One-Pot Synthesis of Amphiphilic Biopolymers from Oxidized Alginate and Self-Assembly as a Carrier for Sustained Release of Hydrophobic Drugs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
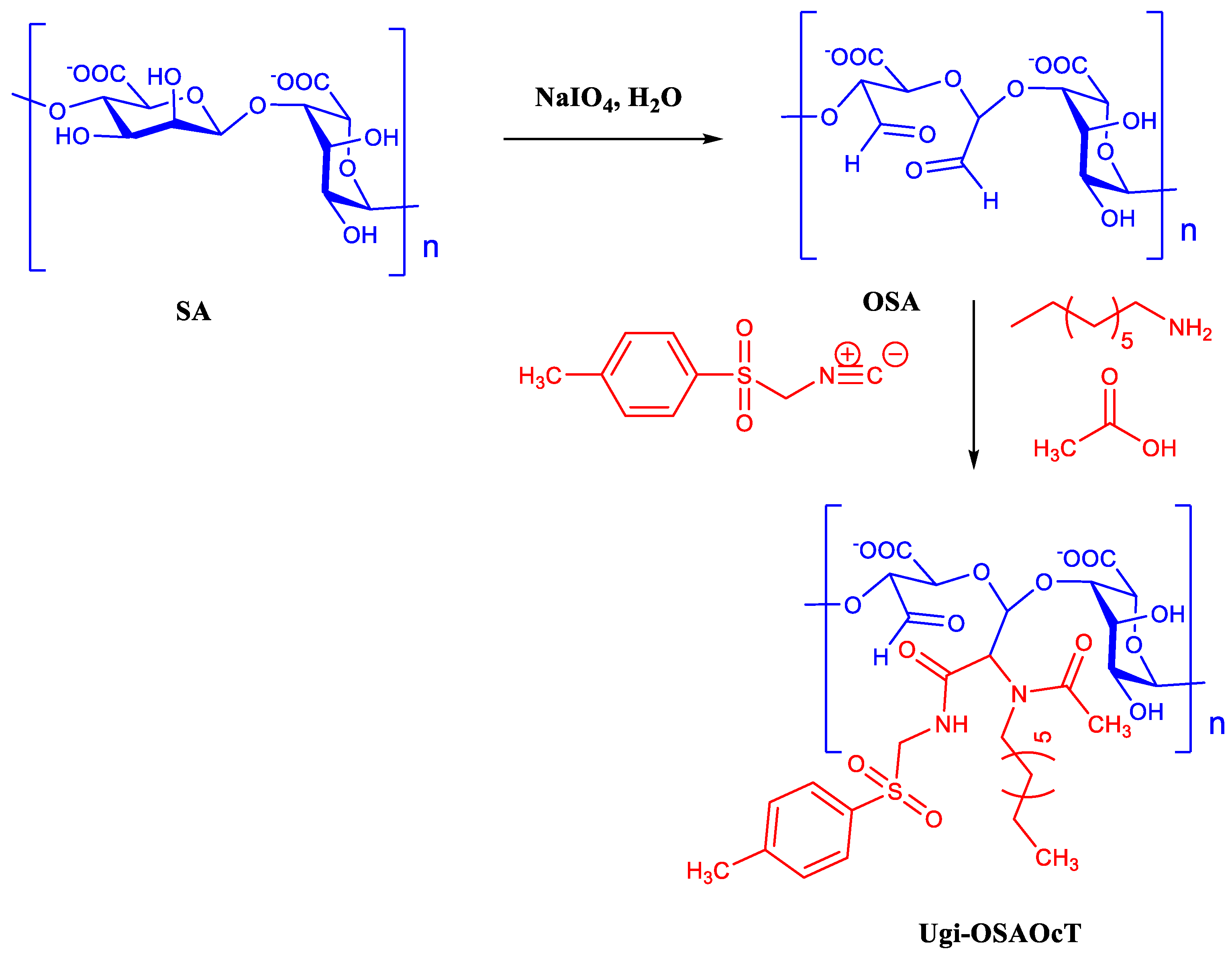
2.2. Synthesis of Ugi-OSAOcT Conjugates
2.2.1. Oxidation of Sodium Alginate
2.2.2. OSA Modification with OCA
2.3. Characterizations of Ugi-OSAOcT Conjugates
2.3.1. FTIR and 1H NMR Spectroscopy
2.3.2. X-ray Diffraction Analysis
2.3.3. Thermogravimetric Analysis
2.3.4. Measurement of Degree of Substitution
2.3.5. Gel Permeation Chromatography Analysis
2.4. Measurement of Critical Micelle Concentration (CMC)
2.5. Preparation of Blank Ugi-OSAOcT Micelles
2.6. Characterization of Polymeric Micelles
2.6.1. Dynamic Light Scattering (DLS)
2.6.2. Transmission Electron Microscopy (TEM)
2.6.3. Storage Stability of Blank Ugi-OSAOcT Micelles
2.7. Preparation of IBU-Loaded Self-Assembled Nanoparticles
2.8. In Vitro Drug Release Studies
2.9. In Vitro Cytotoxicity Assays
2.10. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of Ugi-OSAOcT Conjugates
3.2. Self-Assembly Behavior of Ugi-OSAOcT Conjugate
3.3. Preparation and Characterization of Ugi-OSAOcT Micelles
3.4. Preparation and Characterization of IBU-Loaded Ugi-OSAOcT Micelles
3.5. In Vitro Release of IBU from Ugi-OSAOcT Micelles
3.6. In Vitro Cytotoxicity of Ugi-OSAOcT Conjugates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kausar, A. Polymer and modified chitosan-based nanocomposite: Impending material for technical application. Polym. Plast. Technol. Mater. 2019, 58, 934–947. [Google Scholar] [CrossRef]
- Butowska, K.; Woziwodzka, A.; Borowik, A.; Piosik, J. Polymeric nanocarriers: A transformation in doxorubicin therapies. Materials 2021, 14, 2135. [Google Scholar] [CrossRef] [PubMed]
- Fidaleo, M.; Tacconi, S.; Sbarigia, C.; Passeri, D.; Rossi, M.; Tata, A.M.; Dini, L. Current Nanocarrier Strategies Improve Vitamin B12 Pharmacokinetics, Ameliorate Patients’ Lives, and Reduce Costs. Nanomaterials 2021, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Song, M.M.; Liu, C.G.; Chen, S.Y.; Zhang, W.X. Nanocarrier-Based Drug Delivery for Melanoma Therapeutics. Int. J. Mol. Sci. 2021, 22, 1873. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.G.; Silvestre, A.L.P.; Dos Santos, A.M.; Fonseca-Santos, B.; Rodrigues, W.D.; Gremião, M.P.D.; Chorilli, M.; Villanova, J.C.O. Polymeric-based drug delivery systems for veterinary use: State of the art. Int. J. Pharm. 2021, 604, 120756. [Google Scholar] [CrossRef]
- Antoniou, A.I.; Giofrè, S.; Seneci, P.; Passarella, D.; Pellegrino, S. Stimulus-responsive liposomes for biomedical applications. Drug Discov. Today 2021, 26, 1794–1824. [Google Scholar] [CrossRef]
- Foroughi-Nia, B.; Barar, J.; Memar, M.Y.; Aghanejad, A.; Davaran, S. Progresses in polymeric nanoparticles for delivery of tyrosine kinase inhibitors. Life Sci. 2021, 278, 119642. [Google Scholar] [CrossRef]
- Motiei, M.; Kashanian, S. Novel amphiphilic chitosan nanocarriers for sustained oral delivery of hydrophobic drugs. Eur. J. Pharm. Sci. 2017, 99, 285–291. [Google Scholar] [CrossRef]
- Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santos, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M. Naringenin nano-delivery systems and their therapeutic applications. Pharmaceutics 2021, 13, 291. [Google Scholar] [CrossRef]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef]
- Raval, N.; Maheshwari, R.; Shukla, H.; Kalia, K.; Torchilin, V.P.; Tekade, R.K. Multifunctional polymeric micellar nanomedicine in the diagnosis and treatment of cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112186. [Google Scholar] [CrossRef] [PubMed]
- Atanase, L.I. Micellar drug delivery systems based on natural biopolymers. Polymers 2021, 13, 477. [Google Scholar] [CrossRef] [PubMed]
- Bodratti, A.M.; Alexandridis, P. Amphiphilic block copolymers in drug delivery: Advances in formulation structure and performance. Expert Opin. Drug Deliv. 2018, 15, 1085–1104. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Costil, R.; Leung, F.K.C.; Feringa, B.L. Self-Assembly of Photoresponsive Molecular Amphiphiles in Aqueous Media. Angew. Chem. Int. Ed. 2021, 60, 11604–11627. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Mishra, V.; Singh, S.K.; Gulati, M.; Kapoor, B.; Chellappan, D.K.; Gupta, G.; Dureja, H.; Anand, K.; Dua, K. Harnessing amphiphilic polymeric micelles for diagnostic and therapeutic applications: Breakthroughs and bottlenecks. J. Control. Release 2021, 334, 64–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Jiao, Y.P.; Wang, Y.F.; Zhou, C.R.; Zhang, Z.Y. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Shi, X.Q.; D’arcy, R.; Tirelli, N.; Zhai, G.X. Amphiphilic polysaccharides as building blocks for self-assembled nanosystems: Molecular design and application in cancer and inflammatory diseases. J. Control. Release 2018, 272, 114–144. [Google Scholar] [CrossRef]
- Sabra, S.; Abdelmoneem, M.; Abdelwakil, M.; Mabrouk, M.T.; Anwar, D.; Mohamed, R.; Khattab, S.; Bekhit, A.; Elkhodairy, K.; Freag, M. Self-assembled nanocarriers based on amphiphilic natural polymers for anti-cancer drug delivery applications. Curr. Pharm. Des. 2017, 23, 5213–5229. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, X.L.; Hu, H. Marine Polysaccharides as a Versatile Biomass for the Construction of Nano Drug Delivery Systems. Mar. Drugs 2021, 19, 345. [Google Scholar] [CrossRef]
- Hassani, L.N.; Hendra, F.; Bouchemal, K. Auto-associative amphiphilic polysaccharides as drug delivery systems. Drug Discov. Today 2012, 17, 608–614. [Google Scholar] [CrossRef]
- Boominathan, T.; Sivaramakrishna, A. Recent Advances in the Synthesis, Properties, and Applications of Modified Chitosan Derivatives: Challenges and Opportunities. Top. Curr. Chem. 2021, 379, 1–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, L.X.; Zhang, C.H.; Deng, Y.J.; Xie, P.J.; Liu, L.J.; Cheng, J. Research advances in chemical modifications of starch for hydrophobicity and its applications: A review. Carbohydr. Polym. 2020, 240, 116292. [Google Scholar] [CrossRef] [PubMed]
- Gericke, M.; Schulze, P.; Heinze, T. Nanoparticles based on hydrophobic polysaccharide derivatives–formation principles, characterization techniques, and biomedical applications. Macromol. Biosci. 2020, 20, 1900415. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Q.; Zhang, B.F.; Wen, L.Q.; Liang, Q.Y.; Zhang, L.-M. Amphiphilic cholesteryl grafted sodium alginate derivative: Synthesis and self-assembly in aqueous solution. Carbohydr. Polym. 2007, 68, 218–225. [Google Scholar] [CrossRef]
- de Oliveira Pedro, R.; Hoffmann, S.; Pereira, S.; Goycoolea, F.M.; Schmitt, C.C.; Neumann, M.G. Self-assembled amphiphilic chitosan nanoparticles for quercetin delivery to breast cancer cells. Eur. J. Pharm. Biopharm. 2018, 131, 203–210. [Google Scholar] [CrossRef]
- Herrera, R.P.; Marqués-López, E. Multicomponent Reactions: Concepts and Applications for Design and Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Cioc, R.C.; Ruijter, E.; Orru, R.V.A. Multicomponent reactions: Advanced tools for sustainable organic synthesis. Green Chem. 2014, 16, 2958–2975. [Google Scholar] [CrossRef]
- Rocha, R.O.; Rodrigues, M.O.; Neto, B.A.D. Review on the Ugi multicomponent reaction mechanism and the use of fluorescent derivatives as functional chromophores. ACS Omega 2020, 5, 972–979. [Google Scholar] [CrossRef]
- Tao, Y.; Tao, Y.H. Ugi Reaction of Amino Acids: From Facile Synthesis of Polypeptoids to Sequence-Defined Macromolecules. Macromol. Rapid Commun. 2021, 42, 2000515. [Google Scholar] [CrossRef]
- Lei, J.; Meng, J.-P.; Tang, D.-Y.; Frett, B.; Chen, Z.-Z.; Xu, Z.-G. Recent advances in the development of polycyclic skeletons via Ugi reaction cascades. Mol. Divers. 2018, 22, 503–516. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.C.; Tan, H.P. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.T.; Cheng, J.Q.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef] [PubMed]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From food industry to biomedical applications and management of metabolic disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; da Silva, C.F.; Andrade, L.N.; de Lima Oliveira, D.; Campos, J.; Souto, E.B. Alginate nanoparticles for drug delivery and targeting. Curr. Pharm. Des. 2019, 25, 1312–1334. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Jameel, E.; Mohanta, B.; Dhara, A.K.; Alkahtani, S.; Nayak, A.K. Chapter 1—Alginates: Sources, structure, and properties. In Alginates in Drug Delivery; Nayak, A.K., Hasnain, M.S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–17. [Google Scholar]
- Dwivedi, L.M.; Baranwal, K.; Gupta, S.; Mishra, M.; Sundaram, S.; Singh, V. Antibacterial nanostructures derived from oxidized sodium alginate-ZnO. Int. J. Biol. Macromol. 2020, 149, 1323–1330. [Google Scholar] [CrossRef]
- Boontheekul, T.; Kong, H.-J.; Mooney, D. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 2005, 26, 2455–2465. [Google Scholar] [CrossRef]
- Gomez, C.G.; Rinaudo, M.; Villar, M.A. Oxidation of sodium alginate and characterization of the oxidized derivatives. Carbohydr. Polym. 2007, 67, 296–304. [Google Scholar] [CrossRef]
- Emami, Z.; Ehsani, M.; Zandi, M.; Foudazi, R. Controlling alginate oxidation conditions for making alginate-gelatin hydrogels. Carbohydr. Polym. 2018, 198, 509–517. [Google Scholar] [CrossRef]
- Yan, H.Q.; Chen, X.Q.; Li, J.C.; Feng, Y.H.; Shi, Z.F.; Wang, X.H.; Lin, Q. Synthesis of alginate derivative via the Ugi reaction and its characterization. Carbohydr. Polym. 2016, 136, 757–763. [Google Scholar] [CrossRef]
- Liu, C.G.; Desai, K.G.; Chen, X.G.; Park, H.J. Linolenic acid-modified chitosan for formation of self-assembled nanoparticles. J. Agric. Food Chem. 2005, 53, 437–441. [Google Scholar] [CrossRef]
- Opanasopit, P.; Ngawhirunpat, T.; Chaidedgumjorn, A.; Rojanarata, T.; Apirakaramwong, A.; Phongying, S.; Choochottiros, C.; Chirachanchai, S. Incorporation of camptothecin into N-phthaloyl chitosan-g-mPEG self-assembly micellar system. Eur. J. Pharm. Biopharm. 2006, 64, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Naksuriya, O.; Shi, Y.; Van Nostrum, C.F.; Anuchapreeda, S.; Hennink, W.E.; Okonogi, S. HPMA-based polymeric micelles for curcumin solubilization and inhibition of cancer cell growth. Eur. J. Pharm. Biopharm. 2015, 94, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tai, G.X.; Liu, H.Y.; Ge, J.Y.; Feng, Y.; Chen, F.F.; Yu, F.; Liu, Z.H. Activin A down-regulates the phagocytosis of lipopolysaccharide-activated mouse peritoneal macrophages in vitro and in vivo. Cell. Immunol. 2009, 255, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.L.; Cheng, C.; Ma, Y.L.; Zhao, C.S. Preparation of silver nanoparticles with antimicrobial activities and the researches of their biocompatibilities. J. Mater. Sci. Mater. Med. 2010, 21, 2861–2868. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, D.K.; Kumar, R.; Gupta, A. Controlled release of the fungicide thiram from starch–alginate–clay based formulation. Appl. Clay Sci. 2009, 45, 76–82. [Google Scholar] [CrossRef]
- Yang, J.S.; Ren, H.B.; Xie, Y.J. Synthesis of amidic alginate derivatives and their application in microencapsulation of λ-cyhalothrin. Biomacromolecules 2011, 12, 2982–2987. [Google Scholar] [CrossRef]
- Islam, M.S.; Karim, M.R. Fabrication and characterization of poly(vinyl alcohol)/alginate blend nanofibers by electrospinning method. Colloids Surf. A Physicochem. Eng. Asp. 2010, 366, 135–140. [Google Scholar] [CrossRef]
- Kang, H.-A.; Shin, M.S.; Yang, J.-W. Preparation and characterization of hydrophobically modified alginate. Polym. Bull. 2002, 47, 429–435. [Google Scholar] [CrossRef]
- Dalheim, M.; Vanacker, J.; Najmi, M.A.; Aachmann, F.L.; Strand, B.L.; Christensen, B.E. Efficient functionalization of alginate biomaterials. Biomaterials 2016, 80, 146–156. [Google Scholar] [CrossRef]
- Zhang, W.L.; Li, Y.L.; Liu, L.X.; Sun, Q.Q.; Shuai, X.T.; Zhu, W.; Chen, Y.M. Amphiphilic toothbrushlike copolymers based on poly (ethylene glycol) and poly (ε-caprolactone) as drug carriers with enhanced properties. Biomacromolecules 2010, 11, 1331–1338. [Google Scholar] [CrossRef]
- Luckachan, G.E.; Pillai, C.K.S. Chitosan/oligo L-lactide graft copolymers: Effect of hydrophobic side chains on the physico-chemical properties and biodegradability. Carbohydr. Polym. 2006, 64, 254–266. [Google Scholar] [CrossRef]
- Prabaharan, M.; Gong, S.Q. Novel thiolated carboxymethyl chitosan-g-β-cyclodextrin as mucoadhesive hydrophobic drug delivery carriers. Carbohydr. Polym. 2008, 73, 117–125. [Google Scholar] [CrossRef]
- Zong, Z.; Kimura, Y.; Takahashi, M.; Yamane, H. Characterization of chemical and solid state structures of acylated chitosans. Polym. Bull. 2000, 41, 899–906. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, L.J.; Zhang, Q.; Guo, H.; Wang, A.M.; Xu, W.L.; Wang, Y.L. Structure and properties of carboxymethyl cotton fabric loaded by reduced graphene oxide. Carbohydr. Polym. 2019, 214, 117–123. [Google Scholar] [CrossRef]
- Yan, H.Q.; Chen, X.Q.; Feng, M.X.; Shi, Z.F.; Zhang, W.X.; Wang, Y.; Ke, C.R.; Lin, Q. Entrapment of bacterial cellulose nanocrystals stabilized Pickering emulsions droplets in alginate beads for hydrophobic drug delivery. Colloid Surf. B Biointerfaces 2019, 177, 112–120. [Google Scholar] [CrossRef]
- Yang, X.D.; Zhang, C.G.; Qiao, C.D.; Mu, X.L.; Li, T.D.; Xu, J.K.; Shi, L.; Zhang, D.J. A simple and convenient method to synthesize N-[(2-hydroxyl)-propyl-3-trimethylammonium] chitosan chloride in an ionic liquid. Carbohydr. Polym. 2015, 130, 325–332. [Google Scholar] [CrossRef]
- Lee, K.Y.; Jo, W.H.; Kwon, L.C.; Kim, Y.-H.; Jeong, S.Y. Physicochemical characteristics of self-aggregates of hydrophobically modified chitosans. Langmuir 1998, 14, 2329–2332. [Google Scholar] [CrossRef]
- Feng, H.; Dong, C.-M. Preparation, characterization, and self-assembled properties of biodegradable chitosan− poly (l-lactide) hybrid amphiphiles. Biomacromolecules 2006, 7, 3069–3075. [Google Scholar] [CrossRef]
- Li, F.; Danquah, M.; Mahato, R.I. Synthesis and characterization of amphiphilic lipopolymers for micellar drug delivery. Biomacromolecules 2010, 11, 2610–2620. [Google Scholar] [CrossRef]
- Falamarzian, A.; Lavasanifar, A. Chemical modification of hydrophobic block in poly(ethylene oxide) poly(caprolactone) based nanocarriers: Effect on the solubilization and hemolytic activity of amphotericin B. Macromol. Biosci. 2010, 10, 648–656. [Google Scholar] [CrossRef]
- An, Y.Q.; Chen, M.; Xue, Q.J.; Liu, W.M. Preparation and self-assembly of carboxylic acid-functionalized silica. J. Coll. Interface Sci. 2007, 311, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Jacobs, C.; Kayser, O. Nanosuspensions as particulate drug formulations in therapy. Rationale for development and what we can expect for the future. Adv. Drug Deliv. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef]
- Du, H.L.; Yang, X.Y.; Pang, X.; Zhai, G.X. The synthesis, self-assembling, and biocompatibility of a novel O-carboxymethyl chitosan cholate decorated with glycyrrhetinic acid. Carbohydr. Polym. 2014, 111C, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Snowden, M.J.; Chowdhry, B.Z.; Vincent, B.; Morris, G.E. Colloidal copolymer microgels of N-isopropylacrylamide and acrylic acid: pH, ionic strength and temperature effects. J. Chem. Soc. Faraday Trans. 1996, 92, 5013–5016. [Google Scholar] [CrossRef]
- Lin, W.J.; Juang, L.W.; Lin, C.C. Stability and release performance of a series of pegylated copolymeric micelles. Pharm. Res. 2003, 20, 668–673. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Kim, S.H.; Tan, J.P.; Nederberg, F.; Fukushima, K.; Colson, J.; Yang, C.; Nelson, A.; Yang, Y.Y.; Hedrick, J.L. Hydrogen bonding-enhanced micelle assemblies for drug delivery. Biomaterials 2010, 31, 8063–8071. [Google Scholar] [CrossRef]
- Yokoyama, M.; Fukushima, S.; Uehara, R.; Okamoto, K.; Kataoka, K.; Sakurai, Y.; Okano, T. Characterization of physical entrapment and chemical conjugation of adriamycin in polymeric micelles and their design for in vivo delivery to a solid tumor. J. Control. Release 1998, 50, 79–92. [Google Scholar] [CrossRef]
- Kang, H.; Kim, J.D.; Han, S.H.; Chang, I.S. Self-aggregates of poly(2-hydroxyethyl aspartamide) copolymers loaded with methotrexate by physical and chemical entrapments. J. Control. Release 2002, 81, 135–144. [Google Scholar] [CrossRef]
- Li, Y.Y.; Qiu, X.Q.; Qian, Y.; Xiong, W.L.; Yang, D.J. pH-responsive lignin-based complex micelles: Preparation, characterization and application in oral drug delivery. Chem. Eng. J. 2017, 327, 1176–1183. [Google Scholar] [CrossRef]
- Kevadiya, B.D.; Joshi, G.V.; Patel, H.A.; Ingole, P.G.; Mody, H.M.; Bajaj, H.C. Montmorillonite-alginate nanocomposites as a drug delivery system: Intercalation and in vitro release of vitamin B1 and vitamin B6. J. Biomater. Appl. 2010, 25, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Wee, C.E.; Wai, L.K.; Zin, N.M.; Azmi, F. Biomimetic amphiphilic chitosan nanoparticles: Synthesis, characterization and antimicrobial activity. Carbohydr. Polym. 2021, 254, 117299. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Zhu, Q.M.; Li, Z.Y.; Yan, H.Q.; Lin, Q. The Molecular Structure and Self-Assembly Behavior of Reductive Amination of Oxidized Alginate Derivative for Hydrophobic Drug Delivery. Molecules 2021, 26, 5821. [Google Scholar] [CrossRef] [PubMed]
- Raja, M.A.; Liu, C.; Huang, Z. Nanoparticles based on oleate alginate ester as curcumin delivery system. Curr. Drug Deliv. 2015, 12, 613–627. [Google Scholar] [CrossRef]










| Sample a | b DO (%) | C (% m/m) | H (% m/m) | N (% m/m) | DS (%) |
|---|---|---|---|---|---|
| Ugi-OSA10OcT | 9.51 | 51.5 | 7.45 | 0.89 | 4.8 |
| Ugi-OSA30OcT | 27.76 | 41.69 | 7.17 | 1.96 | 14.8 |
| Ugi-OSA50OcT | 44.25 | 30.36 | 6.77 | 2.1 | 24.3 |
| NOSA10:NOCA:NHAc:NTOSMIC a | t (h) | T (°C) | DS (%) b | Mw c | Mn c | Mw/Mn c | Yield (%) |
|---|---|---|---|---|---|---|---|
| 1:2:2:2.2 | 12 | 25 | 3.3 | 112,671 | 84,132 | 1.34 | 41.3 |
| 1:2.4:2.4:2.6 | 12 | 25 | 4.0 | 121,653 | 92,161 | 1.32 | 52.6 |
| 1:2.8:2.8:3.1 | 12 | 25 | 3.9 | 119,868 | 77,334 | 1.55 | 53.4 |
| 1:2.4:2.4:2.6 | 16 | 25 | 3.8 | 120,848 | 81,654 | 1.48 | 53.3 |
| 1:2.4:2.4:2.6 | 8 | 25 | 2.8 | 111,269 | 73,203 | 1.52 | 46.8 |
| 1:2.4:2.4:2.6 | 12 | 37 | 3.8 | 121,058 | 85,857 | 1.41 | 48.5 |
| 1:2.4:3:3.3 | 12 | 25 | 4.8 | 123,259 | 88,676 | 1.39 | 54.2 |
| 1:2.4:3.5:3.9 | 12 | 25 | 4.8 | 124,512 | 87,685 | 1.42 | 54.7 |
| Sample | DS (%) | CMC (mg/mL) | Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|---|
| Ugi-OSA10OcT | 4.8 | 0.30 | 196.5 ± 3.8 | 0.43 ± 0.04 | −38.2 ± 0.8 |
| Ugi-OSA30OcT | 14.8 | 0.20 | 178.3 ± 4.5 | 0.45 ± 0.03 | −36.8 ± 0.6 |
| Ugi-OSA50OcT | 24.3 | 0.085 | 135.7 ± 2.4 | 0.37 ± 0.02 | −32.8 ± 0.4 |
| Sample | Drug/Polymer (w/w) | DL (%) | EE (%) | Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|---|---|
| Ugi-OSA50OcT | 1:10 | 3.9 ± 0.4 | 40.8 ± 1.6 | 142.5 ± 3.5 | 0.37 ± 0.06 | −34.8 ± 1.4 |
| Ugi-OSA50OcT | 2:10 | 8.2 ± 0.5 | 44.6 ± 1.8 | 150.7 ± 2.3 | 0.41 ± 0.03 | −35.2 ± 1.8 |
| Ugi-OSA50OcT | 3:10 | 14.6 ± 0.3 | 57.2 ± 1.3 | 160.3 ± 5.7 | 0.35 ± 0.02 | −38.8 ± 0.6 |
| Ugi-OSA50OcT | 5:10 | 19.3 ± 1.2 | 52.4 ± 1.5 | 154.6 ± 4.8 | 0.36 ± 0.05 | −36.7 ± 1.5 |
| Ugi-OSA10OcT | 3:10 | 10.9 ± 0.4 | 40.8 ± 1.6 | 210.8 ± 5.2 | 0.45 ± 0.03 | −42.5 ± 0.3 |
| Ugi-OSA30OcT | 3:10 | 13.2 ± 0.5 | 50.6 ± 1.8 | 198.6 ± 4.8 | 0.43 ± 0.03 | −42.3 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Chen, X.; Huang, Z.; Wang, H.; Cao, S.; Liu, C.; Yan, H.; Lin, Q. One-Pot Synthesis of Amphiphilic Biopolymers from Oxidized Alginate and Self-Assembly as a Carrier for Sustained Release of Hydrophobic Drugs. Polymers 2022, 14, 694. https://doi.org/10.3390/polym14040694
Liu Z, Chen X, Huang Z, Wang H, Cao S, Liu C, Yan H, Lin Q. One-Pot Synthesis of Amphiphilic Biopolymers from Oxidized Alginate and Self-Assembly as a Carrier for Sustained Release of Hydrophobic Drugs. Polymers. 2022; 14(4):694. https://doi.org/10.3390/polym14040694
Chicago/Turabian StyleLiu, Zhaowen, Xiuqiong Chen, Zhiqin Huang, Hongcai Wang, Shirui Cao, Chunyang Liu, Huiqiong Yan, and Qiang Lin. 2022. "One-Pot Synthesis of Amphiphilic Biopolymers from Oxidized Alginate and Self-Assembly as a Carrier for Sustained Release of Hydrophobic Drugs" Polymers 14, no. 4: 694. https://doi.org/10.3390/polym14040694
APA StyleLiu, Z., Chen, X., Huang, Z., Wang, H., Cao, S., Liu, C., Yan, H., & Lin, Q. (2022). One-Pot Synthesis of Amphiphilic Biopolymers from Oxidized Alginate and Self-Assembly as a Carrier for Sustained Release of Hydrophobic Drugs. Polymers, 14(4), 694. https://doi.org/10.3390/polym14040694






