Diffusion- and Chemometric-Based Separation of Complex Electrochemical Signals That Originated from Multiple Redox-Active Molecules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
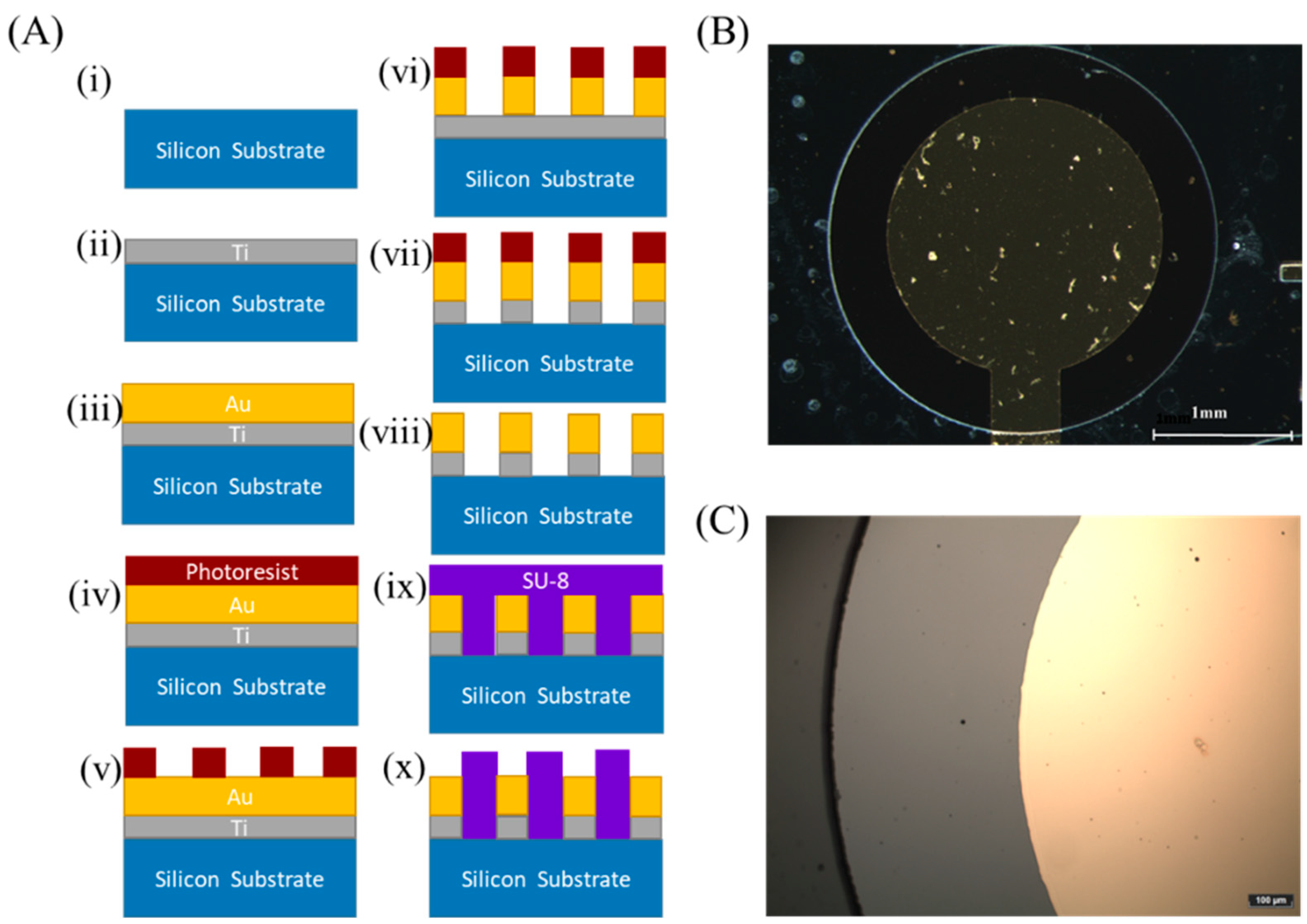
2.2. Fabrication of the Gold Electrodes
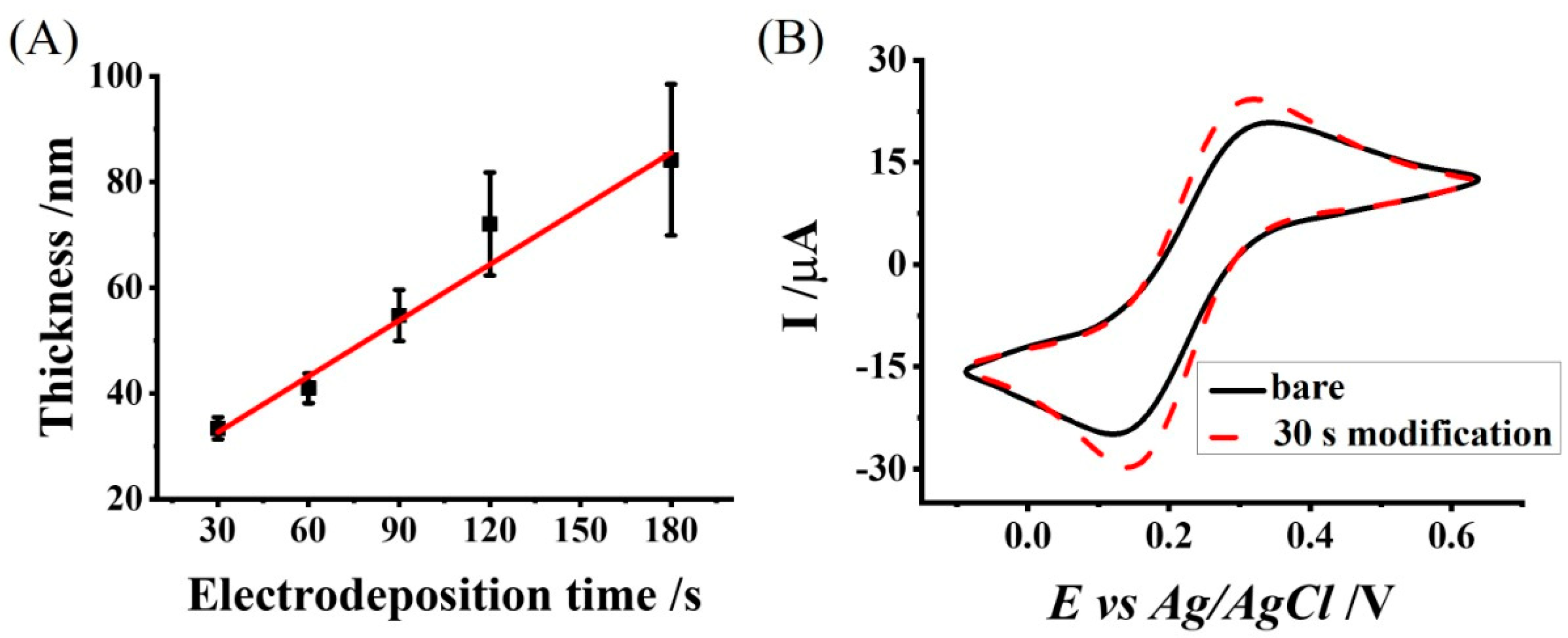
2.3. Chitosan Electrodeposition onto the Electrodes
2.4. Electrode Surface Characterization
2.5. Electrode Electrochemical Characterization
3. Results
3.1. Electrochemical Characterization of the Microfabricated Gold Electrodes
3.2. Characterization of the Chitosan Electrodeposition Process and the Resulting Chitosan-Modified Electrode
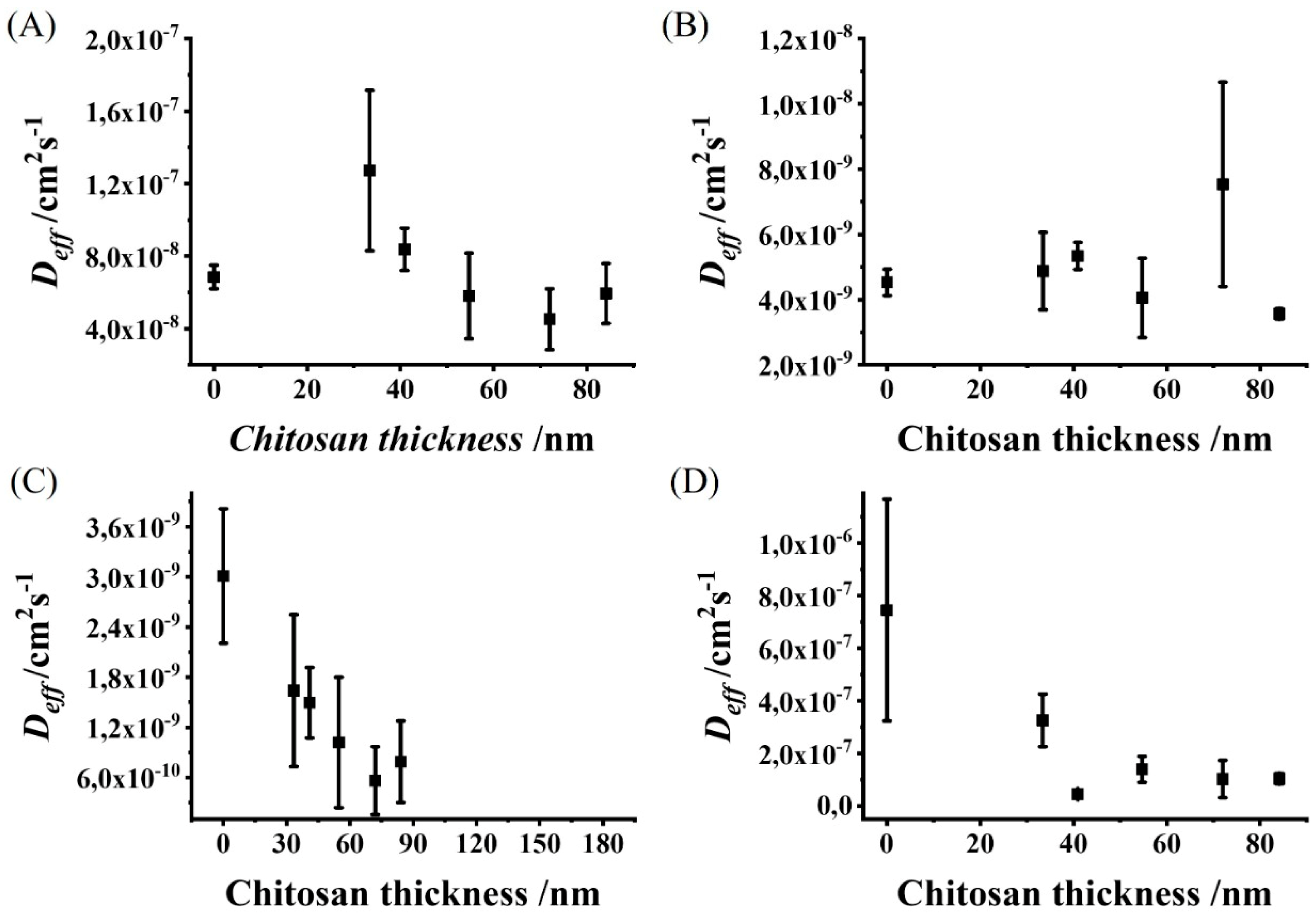
3.3. Chitosan Film Thickness Affects the Effective Diffusion Coefficients of Redox Molecules with Different Physiological Charges and Molecular Weights
3.4. Chitosan Film Can Differentially Affect the Diffusion Rate of Differentially Charged Redox Molecules
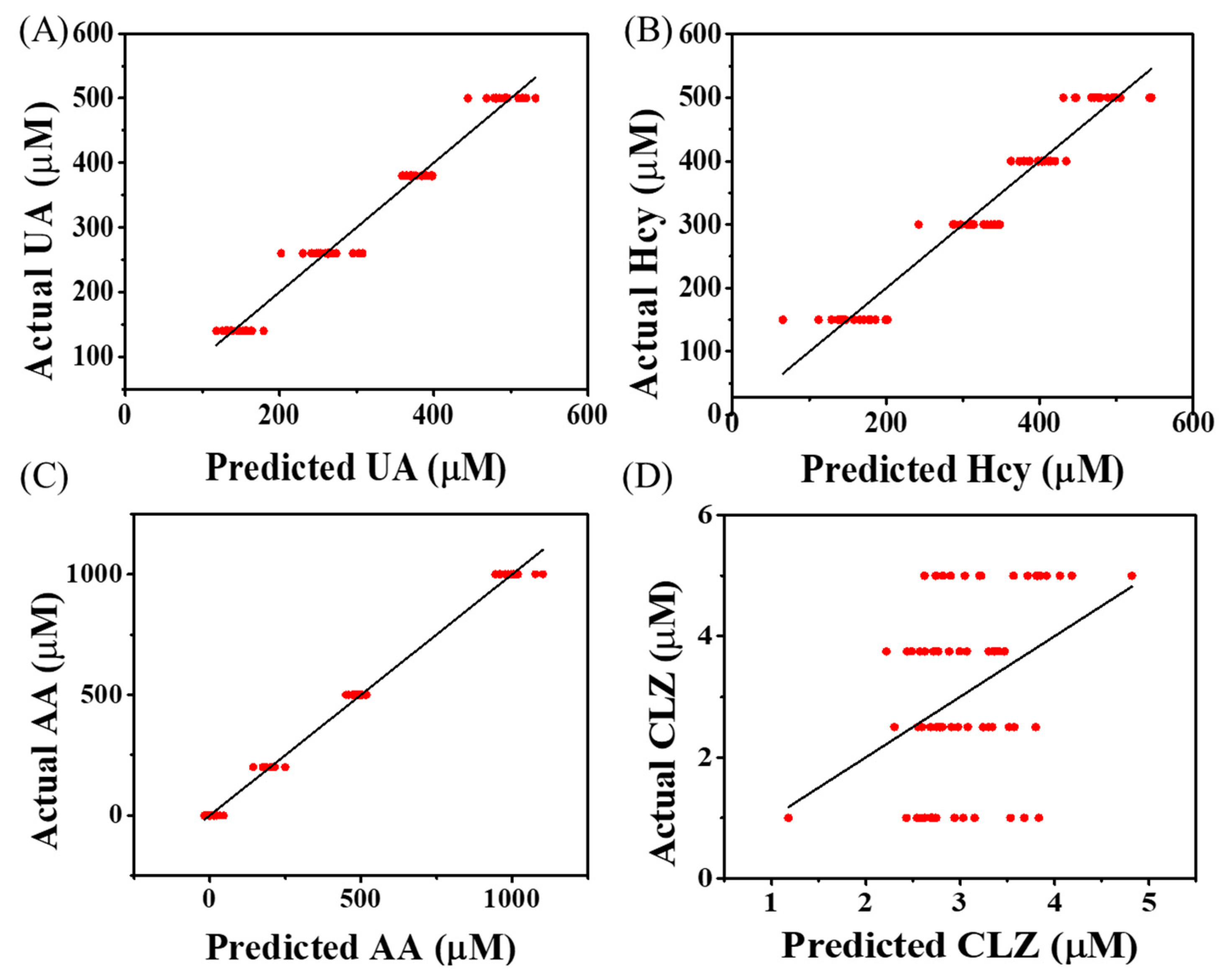
3.5. Simultaneous Prediction of Molecules’ Concentration from Mixture Samples
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.K.; Patel, A.K.; Shah, N.; Choudhary, A.K.; Jha, U.K.; Yadav, U.C.; Gupta, P.K.; Pakuwal, U. Oxidative stress and antioxidants in disease and cancer: A review. Asian Pac. J. Cancer Prev. 2014, 15, 4405–4409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T. Clinical relevance of biomarkers of oxidative stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristal, B.S.; Vigneau-Callahan, K.E.; Matson, W.R. Simultaneous analysis of the majority of low-molecular-weight, redox-active compounds from mitochondria. Anal. Biochem. 1998, 263, 18–25. [Google Scholar] [CrossRef]
- Subaihi, A.; Almanqur, L.; Muhamadali, H.; AlMasoud, N.; Ellis, D.I.; Trivedi, D.K.; Hollywood, K.A.; Xu, Y.; Goodacre, R. Rapid, accurate, and quantitative detection of propranolol in multiple human biofluids via surface-enhanced raman scattering. Anal. Chem. 2016, 88, 10884–10892. [Google Scholar] [CrossRef]
- Pitt, J.J. Principles and applications of liquid chromatography-mass spectrometry in clinical biochemistry. Clin. Biochem. Rev. 2009, 30, 19. [Google Scholar]
- Gouveia, M.J.; Brindley, P.J.; Santos, L.L.; da Costa, J.M.C.; Gomes, P.; Vale, N. Mass spectrometry techniques in the survey of steroid metabolites as potential disease biomarkers: A review. Metab. Clin. Exp. 2013, 62, 1206–1217. [Google Scholar] [CrossRef] [Green Version]
- Weltin, A.; Kieninger, J.; Urban, G.A. Microfabricated, amperometric, enzyme-based biosensors for in vivo applications. Anal. Bioanal. Chem. 2016, 408, 4503–4521. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Feng, L.; Ren, J.; Qu, X. Electrochemical detection of dopamine using porphyrin-functionalized graphene. Biosens. Bioelectron. 2012, 34, 57–62. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jia, W.; Wang, J. Tattoo-based wearable electrochemical devices: A review. Electroanalysis 2015, 27, 562–572. [Google Scholar] [CrossRef]
- Rotariu, L.; Lagarde, F.; Jaffrezic-Renault, N.; Bala, C. Electrochemical biosensors for fast detection of food contaminants–trends and perspective. TrAC Trends Anal. Chem. 2016, 79, 80–87. [Google Scholar] [CrossRef]
- Corrie, S.R.; Coffey, J.W.; Islam, J.; Markey, K.A.; Kendall, M. Blood, sweat, and tears: Developing clinically relevant protein biosensors for integrated body fluid analysis. Analyst 2015, 140, 4350–4364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahadır, E.B.; Sezgintürk, M.K. Applications of graphene in electrochemical sensing and biosensing. TrAC Trends Anal. Chem. 2016, 76, 1–14. [Google Scholar] [CrossRef]
- Zhang, W.; Zong, L.; Geng, G.; Li, Y.; Zhang, Y. Enhancing determination of quercetin in honey samples through electrochemical sensors based on highly porous polypyrrole coupled with nanohybrid modified GCE. Sens. Actuators B Chem. 2018, 257, 1099–1109. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Adekunle, A.S.; Kumara Swamy, B.E.; Ebenso, E.E. Electrochemical sensor for the detection of dopamine in real samples using polyaniline/NiO, ZnO, and Fe3O4 nanocomposites on glassy carbon electrode. J. Electroanal. Chem. 2018, 818, 236–249. [Google Scholar] [CrossRef]
- Kim, D.; Lee, S.; Piao, Y. Electrochemical determination of dopamine and acetaminophen using activated graphene-nafion modified glassy carbon electrode. J. Electroanal. Chem. 2017, 794, 221–228. [Google Scholar] [CrossRef]
- Jang, H.; Kim, D.; Lee, C.; Yan, B.; Qin, X.; Piao, Y. Nafion coated au nanoparticle-graphene quantum dot nanocomposite modified working electrode for voltammetric determination of dopamine. Inorg. Chem. Commun. 2019, 105, 174–181. [Google Scholar] [CrossRef]
- Cetó, X.; Voelcker, N.H.; Prieto-Simón, B. Bioelectronic tongues: New trends and applications in water and food analysis. Biosens. Bioelectron. 2016, 79, 608–626. [Google Scholar] [CrossRef]
- Ha, D.; Sun, Q.; Su, K.; Wan, H.; Li, H.; Xu, N.; Sun, F.; Zhuang, L.; Hu, N.; Wang, P. Recent achievements in electronic tongue and bioelectronic tongue as taste sensors. Sens. Actuators B Chem. 2015, 207, 1136–1146. [Google Scholar] [CrossRef]
- Twomey, K.; De Eulate, E.A.; Alderman, J.; Arrigan, D. Fabrication and characterization of a miniaturized planar voltammetric sensor array for use in an electronic tongue. Sens. Actuators B Chem. 2009, 140, 532–541. [Google Scholar] [CrossRef]
- Tian, S.; Deng, S.; Chen, Z. Multifrequency large amplitude pulse voltammetry: A novel electrochemical method for electronic tongue. Sens. Actuators B Chem. 2007, 123, 1049–1056. [Google Scholar] [CrossRef]
- Arrieta, Á.A.; Rodríguez-Méndez, M.L.; De Saja, J.A.; Blanco, C.A.; Nimubona, D. Prediction of bitterness and alcoholic strength in beer using an electronic tongue. Food Chem. 2010, 123, 642–646. [Google Scholar] [CrossRef]
- Cetó, X.; Gutiérrez, J.M.; Moreno-Barón, L.; Alegret, S.; Del Valle, M. Voltammetric electronic tongue in the analysis of cava wines. Electroanalysis 2011, 23, 72–78. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, X.; Betz, J.; Buckhout-White, S.; Bekdash, O.; Payne, G.F.; Bentley, W.E.; Rubloff, G.W. In situ quantitative visualization and characterization of chitosan electrodeposition with paired sidewall electrodes. Soft Matter 2010, 6, 3177–3183. [Google Scholar] [CrossRef]
- Redepenning, J.; Venkataraman, G.; Chen, J.; Stafford, N. Electrochemical preparation of chitosan/hydroxyapatite composite coatings on titanium substrates. J. Biomed. Mater. Res. Part A 2003, 66, 411–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Altomare, L.; Draghi, L.; Chiesa, R.; De Nardo, L. Morphology tuning of chitosan films via electrochemical deposition. Mater. Lett. 2012, 78, 18–21. [Google Scholar] [CrossRef]
- Hsieh, W.; Chang, C.; Lin, S. Morphology and characterization of 3D micro-porous structured chitosan scaffolds for tissue engineering. Colloids Surfaces B Biointerfaces 2007, 57, 250–255. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Z.; Lai, Z.; Wang, R.; Guo, X.; Wu, X.; Zhang, G.; Zhang, Z.; Wang, Y.; Chen, Z. Planar Amperometric glucose sensor based on glucose oxidase immobilized by chitosan film on prussian blue layer. Sensors 2002, 2, 127–136. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Zhu, R.; Zhu, Z.; Lai, Z.; Chen, Z. Chitosan/prussian blue-based biosensors. Measurement Sci. Technol. 2003, 14, 831. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Jara, C.C.; Cerro-Lopez, M.; Quiroz, M.A. Chitosan-modified glassy carbon electrodes: Electrochemical behaviour as a function of the preparation method and pH. Can. J. Anal. Sci. Spectrosc. 2009, 54, 53–62. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Fundamentals and applications. Electrochem. Methods 2001, 2, 580–632. [Google Scholar]
- Psychogios, N.; Hau, D.D.; Peng, J.; Guo, A.C.; Mandal, R.; Bouatra, S.; Sinelnikov, I.; Krishnamurthy, R.; Eisner, R.; Gautam, B. The human serum metabolome. PLoS ONE 2011, 6, e16957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stark, A.; Scott, J. A Review of the use of clozapine levels to guide treatment and determine cause of death. Aust. N. Z. J. Psychiatry 2012, 46, 816–825. [Google Scholar] [CrossRef]
- Konopka, S.J.; McDuffie, B. Diffusion coefficients of ferri-and ferrocyanide ions in aqueous media, using twin-electrode thin-layer electrochemistry. Anal. Chem. 1970, 42, 1741–1746. [Google Scholar] [CrossRef]
- Zangmeister, R.A.; Park, J.J.; Rubloff, G.W.; Tarlov, M.J. Electrochemical study of chitosan films deposited from solution at reducing potentials. Electrochim. Acta 2006, 51, 5324–5333. [Google Scholar] [CrossRef]
- Kim, E.; Liu, Y.; Ben-Yoav, H.; Winkler, T.E.; Yan, K.; Shi, X.; Shen, J.; Kelly, D.L.; Ghodssi, R.; Bentley, W.E. Fusing sensor paradigms to acquire chemical information: An integrative role for smart biopolymeric hydrogels. Adv. Healthc. Mater. 2016, 5, 2595–2616. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, J.; Tschirhart, T.; Terrell, J.L.; Kim, E.; Tsao, C.; Kelly, D.L.; Bentley, W.E.; Payne, G.F. Connecting biology to electronics: Molecular communication via redox modality. Adv. Healthc. Mater. 2017, 6, 1700789. [Google Scholar] [CrossRef]
- Liu, A.; Wei, M.D.; Honma, I.; Zhou, H. Biosensing properties of titanatenanotube films: Selective detection of dopamine in the presence of ascorbate and uric acid. Adv. Funct. Mater. 2006, 16, 371–376. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Winkler, T.E.; Lederer, S.L.; Kim, E.; Ben-Yoav, H.; Kelly, D.L.; Payne, G.F.; Ghodssi, R. Molecular processes in an electrochemical clozapine sensor. Biointerphases 2017, 12, 02B401. [Google Scholar] [CrossRef] [PubMed]
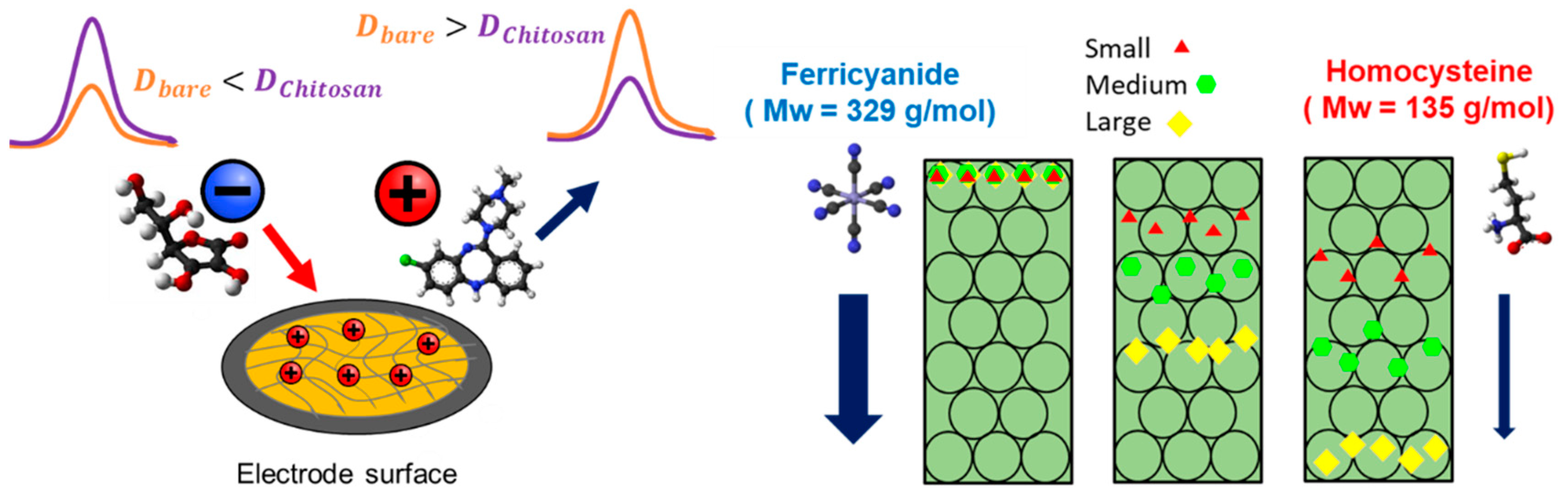


| Ferri | AA | HCy | CLZ | |
|---|---|---|---|---|
| Mw [g mol−1] | 329.24 | 176.12 | 135.18 | 326.82 |
| Physiological charge | −3 [39] | −1 [39] | 0 [40] | +1 [41] |
| AA | CLZ | UA | HCy | |
|---|---|---|---|---|
| RMSE (µM) | 125 | 1.63 | 51.8 | 55.7 |
| PCC | 0.947 | −0.093 | 0.877 | 0.903 |
| R2 | 0.894 | 0 | 0.753 | 0.809 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biton Hayun, S.; Shukla, R.P.; Ben-Yoav, H. Diffusion- and Chemometric-Based Separation of Complex Electrochemical Signals That Originated from Multiple Redox-Active Molecules. Polymers 2022, 14, 717. https://doi.org/10.3390/polym14040717
Biton Hayun S, Shukla RP, Ben-Yoav H. Diffusion- and Chemometric-Based Separation of Complex Electrochemical Signals That Originated from Multiple Redox-Active Molecules. Polymers. 2022; 14(4):717. https://doi.org/10.3390/polym14040717
Chicago/Turabian StyleBiton Hayun, Stav, Rajendra P. Shukla, and Hadar Ben-Yoav. 2022. "Diffusion- and Chemometric-Based Separation of Complex Electrochemical Signals That Originated from Multiple Redox-Active Molecules" Polymers 14, no. 4: 717. https://doi.org/10.3390/polym14040717
APA StyleBiton Hayun, S., Shukla, R. P., & Ben-Yoav, H. (2022). Diffusion- and Chemometric-Based Separation of Complex Electrochemical Signals That Originated from Multiple Redox-Active Molecules. Polymers, 14(4), 717. https://doi.org/10.3390/polym14040717








