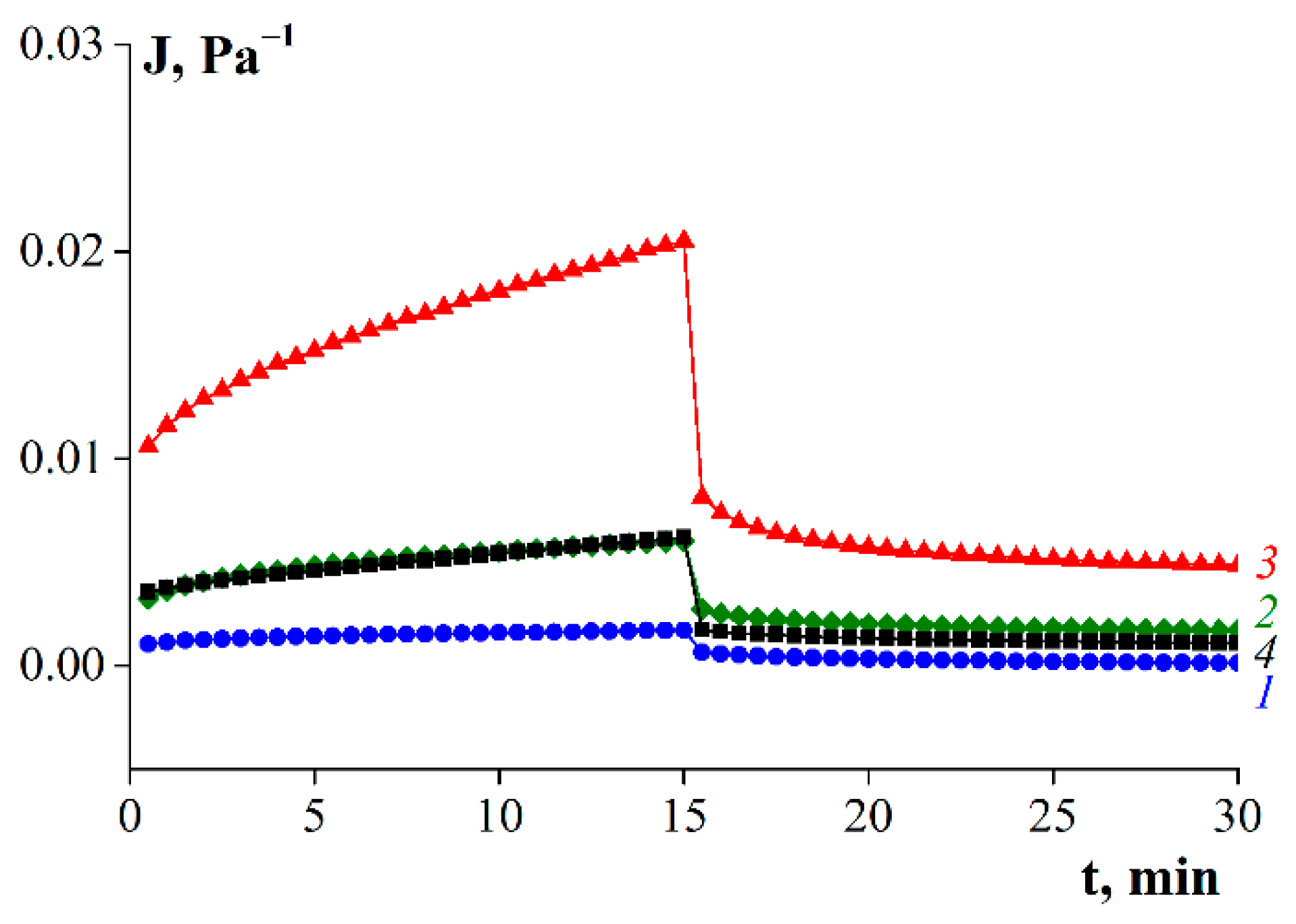
- Isothermal creep and elastic recovery at 6 °C and constant stress in the range of 5–300 Pa for loading for 15 min and recovery for 15 min.
3. Results
3.1. Chemical Composition
3.2. Amino Acid Composition
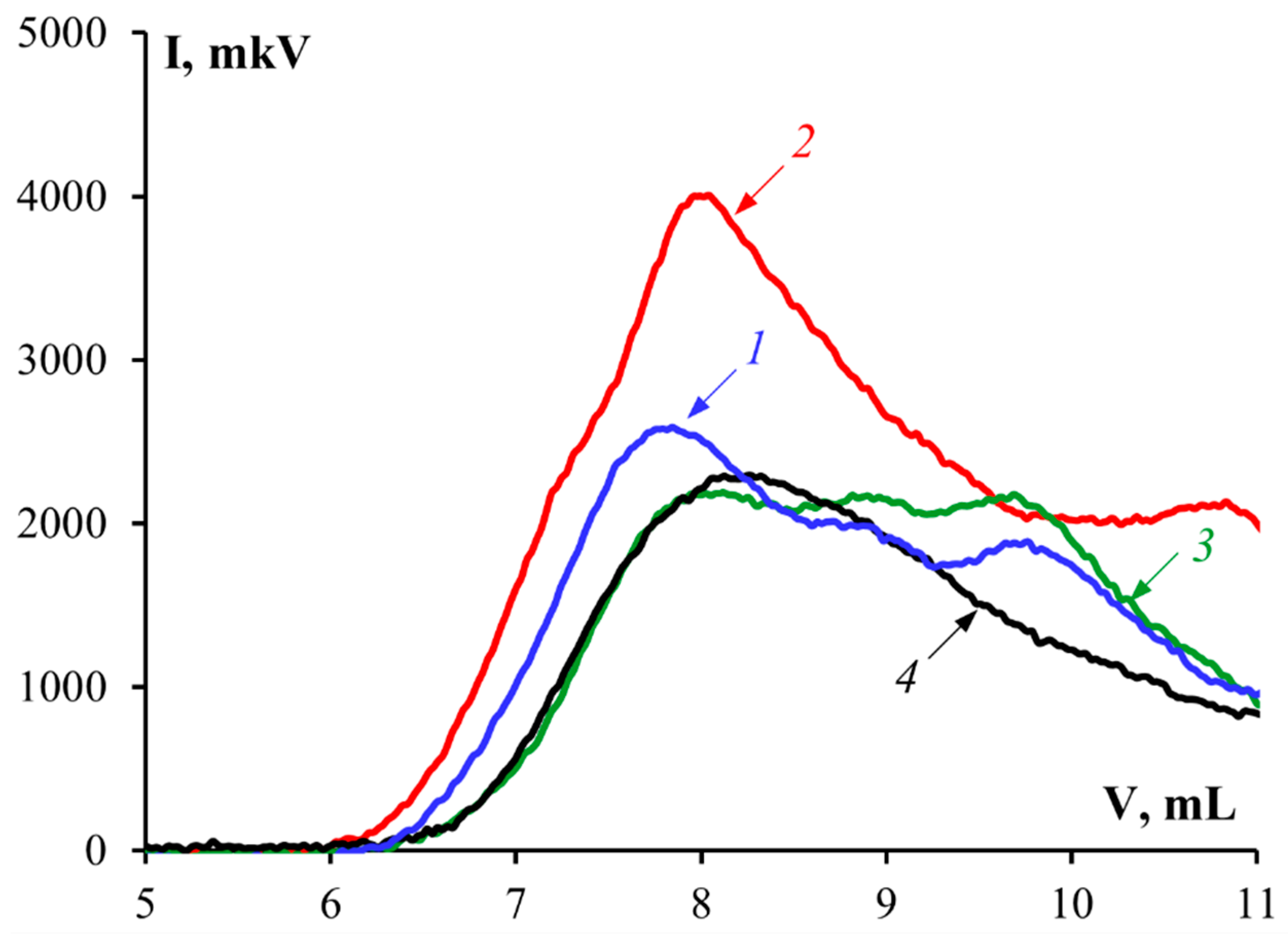
3.3. Molecular Weight
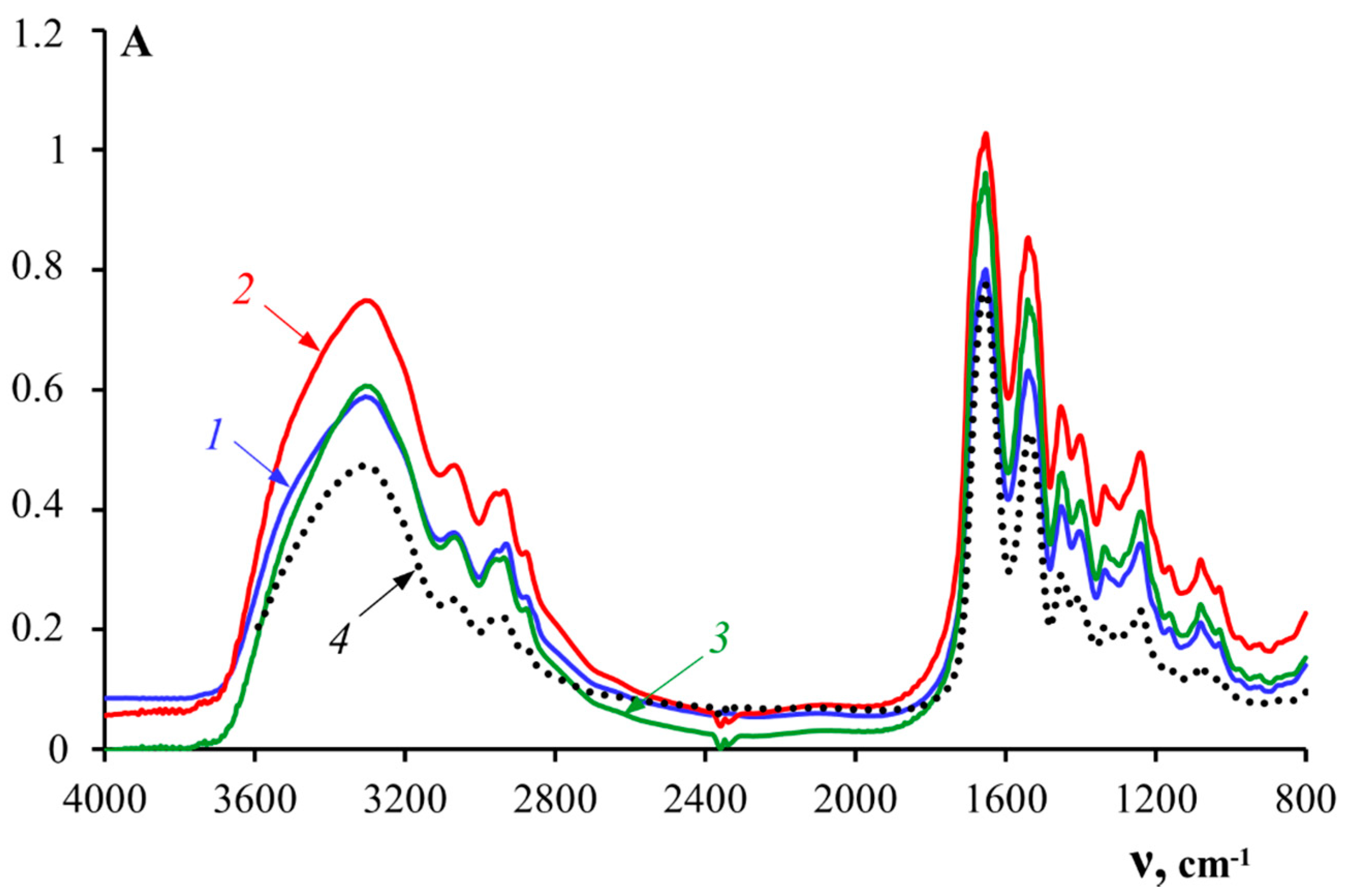
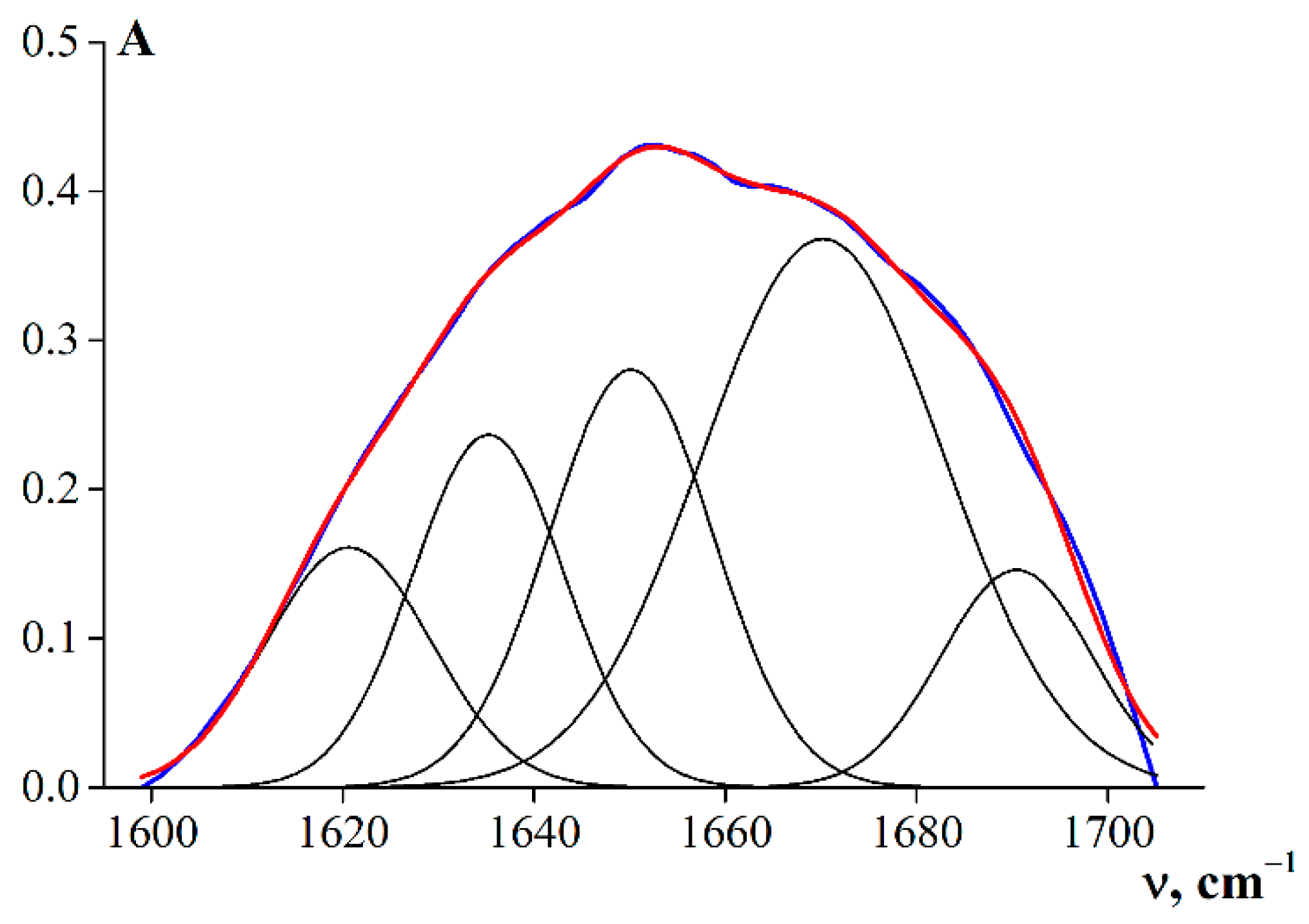
3.4. Secondary Structures of the Gelatin Macromolecules
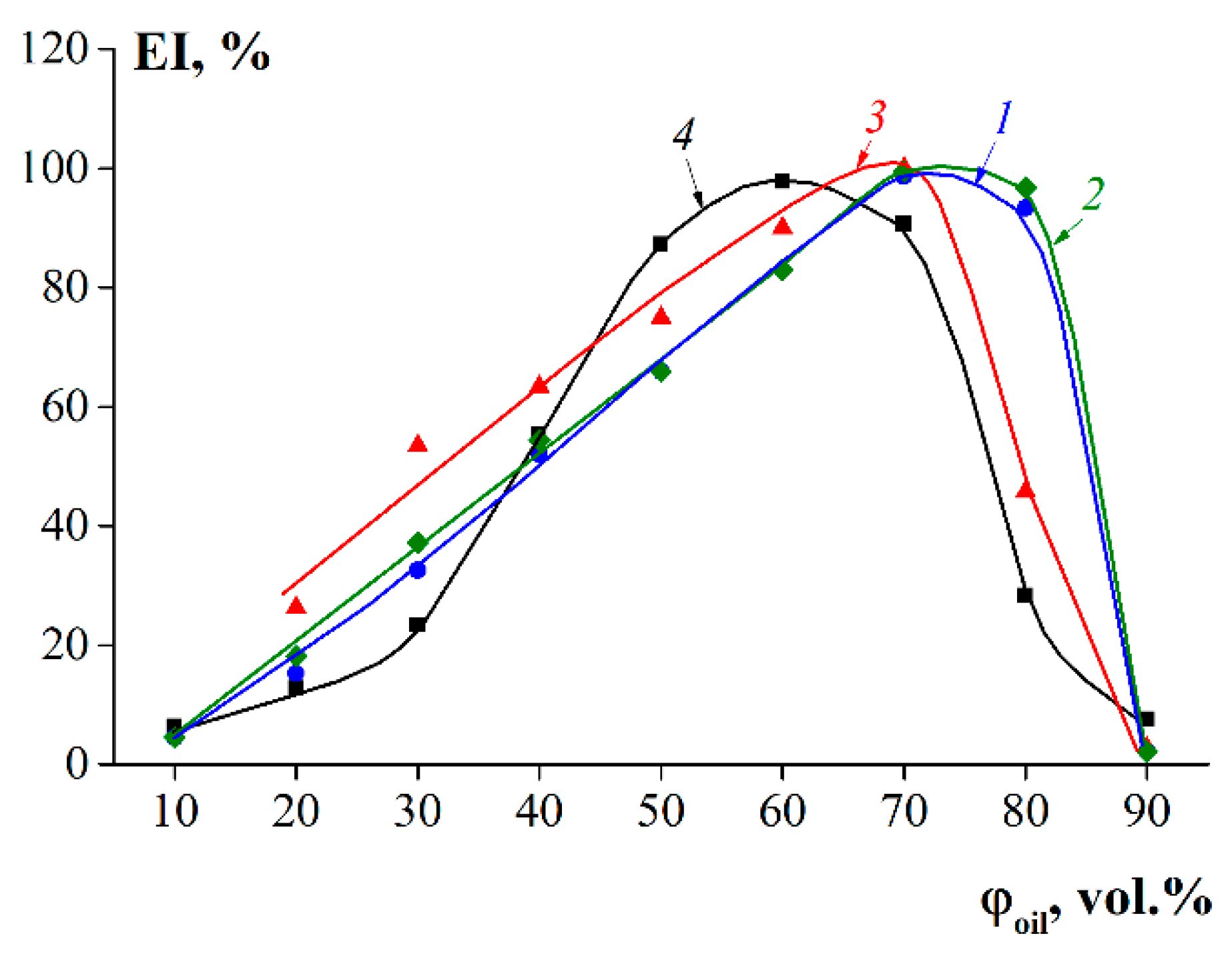
3.5. Emulsification Capability
3.6. Thermal Properties of Gelatin
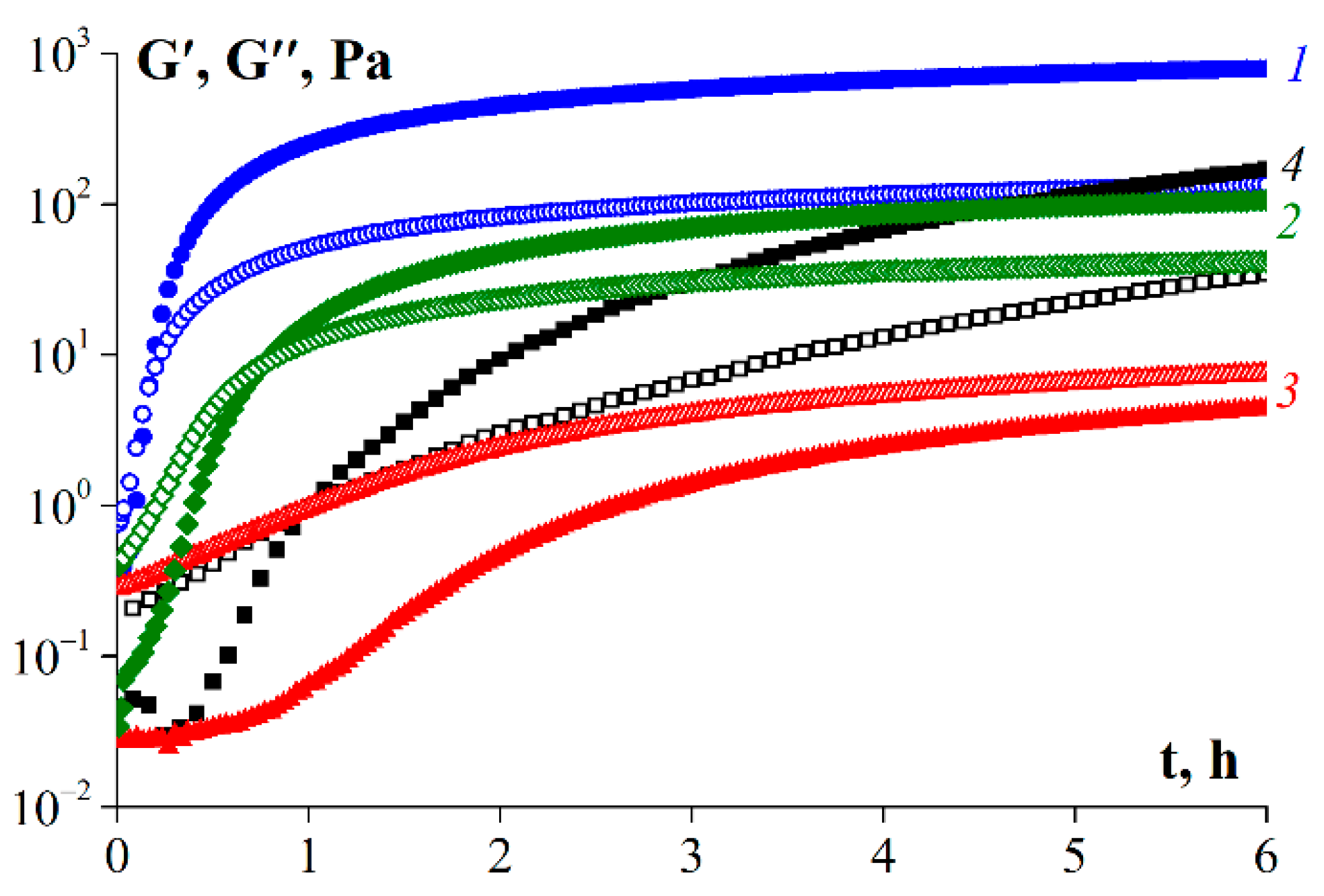
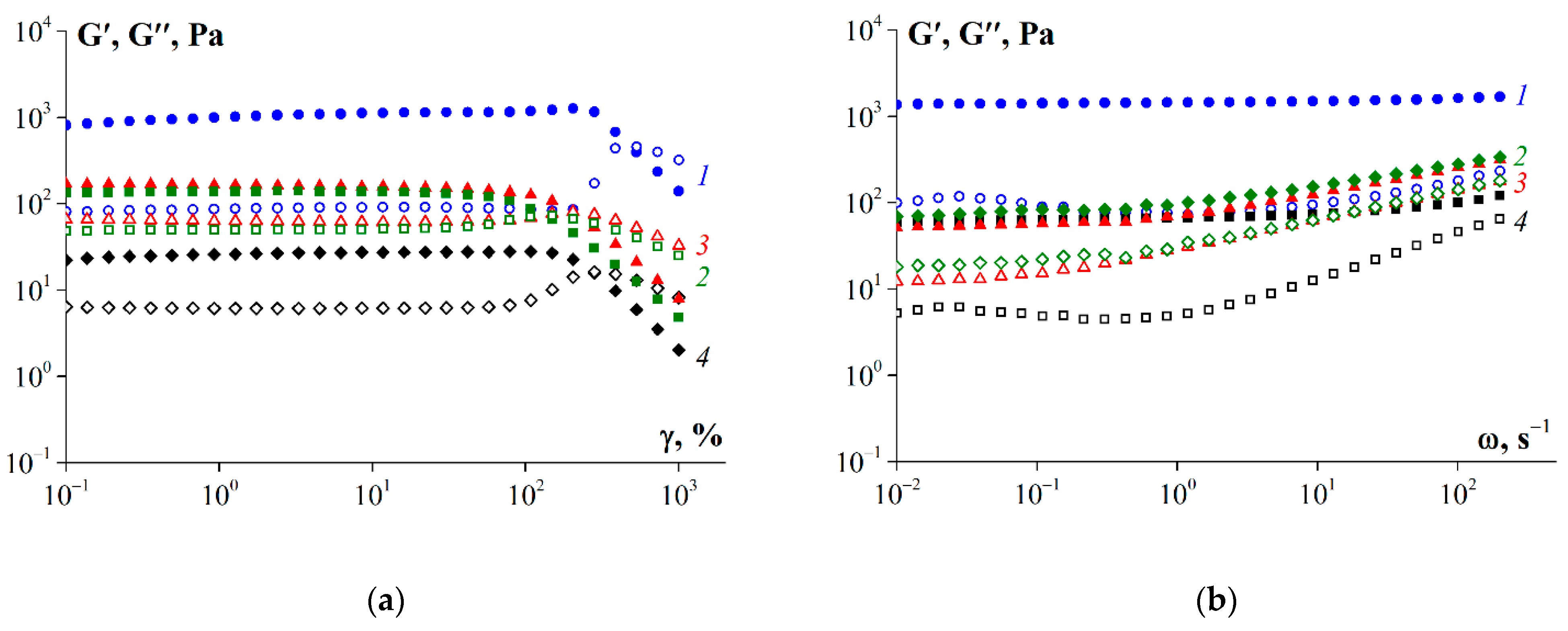
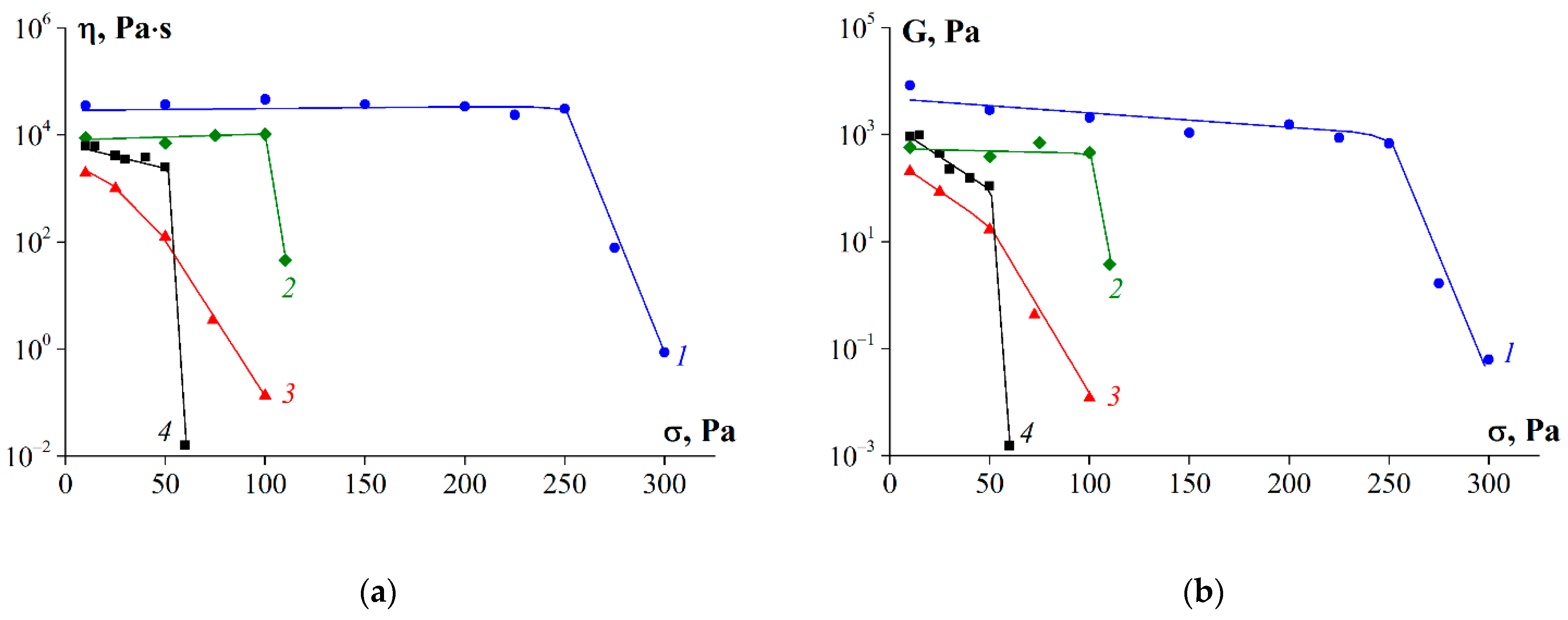
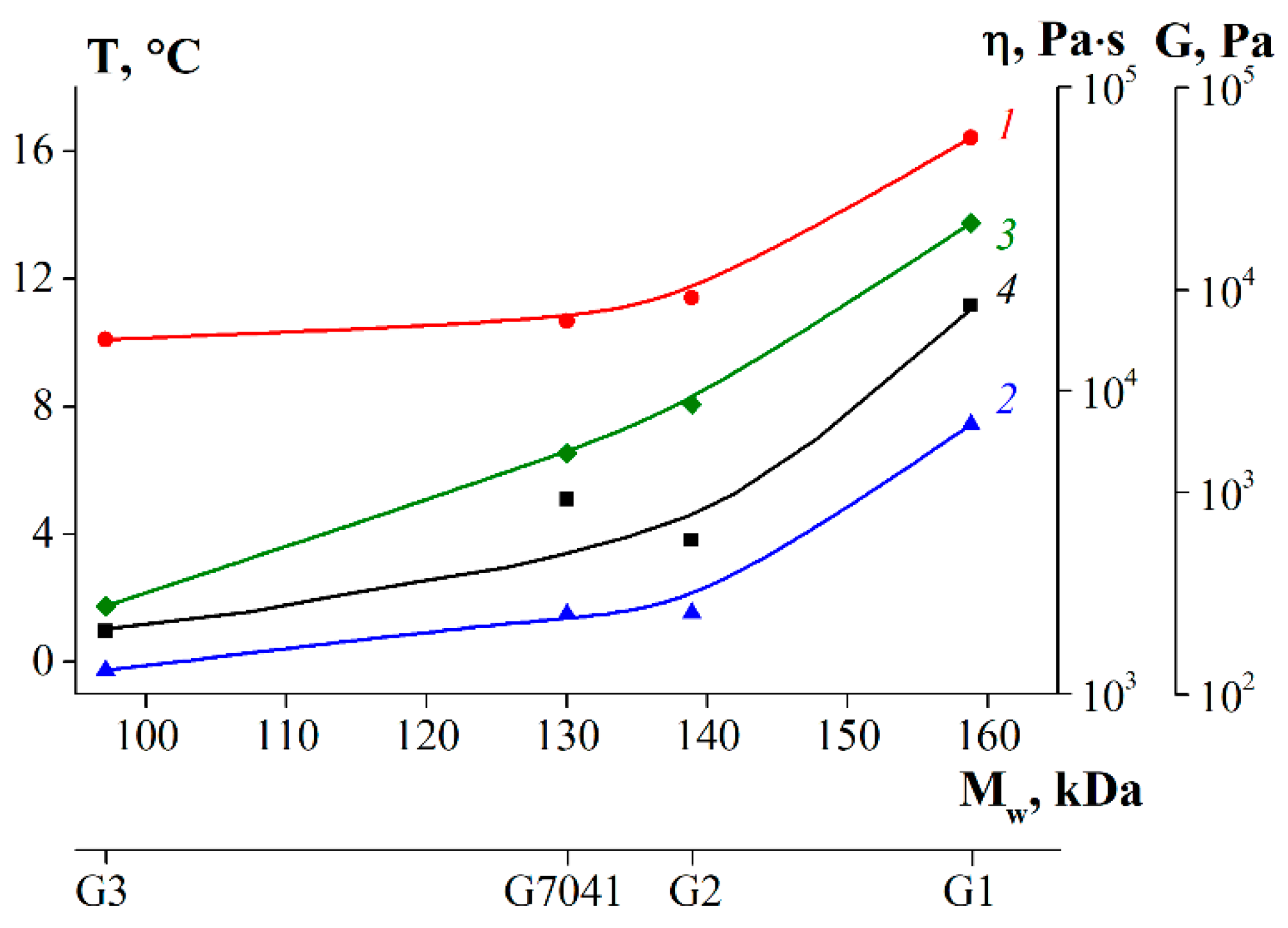
3.7. Rheological Properties of Gelatin Gels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Djagny, K.B.; Wang, Z.; Xu, S. Gelatin: A valuable protein for food and pharmaceutical industries. Crit. Rev. Food Sci. Nutr. 2001, 41, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K.; Muhammad, L.; Arin, F.; Kamilah, H.; Razak, A.; Sulaiman, S. Review of Fish Gelatin Extraction, Properties and Packaging Applications. Food Sci. Qual. Manag. 2016, 56, 47–59. [Google Scholar]
- Zhou, P.; Mulvaney, S.J.; Regenstein, J.M. Properties of Alaska pollock skin gelatin: A comparison with tilapia and pork skin gelatins. J. Food Sci. 2006, 71, 313–321. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S. Modified Fish Gelatin as an Alternative to Mammalian Gelatin in Modern Food Technologies. Polymers 2020, 12, 3051. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Pérez-Mateos, M.; Gómez-Estaca, J.; López-Caballero, E.; Giménez, B.; Montero, P. Fish gelatin: A renewable material for developing active biodegradable films. Trends Food Sci. Technol. 2009, 20, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Siburian, W.Z.; Rochima, E.; Andriani, Y.; Praseptiangga, D. Fish gelatin (definition, manufacture, analysis of quality characteristics, and application): A review. Int. J. Fish. Aquat. 2020, 8, 90–95. [Google Scholar]
- Lionetto, F.; Corcione, C.E. Recent applications of biopolymers derived from fish industry waste in food packaging. Polymers 2021, 13, 2337. [Google Scholar] [CrossRef]
- Derkach, S.R.; Kuchina, Y.A.; Baryshnikov, A.V.; Kolotova, D.S.; Voron’ko, N.G. Tailoring cod gelatin structure and physical properties with acid and alkaline extraction. Polymers 2019, 11, 1724. [Google Scholar] [CrossRef] [Green Version]
- Karim, A.A.; Bhat, R. Gelatin alternatives for the food industry: Recent developments, challenges and prospects. Trends Food Sci. Technol. 2008, 19, 644–656. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/home/en/ (accessed on 10 November 2021).
- Wasswa, J.B.; Tang, J.; Gu, X. Utilization of Fish processing By-Products in the Gelatin Industry. Food Rev. Int. 2007, 23, 159–174. [Google Scholar] [CrossRef]
- Asghar, A.; Henrickson, R.L. Chemical, biochemical, functional, and nutritional characteristics of collagen in food systems. Adv. Food Res. 1982, 28, 231–372. [Google Scholar] [PubMed]
- Bateman, J.F.; Lamande, S.R.; Ramshaw, J.A. Collagen superfamily. In Extracellular Matrix; Comper, W.D., Ed.; Harwood Academic Publishers GmbH: Amsterdam, The Netherlands, 1996; Volume 2, pp. 22–67. [Google Scholar]
- Veis, A. The Macromolecular Chemistry of Gelatin; Academic Press: New York, NY, USA, 1964. [Google Scholar]
- Djabourov, M.; Lechaire, J.P.; Gaill, F. Structure and rheology of gelatin and collagen gels. Biorheology 1993, 30, 191–205. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Guillén, M.C.; Turnay, J.; Fer-nández-Dıaz, M.D.; Ulmo, N.; Lizarbe, M.A.; Montero, P. Structural and physical properties of gelatin extracted from different marine species: A comparative study. Food Hydrocoll. 2002, 16, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Johnston-Banks, F.A. Gelatine. In Food Gels; Springer: Dordrecht, The Netherlands, 1990; pp. 233–289. [Google Scholar]
- Franca-Oliveira, G.; Fornari, T.; Hernández-Ledesma, B. A Review on the Extraction and Processing of Natural Source-Derived Proteins through Eco-Innovative Approaches. Processes 2021, 9, 1626. [Google Scholar] [CrossRef]
- Ideia, P.; Pinto, J.; Ferreira, R.; Figueiredo, L.; Spínola, V.; Castilho, P.C. Fish processing industry residues: A review of valuable products extraction and characterization methods. Waste Biomass Valorization 2020, 11, 3223–3246. [Google Scholar] [CrossRef]
- Marti-Quijal, F.J.; Remize, F.; Meca, G.; Ferrer, E.; Ruiz, M.J.; Barba, F.J. Fermentation in fish and by-products processing: An overview of current research and future prospects. Curr. Opin. Food Sci. 2020, 31, 9–16. [Google Scholar] [CrossRef]
- Hosseini-Parvar, S.H.; Keramat, J.; Kadivar, M.; Khanipour, E.; Motamedzadegan, A. Optimising conditions for enzymatic extraction of edible gelatin from the cattle bones using response surface methodology. Int. J. Food Sci. Technol. 2009, 44, 467–475. [Google Scholar] [CrossRef]
- Sae-leaw, T.; O’Callaghan, Y.C.; Benjakul, S.; O’Brien, N.M. Antioxidant Activities and Selected Characteristics of Gelatin Hydrolysates from Seabass (Lates calcarifer) Skin as Affected by Production Processes. J. Food Sci. Technol. 2016, 53, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Diniz, F.M.; Martin, A.M. Fish Protein Hydrolysates by Enzymatic Processing. Agro Food Ind. Hi Tech 1997, 8, 9–13. [Google Scholar]
- Guerard, F.; Guimas, L.; Binet, A. Production of Tuna Waste Hydrolysates by a Commercial Neutral Protease Preparation. J. Mol. Catal. B Enzym. 2002, 19–20, 489–498. [Google Scholar] [CrossRef]
- Petersen, B.R.; Yates, J.R. Gelatin Extraction. U.S. Patent 4,064,008, 20 December 1977. [Google Scholar]
- Lukin, A. Application and comparison of proteolytic enzyme preparations in technology of protein hydrolyzates. Food Sci. Technol. 2019, 40, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Paez, V.; Barrett, W.B.; Deng, X.; Diaz-Amigo, C.; Fiedler, K.; Fuerer, C.; Coates, S.G. AOAC SMPR® 2016.002. J. AOAC Int. 2016, 99, 1122–1124. [Google Scholar] [CrossRef] [PubMed]
- Laurent, T.C.; Killander, J. A theory of gel filtration and its experimental verification. J. Chromatogr. A 1964, 14, 317–330. [Google Scholar] [CrossRef]
- Prystupa, D.A.; Donald, A.M. Infrared study of gelatin conformations in the gel and sol states. Polym. Gels Netw. 1996, 4, 87–110. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Timgren, A.; Rayner, M.; Sjöö, M.; Dejmek, P. Starch particles for food based Pickering emulsions. Procedia Food Sci. 2011, 1, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Malkin, A.Y.; Isayev, A.I. Rheology: Concepts, Methods, and Applications, 3rd ed.; ChemTec Publishing: Toronto, ON, Canada, 2017. [Google Scholar]
- GOST 11293-2017; Gelatin. Tekhnicheskie Usloviya [State Standard 11293–2017. Gelatin. Technical Specification]. Standartinform Publ.: Moscow, Russia, 2020. 26p. (In Russian)
- Azilawati, M.I.; Hashim, D.M.; Jamilah, B.; Amin, I. RP-HPLC method using 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate incorporated with normalization technique in principal component analysis to differentiate the bovine, porcine and fish gelatins. Food Chem. 2015, 172, 368–376. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2004. [Google Scholar]
- Ali, A.M.M.; Kishimura, H.; Benjakul, S. Physicochemical and molecular properties of gelatin from skin of golden carp (Probarbus jullieni) as influenced by acid pretreatment and prior-ultrasonication. Food Hydrocoll. 2018, 82, 164–172. [Google Scholar] [CrossRef]
- Sila, A.; Martinez-Alvarez, O.; Krichen, F.; Gómez-Guillén, M.C.; Bougatef, A. Gelatin prepared from European eel (Anguilla anguilla) skin: Physicochemical, textural, viscoelastic and surface properties. Colloids Surf. A Physicochem. Eng. 2017, 529, 643–650. [Google Scholar] [CrossRef] [Green Version]
- Dong, A.; Huang, P.; Caughey, W.S. Protein secondary structures in water from second-derivative amide I infrared spectra. Biochemistry 1990, 29, 3303–3308. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Zheng, H.; Su, W. Characterization of edible films based on tilapia (Tilapia zillii) scale gelatin with different extraction pH. Food Hydrocoll. 2014, 41, 19–26. [Google Scholar] [CrossRef]
- Stainsby, G. Gelatin gels. In Advances in Meat Research: Collagen as a Food; Pearson, A.M., Dutson, T.R., Bailey, A.J., Eds.; Van Nostrand Reinhold Company Inc.: New York, NY, USA, 1987. [Google Scholar]
- Hudson, H.M.; Daubert, C.R.; Foegeding, E.A. Rheological and physical properties of derivitized whey protein isolate powders. J. Agric. Food Chem. 2000, 48, 3112–3119. [Google Scholar] [CrossRef] [PubMed]


| Sample | Conditions |
|---|---|
| G1 | Hydromodulus 1:4 pH = 7.0–7.5; T = 40 ± 1 °C; t = 5 h; without enzyme |
| G2 | Hydromodulus 1:4 pH = 7.0–7.5; T = 40 ± 1 °C; t = 3 h; Enzyme—Protosubtilin (CE = 0.025 g/1 kg of raw material) |
| G3 | Hydromodulus 1:4 pH = 7.0–7.5; T = 40 ± 1 °C; t = 3 h; Enzyme—Pancreatin (CE = 0.025 g/1 kg of raw material) |
| Sample | Moisture Content X, % | Total Nitrogen NT, % | Protein * P, % | Ash, % | Yield B, % |
|---|---|---|---|---|---|
| G1 | 4.8 ± 0.5 | 17.0 ± 0.1 | 94.4 ± 0.5 | 0.9 ± 0.1 | 51 ± 1 |
| G2 | 5.6 ± 0.5 | 16.9 ± 0.1 | 93.8 ± 0.5 | 0.5 ± 0.1 | 60 ± 1 |
| G3 | 6.8 ± 0.5 | 16.6 ± 0.1 | 92.0 ± 0.5 | 1.1 ± 0.1 | 58 ± 1 |
| G 7041 | 5.5 ± 0.5 | 17.0 ± 0.1 | 93.5 ± 0.5 | - | - |
| Amino Acid | Abbreviation | Content, g/100 g | ||||
|---|---|---|---|---|---|---|
| G1 | G2 | G3 | Cod Skin | G 7041 | ||
| Glycine | GLY | 19.0 | 18.5 | 18.2 | 18.4 | 18.6 |
| Proline | PRO | 8.0 | 7.9 | 7.6 | 8.3 | 12.9 |
| Alanine | ALA | 10.0 | 9.1 | 9.6 | 9.3 | 9.4 |
| Glutamic acid | GLU | 10.4 | 10.7 | 10.0 | 10.0 | 9.3 |
| Arginine | ARG | 10.9 | 11.0 | 10.3 | 10.0 | 7.6 |
| Aspartic acid | ASP | 6.8 | 7.1 | 6.7 | 7.1 | 5.6 |
| Serine | SER | 6.3 | 6.4 | 6.0 | 6.0 | 6.3 |
| Leucine * | LEU | 3.3 | 3.4 | 3.3 | 3.8 | 2.8 |
| Threonine * | THR | 2.7 | 2.8 | 2.6 | 2.8 | 2.6 |
| Phenylalanine * | PHE | 2.3 | 2.3 | 2.2 | 2.4 | 2.4 |
| Lysine * | LYS | 4.6 | 4.8 | 4.6 | 4.5 | 2.3 |
| Valine * | VAL | 2.2 | 2.4 | 2.3 | 2.7 | 2.1 |
| Methionine * | MET | 0.8 | 0.7 | 0.7 | 1.8 | 1.6 |
| Isoleucine * | ILE | 1.8 | 1.8 | 1.7 | 1.9 | 1.5 |
| Tyrosine * | TYR | 0.9 | 0.8 | 0.9 | 1.2 | 0.8 |
| Histidine * | HIS | 1.5 | 1.2 | 1.6 | 1.6 | 1.7 |
| Total | 91.5 | 90.9 | 88.3 | 91.8 | 87.5 | |
| Sample | Mwf, kDa | ω, % * | Mw, kDa ** |
|---|---|---|---|
| G1 | 72.4 | 35.9 | 158.8 |
| 207.2 | 64.1 | ||
| G2 | 45.2 | 39.0 | 138.9 |
| 198.8 | 61.0 | ||
| G3 | 45.2 | 53.6 | 97.1 |
| 157.1 | 46.4 | ||
| G 7041 | 131.5 |
| Group | Wave Number ν, cm−1 | Type of Vibration |
|---|---|---|
| Amide A | 3400–3300 | N–H stretching vibrations |
| Amide B | 3000–2900 | N–H stretching vibrations |
| Amide I | 1700–1600 | C=O stretching vibrations—80%, and C–N stretching vibrations |
| Amide II | 1575–1480 | N–H deformation vibrations—80%, and C–N stretching vibrations |
| Amide III | 1300–1230 | C–N stretching vibrations |
| Secondary Structure Element | Wave Number ν, cm−1 | G1 | G2 | G3 | G 7041 |
|---|---|---|---|---|---|
| β-turn/β-sheet | 1620–1624 | 8.3 | 12.1 | 10.4 | 13.6 |
| 1635–1637 | 22.4 | 16.8 | 20.0 | 20.7 | |
| Random coil | 1651–1653 | 17.2 | 21.1 | 28.7 | 23.1 |
| Triple helix | 1668–1670 | 42.9 | 40.6 | 22.4 | 24.2 |
| β-turn/β-sheet | 1686–1690 | 9.2 | 9.4 | 18.5 | 18.4 |
| Sample | Tm, °C | Tg, °C |
|---|---|---|
| G1 | 16.4 | 7.4 |
| G2 | 11.4 | 1.5 |
| G3 | 10.1 | −0.3 |
| G 7041 | 10.7 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derkach, S.R.; Kolotova, D.S.; Kuchina, Y.A.; Shumskaya, N.V. Characterization of Fish Gelatin Obtained from Atlantic Cod Skin Using Enzymatic Treatment. Polymers 2022, 14, 751. https://doi.org/10.3390/polym14040751
Derkach SR, Kolotova DS, Kuchina YA, Shumskaya NV. Characterization of Fish Gelatin Obtained from Atlantic Cod Skin Using Enzymatic Treatment. Polymers. 2022; 14(4):751. https://doi.org/10.3390/polym14040751
Chicago/Turabian StyleDerkach, Svetlana R., Daria S. Kolotova, Yuliya A. Kuchina, and Nadezhda V. Shumskaya. 2022. "Characterization of Fish Gelatin Obtained from Atlantic Cod Skin Using Enzymatic Treatment" Polymers 14, no. 4: 751. https://doi.org/10.3390/polym14040751
APA StyleDerkach, S. R., Kolotova, D. S., Kuchina, Y. A., & Shumskaya, N. V. (2022). Characterization of Fish Gelatin Obtained from Atlantic Cod Skin Using Enzymatic Treatment. Polymers, 14(4), 751. https://doi.org/10.3390/polym14040751







