Influences of Modified Sm2O3 on Thermal Stability, Mechanical and Neutron Shielding Properties of Aminophenol Trifunctional Epoxy Resin
Abstract
:1. Introduction
2. Experimental
2.1. Materials
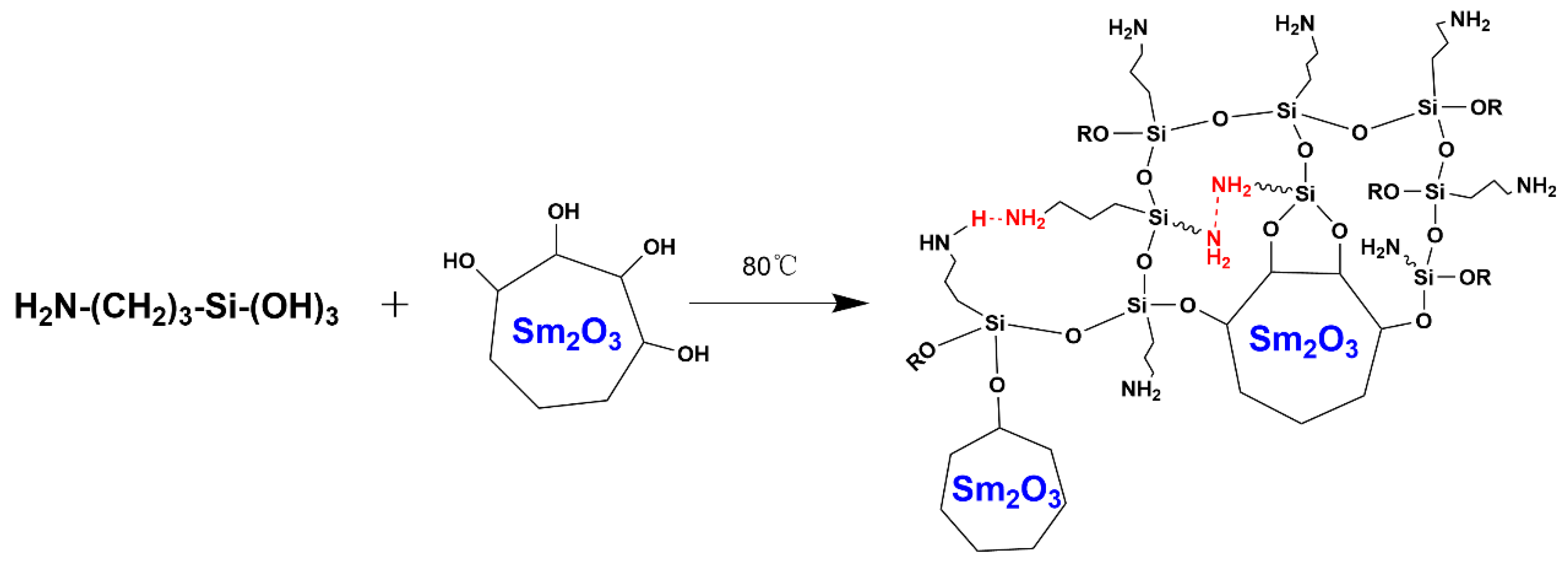
2.2. Surface Modification of Sm2O3 Powder
2.3. Fabrication of Sm2O3-APTES/AFG-90H
2.4. Characterization Methods
3. Results and Discussion
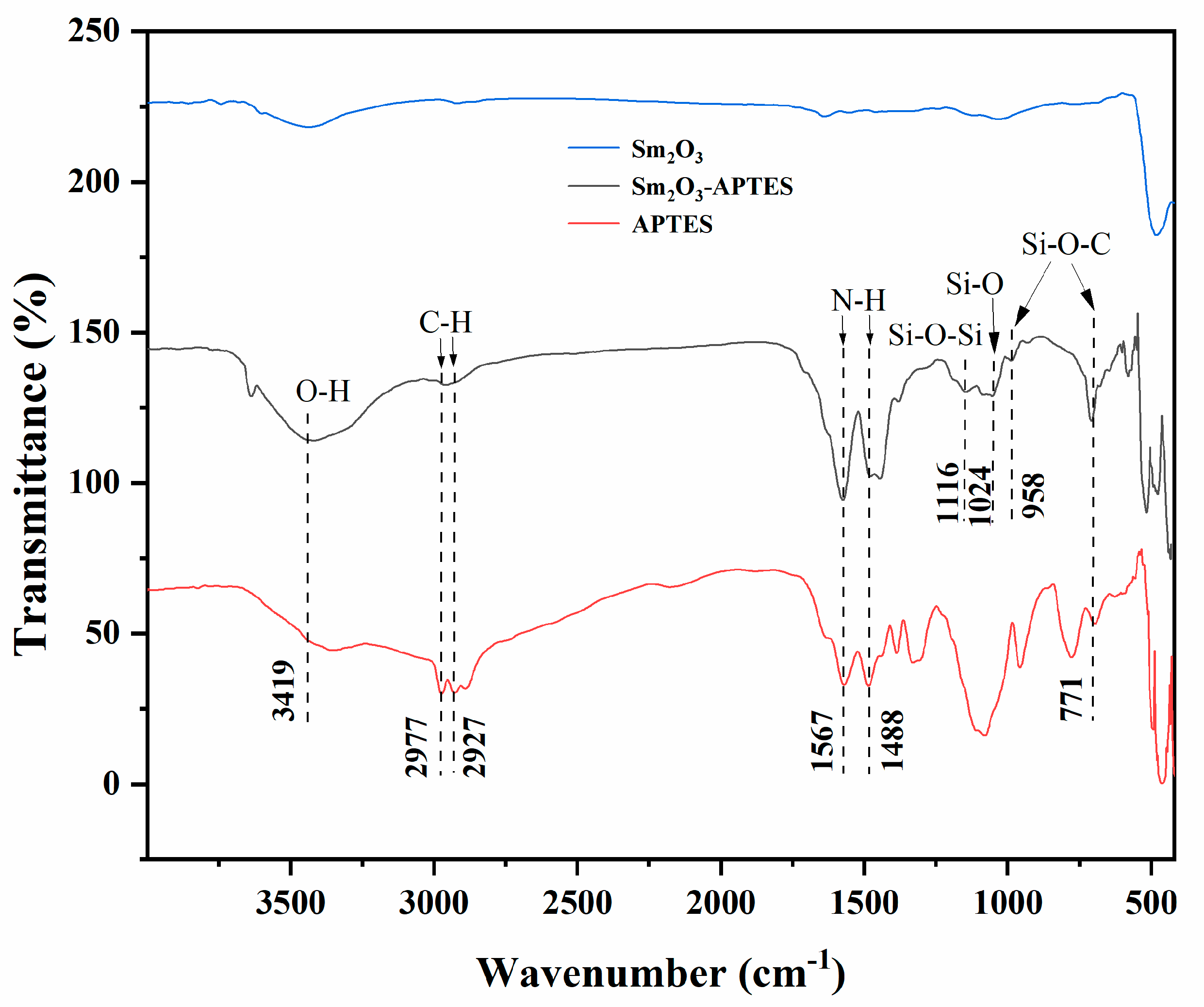
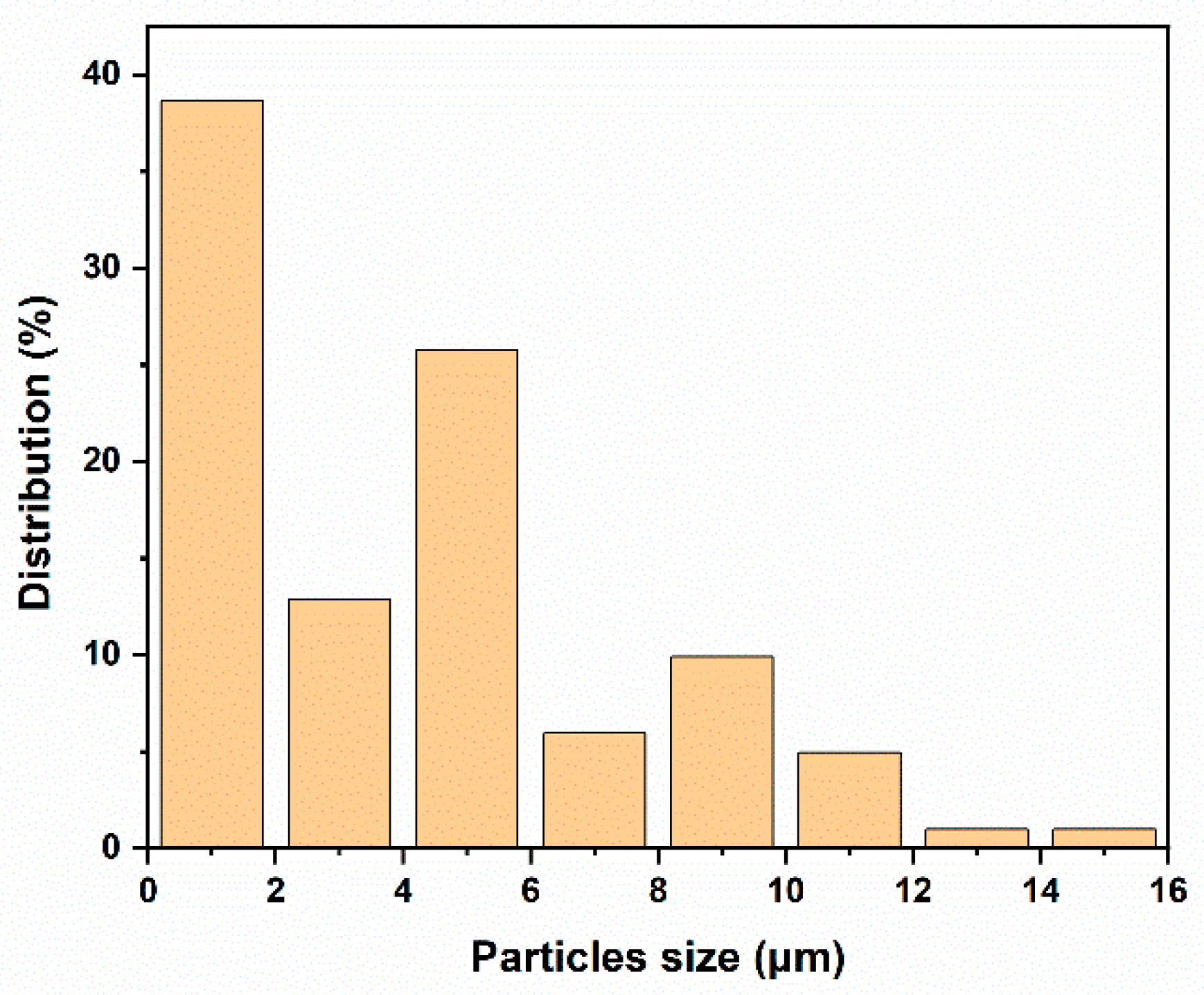
3.1. Surface Modification of Sm2O3 Powders
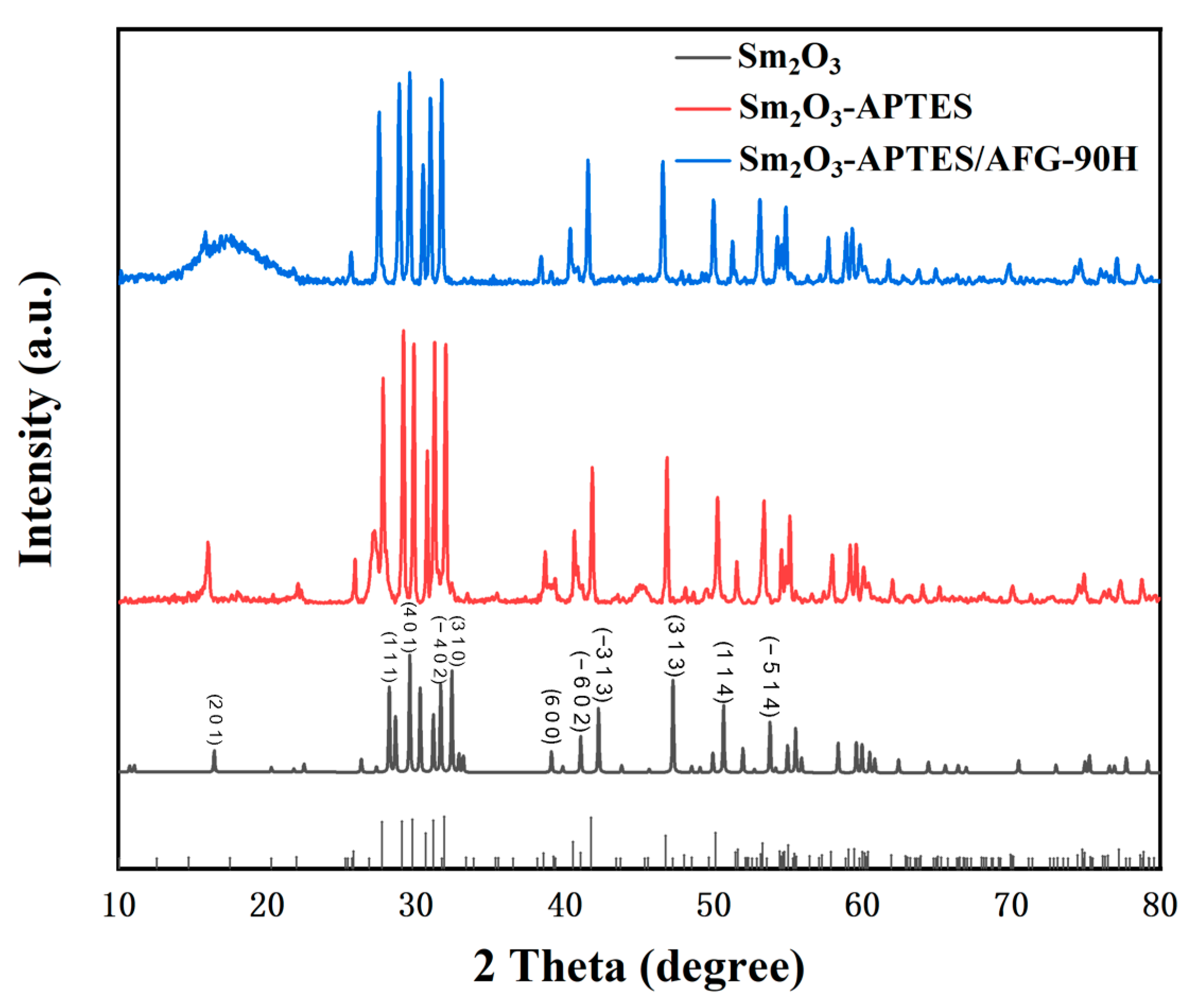
3.2. XRD Patterns of Sm2O3 and Sm2O3-APTES/AFG-90H Composites
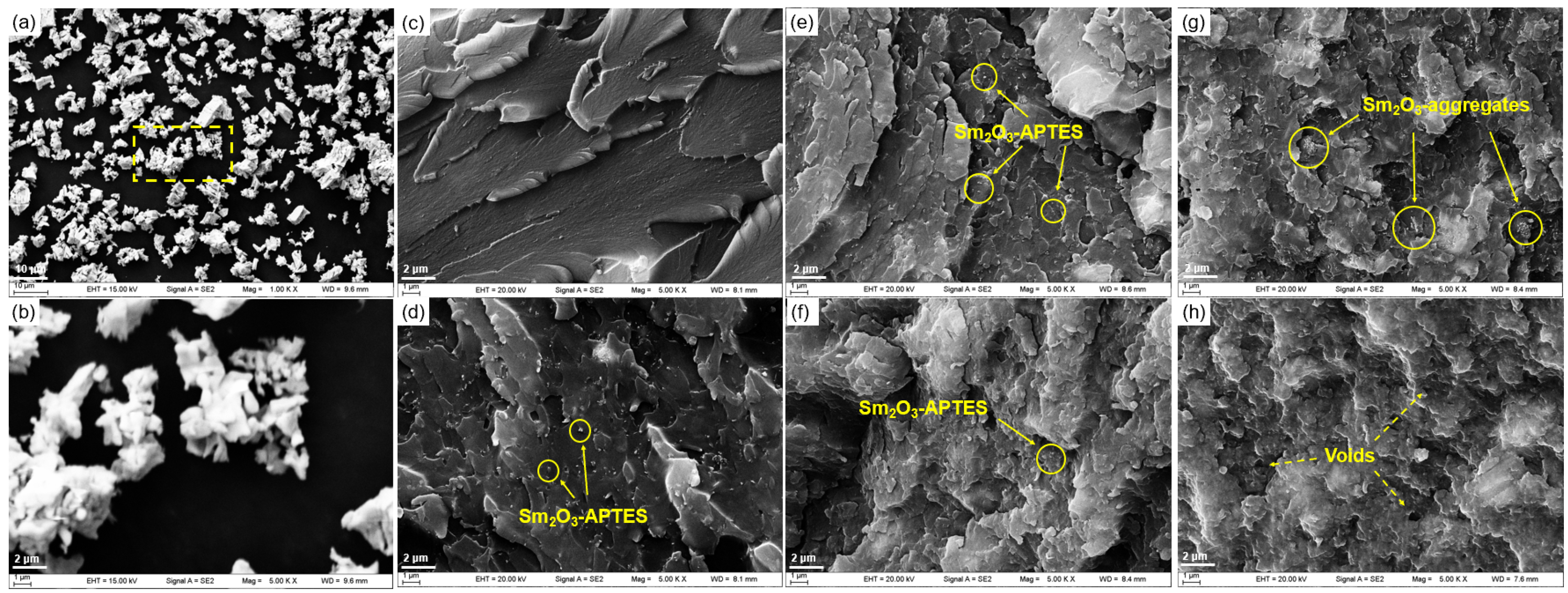
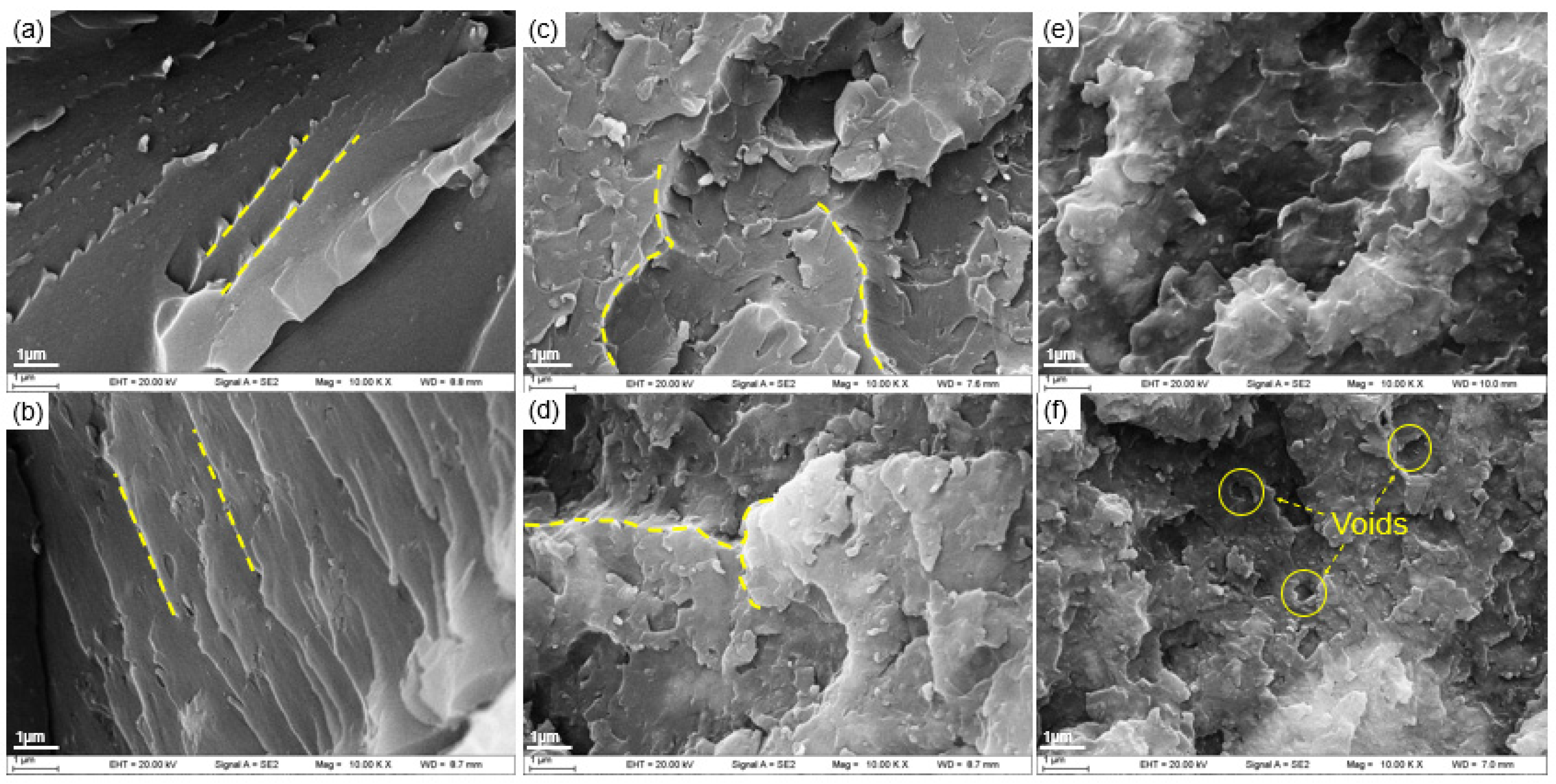
3.3. Morphology Analysis
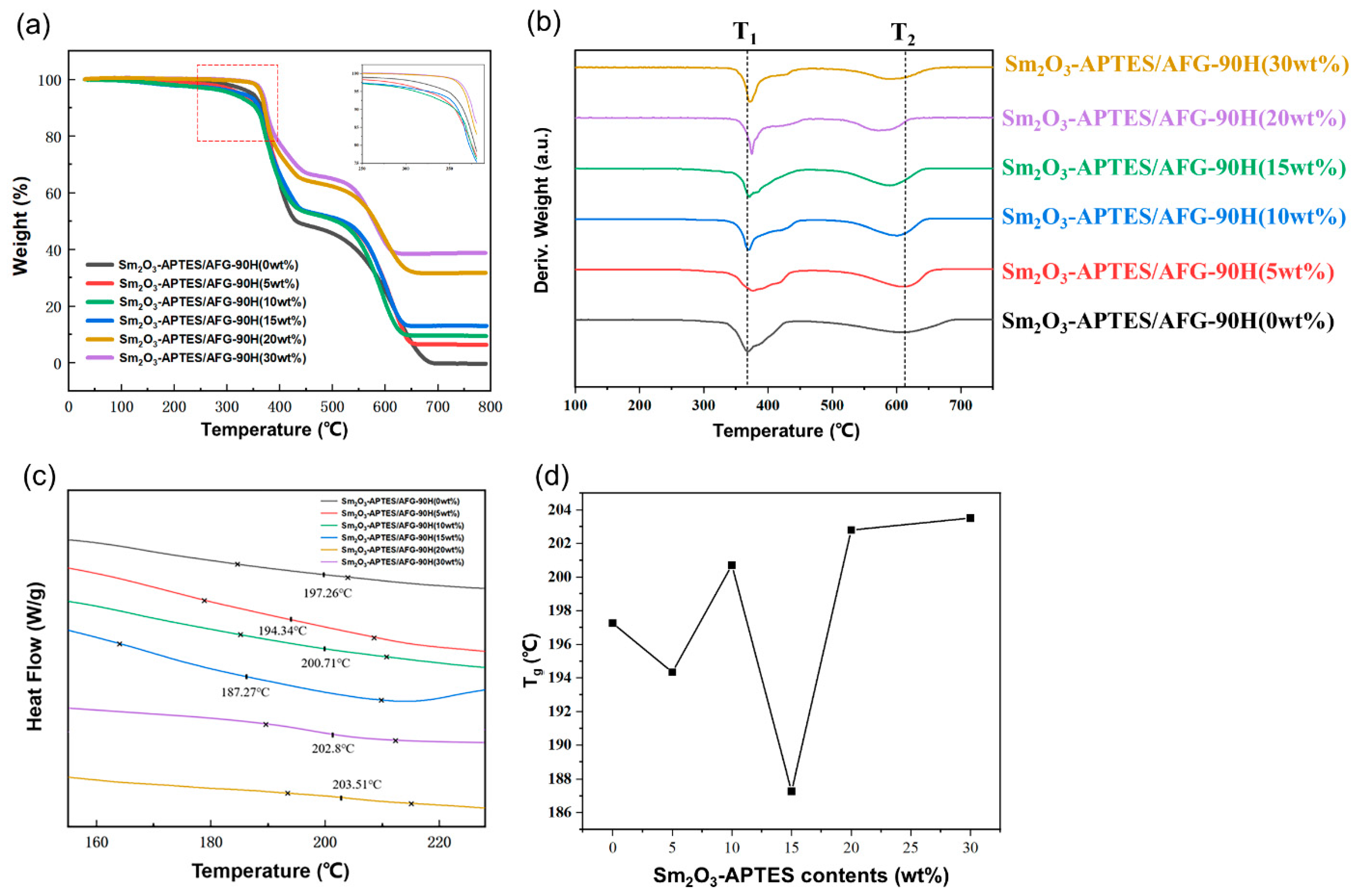
3.4. Thermal Performance
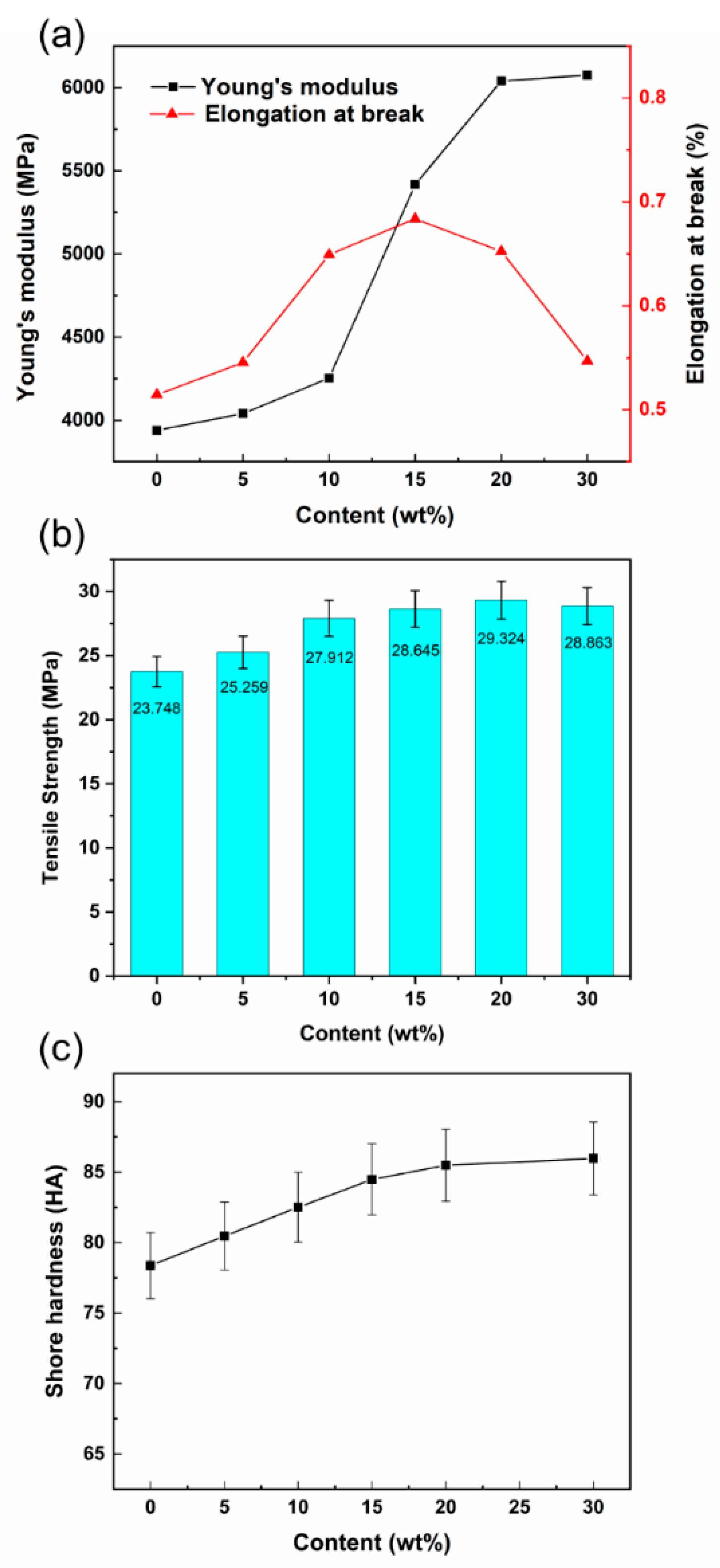
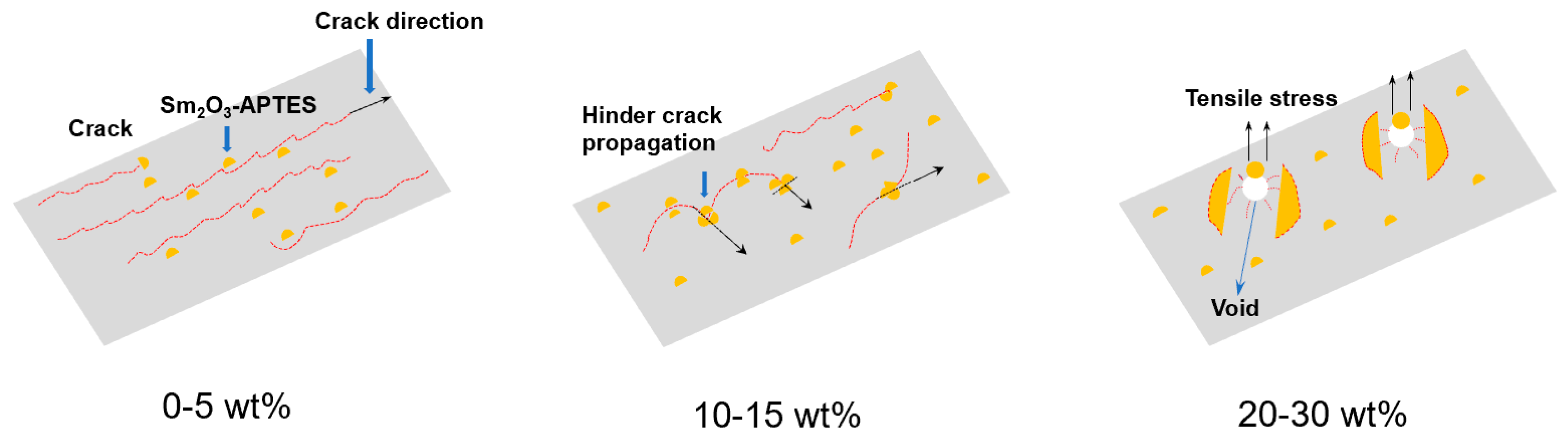
3.5. Mechanical Properties of Sm2O3-APTES/AFG-90H
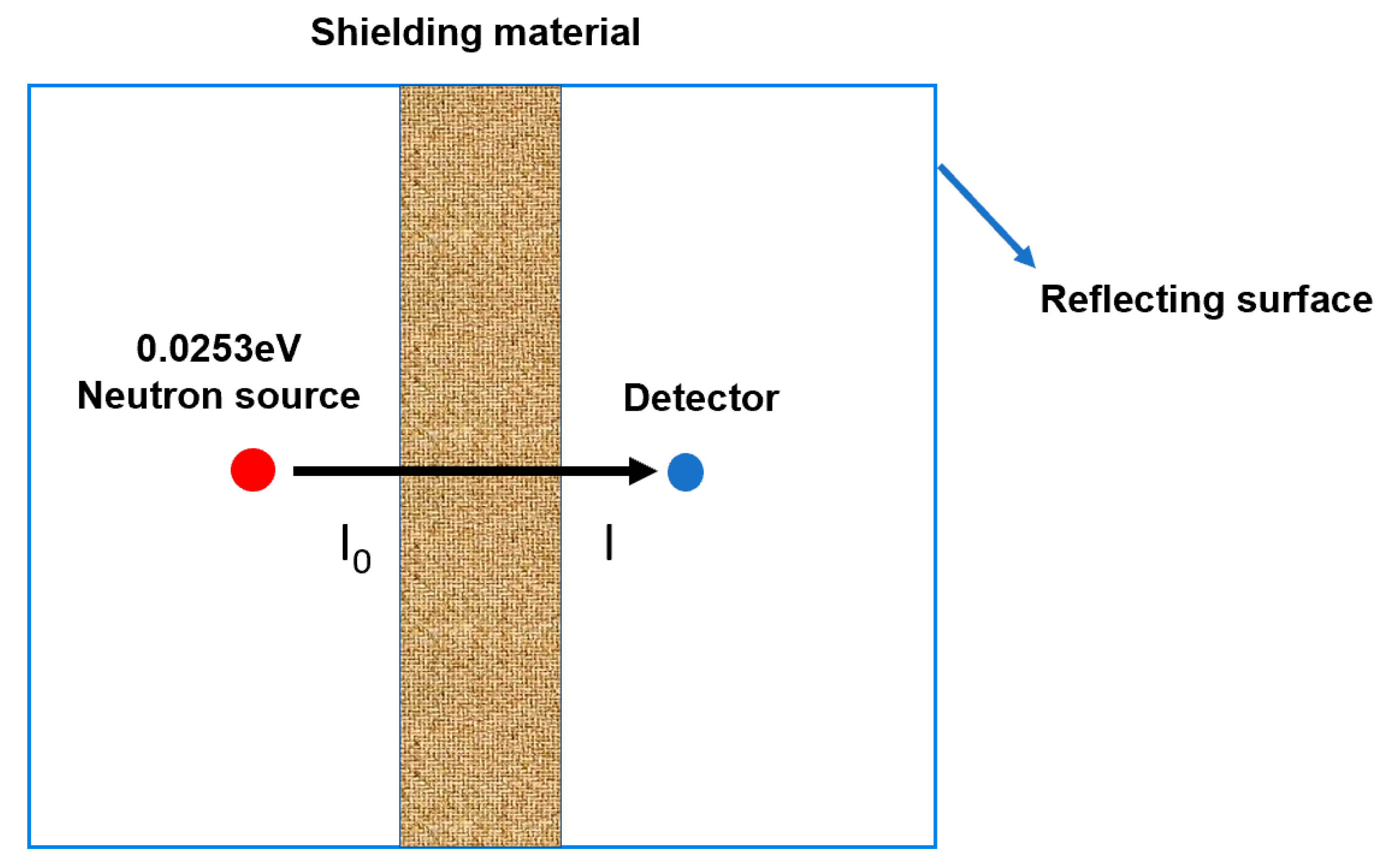
3.6. Neutronics Simulation
4. Conclusions
- (1)
- The uniform dispersion of Sm2O3 particles and good interface compatibility improved the strength and stiffness of Sm2O3-APTES/AFG-90H composites. When the Sm2O3-APTES content is 15 wt%, the composites have higher fracture toughness.
- (2)
- The Sm2O3-APTES hindered the thermal motion of molecular chains and improved the thermal stability of the composite at 340–380 °C. Simultaneously, the glass transition temperature Tg of the composites is slightly increased.
- (3)
- The shielding simulation showed that 30 wt% Sm2O3-APTES/AFG-90H had a higher neutron shielding performance than the AFG-90H matrix. After capturing neutrons, the secondary γ dose was 8.5 × 10−15 Gy.
- (4)
- The appropriate range of Sm2O3-APTES content in the epoxy matrix is 20–25 wt% to obtain higher properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abu-Khader, M.M. Recent advances in nuclear power: A review. Prog. Nucl. Energy 2009, 51, 225–235. [Google Scholar] [CrossRef]
- Nambiar, S.; Yeow, J.T.W. Polymer-composite materials for radiation protection. ACS Appl. Mater. Inter. 2012, 4, 5717–5726. [Google Scholar] [CrossRef]
- Korkut, T.; Gencel, O.; Kam, E.; Brostow, W. X-Ray, Gamma, and neutron radiation tests on epoxy-ferrochromium slag composites by experiments and Monte Carlo simulations. Int. J. Polym. Anal. Charact. 2013, 18, 224–231. [Google Scholar] [CrossRef]
- More, C.V.; Alsayed, Z.; Badawi, M.S. Polymeric composite materials for radiation shielding: A review. Environ. Chem. Lett. 2021, 19, 2057–2090. [Google Scholar] [CrossRef]
- Hu, C.; Huang, Q.; Zhai, Y. Thermal, mechanical investigation and neutron shielding analysis for Gd-MOF/polyimide materials. RSC Adv. 2021, 11, 40148–40158. [Google Scholar] [CrossRef]
- Abdo, A.E.S.; Ali, M.A.M.; Ismail, M.R. Natural fiber high-density polyethylene and lead oxide composites for radiation shielding. Radiat. Phys. Chem. 2003, 66, 185–195. [Google Scholar] [CrossRef]
- AbdelAziz, M.M.; Gwaily, S.E.; Makarious, A.S.; Abdo EI-Sayed, A. Ethylene-propylene dine rubber low density poly-ethylene boron carbide composites as neutron shields. Polym. Degrad. Stab. 1995, 50, 235–240. [Google Scholar] [CrossRef]
- Karaevsky, S.K.; Potashev, S.I.; Drachev, A.I.; Burmistrov, Y.M. Data correction method for improving the spatial resolution of a position-sensitive 10B neutron detector. Phys. At. Nucl. 2019, 82, 1686–1689. [Google Scholar] [CrossRef]
- Divya, M.; Albert, S.K.; Paul, V.T. Characterization of eutectic borides formed during solidification of borated stainless steel 304B4. Weld. World 2019, 63, 1681–1693. [Google Scholar] [CrossRef]
- Maurya, P.; Maurya, P.; Sen, I.; Roy, S. A critical assessment of the processing parameters yielding an optimum combination of mechanical properties in cast Al-B4C composites. Trans. Indian Inst. Met. 2021, 74, 1279–1294. [Google Scholar] [CrossRef]
- Chidiac, S.E.; El-Samrah, M.G.; Reda, M.A. Mechanical and radiation shielding properties of concrete containing commercial boron carbide powder. Constr. Build. Mater. 2021, 313, 125466. [Google Scholar] [CrossRef]
- Sevim, U.K.; Lumen, Y. Strength and fresh properties of borogypsum concrete. Constr. Build. Mater. 2013, 48, 342–347. [Google Scholar] [CrossRef]
- Shin, J.W.; Lee, J.W.; Yu, S.; Baek, B.K.; Hong, J.P.; Seo, Y.; Kim, W.N.; Hong, S.M.; Koo, C.M. Polyethylene/boron-containing composites for radiation shielding. Thermochim. Acta 2014, 585, 5–9. [Google Scholar] [CrossRef]
- Uddin, Z.; Yasin, T.; Shafiq, M.; Raza, A.; Zahur, A. On the physical, chemical and neutron shielding properties of polyethylene/boron carbide composites. Radiat. Phys. Chem. 2020, 166, 108450. [Google Scholar] [CrossRef]
- Abdulrahman, S.T.; Ahmad, Z.; Thomas, S.; Maria, H.J.; Rahman, A.A. Viscoelastic and thermal properties of natural rubber low-density polyethylene composites with boric acid and borax. J. Appl. Polym. Sci. 2020, 137, e49372. [Google Scholar] [CrossRef]
- Shang, Y.; Yang, G.; Su, F.; Feng, Y.Z.; Ji, X.Y.; Liu, D.; Yin, R.; Liu, C.T.; Shen, C.Y. Multilayer polyethylene/ hexagonal boron nitride composites showing high neutron shielding efficiency and thermal conductivity. Compos. Commun. 2020, 19, 147–153. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Abdalsalam, A.H.; Taki, M.M.; Mhareb, M.H.A.; Alim, B.; Baltakesmez, A.; Sakar, E. MoO3 reinforced ultra-high molecular weight PE for neutrons shielding applications. Radiat. Phys. Chem. 2020, 172, 108852. [Google Scholar] [CrossRef]
- Hu, C.; Zhai, Y.T.; Song, L.L.; Mao, X.D. Structure-thermal activity relationship in a novel polymer/MOF-based neutron-shielding material. Polym. Compos. 2020, 41, 1418–1427. [Google Scholar] [CrossRef]
- Aygun, B.; Korkut, T.; Karabulut, A.; Gencel, O.; Karabulut, A. Production and neutron irradiation tests on a new epoxy/molybdenum composite. Int. J. Polym. Anal. Charact. 2015, 20, 323–329. [Google Scholar] [CrossRef]
- Güngör, A.; Akbay, I.K.; Özdemir, T. EPDM rubber with hexagonal boron nitride: A thermal neutron shielding composite. Radiat. Phys. Chem. 2019, 165, 108391. [Google Scholar] [CrossRef]
- Baykara, O.; İrim, S.G.; Wis, A.A.; Keskin, M.A.; Ozkoc, G.; Avc, A.; Dogru, M. Polyimide nanocomposites in ternary structure: A novel simultaneous neutron and gamma-ray shielding material. Polym. Adv. Technol. 2020, 31, 2466–2479. [Google Scholar] [CrossRef]
- Li, X.M.; Wu, J.Y.; Tang, C.Y.; He, Z.K.; Yuan, P.; Sun, Y.; Lau, W.M.; Zhang, K.; Mei, J.; Huang, Y.H. High temperature resistant polyimide/boron carbide composites for neutron radiation shielding. Compos. Part B-Eng. 2019, 159, 355–361. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, G.; Zhan, M.; Yang, J. High heat resistant carbon fiber/polyimide composites with neutron shielding performance. Prog. Org. Coat. 2019, 132, 184–190. [Google Scholar] [CrossRef]
- Jiang, Y.F.; Luan, W.; Zhang, X.; Han, Y.L.; Sun, K. Preparation of AFG-90H epoxy resin-based temperature-resistant neutron shielding composite. Nucl. Tech. 2015, 38, 120202. [Google Scholar]
- Adeli, R.; Shirmardi, S.P.; Ahmadi, S.J. Neutron irradiation tests on B4C/epoxy composite for neutron shielding application and the parameters assay. Radiat. Phys. Chem. 2016, 127, 140–146. [Google Scholar] [CrossRef]
- Okuno, K. Neutron shielding material based on colemanite and epoxy resin. Radiat. Prot. Dosim. 2005, 115, 258–261. [Google Scholar] [CrossRef]
- Talamo, A.; Gudowski, W. Comparative studies of JENDL-3.3, JENDL-3.2, JEFF-3, JEF-2.2 AND ENDF/B-6.8 data libraries on the Monte Carlo continuous energy modeling of the gas turbine-modular helium reactor operating with thorium fuels. J. Nucl. Sci. Technol. 2005, 42, 1040–1053. [Google Scholar] [CrossRef]
- Dumazert, J.; Coulon, R.; Lecomte, Q.; Bertrand, G.H.V.; Hamel, M. Gadolinium for neutron detection in current nuclear instrumentation research: A review. Nucl. Instrum. Methods A 2018, 882, 53–68. [Google Scholar] [CrossRef]
- Allen, T.; Busby, J.; Meyer, M.; Petti, D. Materials challenges for nuclear systems. Mater. Today 2010, 13, 14–23. [Google Scholar] [CrossRef]
- Muhammad, F. Effects of high density dispersion fuel loading on the control rod worth of a low enriched uranium fueled material test research reactor. Ann. Nucl. Energy 2013, 58, 19–24. [Google Scholar] [CrossRef]
- Lucas, J.; Lucas, P.; le Mercier, T.; Rollat, A.; Davenport, W. Rare Earths; Elsevier: Amsterdam, The Netherlands, 2015; p. 370. [Google Scholar]
- Harris, J. Nature’s Building Blocks: An A-Z Guide to the Elements; Maney Publishing: Leeds, UK, 2002; p. 932. [Google Scholar]
- Fernandez, V. Rare-earth elements market: A historical and financial perspective. Resour. Policy 2017, 53, 26–45. [Google Scholar] [CrossRef]
- Florez, R.; Colorado, H.A.; Giraldo, C.H.C.; Alajo, A. Preparation and characterization of Portland cement pastes with Sm2O3 microparticle additions for neutron shielding applications. Constr. Build. Mater. 2018, 191, 498–506. [Google Scholar] [CrossRef]
- Chen, R.C.; Zhang, Q.P.; Ke, K.; Sun, N.; Xu, W.D.; Liu, D.L.; Yang, W.B.; Li, Y.T.; Zhou, Y.L.; Yang, M.B.; et al. Chemically bonding BaTiO3 nanoparticles in highly filled polymer nanocomposites for greatly enhanced dielectric properties. J. Mater. Chem. C 2020, 8, 8786–8795. [Google Scholar] [CrossRef]
- Zhu, S.; Qian, Y.; Hassan, E.A.M.; Shi, R.J.; Yang, L.L.; Cao, H.J.; Zhou, J.F.; Ge, D.T.; Yu, M.H. Enhanced interfacial interactions by PEEK-grafting and coupling of acylated CNT for GF/PEEK composites. Compos. Commun. 2020, 18, 43–48. [Google Scholar] [CrossRef]
- Wu, Y.C.; Song, J.; Zheng, H.Q. CAD-based Monte Carlo program for integrated simulation of nuclear system SuperMC. Ann. Nucl. Energy 2015, 82, 161–168. [Google Scholar] [CrossRef]
- Xu, D.Z.; Gao, C.J.; Wu, Y.C. Development on hybrid evaluated nuclear data library HENDL1.0/MG/MC. Nucl. Phys. Rev. 2004, 21, 415–418. [Google Scholar]
- Zhi, X.; Mao, Y.Y.; Yu, Z.Z. γ-Aminopropyl triethoxysilane functionalized graphene oxide for composites with high dielectric constant and low dielectric loss. Compos. Part A-Appl. Sci. 2015, 76, 194–202. [Google Scholar] [CrossRef]
- Meroni, D.; Lo Presti, L.; Di Liberto, G. A close look at the structure of the tio2-aptes interface in hybrid nanomaterials and its degradation pathway: An experimental and theoretical study. Langmuir 2017, 121, 430–440. [Google Scholar] [CrossRef]
- Goyat, M.S.; Rana, S.; Halder, S.; Ghosh, P.K. Facile fabrication of epoxy-TiO2 nanocomposites: A critical analysis of TiO2 impact on mechanical properties and toughening mechanisms. Ultrason. Sonochemistry 2018, 40, 861–873. [Google Scholar] [CrossRef]
- Duan, J.K.; Kim, C.N.; Jiang, P.K. On-line monitoring of cycloaliphatic epoxy/acrylate interpenetrating polymer networks formation and characterization of their mechanical properties. J. Polym. Res. 2009, 16, 45–54. [Google Scholar] [CrossRef]
- Duan, J.K.; Kim, C.N.; Yun, Z.; Jiang, P.K. Morphology and thermal and dielectric behavior of cycloaliphatic epoxy/trimethacrylate interpenetrating polymer networks for vacuum-pressure-impregnation electrical insulation. J. Appl. Polym. Sci. 2008, 110, 3096–3106. [Google Scholar] [CrossRef]
- Mahdi, E.M.; Tan, J.C. Dynamic molecular interactions between polyurethane and ZIF-8 in a polymer-MOF nanocomposite: Microstructural, thermo-mechanical and viscoelastic effects. Polymer 2016, 97, 31–43. [Google Scholar] [CrossRef]
- Permal, A.; Devarajan, M.; Huong, L.H.; Zahner, T.; Lacey, D.; Ibrahim, K. Enhanced thermal and mechanical properties of epoxy composites filled with hybrid filler system of aluminium nitride and boron nitride. Polym. Compos. 2018, 39, E1372–E1380. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Zhang, Z.Q.; Moon, K.S.; Wong, C.P. Glass transition and relaxation behavior of epoxy nanocomposites. J. Polym. Sci. Pol. Phys. 2004, 21, 3849–3858. [Google Scholar] [CrossRef]
- Hou, J.; Li, G.H.; Yang, N.; Qin, L.L.; Grami, M.E.; Zhang, Q.X.; Wang, N.Y.; Qu, X.W. Preparation and characterization of surface modified boron nitride epoxy composites with enhanced thermal conductivity. RSC Adv. 2014, 4, 44282–44290. [Google Scholar] [CrossRef]
- Liang, Z.M.; Yin, J.; Wu, J.H.; He, F.F. Polyimide/montmorillonite nanocomposites with photolithographic properties. Eur. Polym. J. 2004, 40, 307–314. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Zhang, X.M.; Chen, W.X.; Feng, L.F. Synthesis, characterization, and properties of polystyrene/SiO2 hybrid materials via sol-gel process. Polym. Compos. 2015, 36, 482–488. [Google Scholar] [CrossRef]
- Ma, S.Q.; Liu, W.Q.; Li, H.J. Morphologies and mechanical and thermal properties of epoxy resins modified by a novel polysiloxane capped with silane coupling agent, epoxide, and imino groups. J. Macromol. Sci. Part B 2011, 50, 975–987. [Google Scholar] [CrossRef]
- Fu, S.Y.; Feng, X.Q.; Lauke, B.; Mai, Y.W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate-polymer composites. Compos. Part B-Eng. 2004, 42, 3849–3858. [Google Scholar] [CrossRef]
- Zaman, I.; Kuan, H.C.; Dai, J.F.; Kawashima, N.; Michelmore, A.; Sovi, A.; Dong, S.Y.; Luong, L.; Ma, J. From carbon nanotubes and silicate layers to graphene platelets for polymer nanocomposites. Nanoscale 2012, 4, 4578–4586. [Google Scholar] [CrossRef] [Green Version]
- Chisholm, N.; Mahfuz, H.; Rangari, V.K. Fabrication and mechanical characterization of carbon/SiC-epoxy nanocomposites. Compos. Struct. 2005, 67, 115–124. [Google Scholar] [CrossRef]
- Rubab, Z.; Afzal, A.; Siddiqi, H.M.; Saeed, S. Preparation, Characterization, and Enhanced Thermal and Mechanical Properties of Epoxy-Titania Composites. Sci. World J. 2014, 515739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wazzan, A.A.; Al-Turaif, H.A.; Abdelkader, A.F. Influence of submicron TiO2 particles on the mechanical properties and fracture characteristics of cured epoxy resin. Polym. Plast. Technol. 2006, 45, 1155–1161. [Google Scholar] [CrossRef]
- Lemaire, S.; Talou, P.; Kawano, T.; Chadwick, M.B.; Madland, D.G. Monte Carlo approach to sequential neutron emission from fission fragments. Phys. Rev. C 2005, 72, 024601. [Google Scholar] [CrossRef]
- Nollett, K.M.; Pieper, S.C.; Wiringa, R.B.; Carlson, J.; Hale, G.M. Quantum Monte Carlo calculations of neutron-α scattering. Phys. Rev. Lett. 2007, 99, 022502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Tang, X.; Chai, H.; Chen, D.; Qiu, Y.L. Design, fabrication and properties of a continuous carbon-fiber reinforced Sm2O3/polyimide gamma ray/neutron shielding material. Fusion Eng. Des. 2015, 101, 218–255. [Google Scholar] [CrossRef]
- Sanusi, M.S.M.; Hassan, W.M.S.W.; Hashim, S.; Ramli, A.T. Tabulation of organ dose conversion factors for terrestrial radioactivity monitoring program. Appl. Radiat. Isot. 2021, 174, 109791. [Google Scholar] [CrossRef]
- Kiani, M.A.; Ahmadi, S.J.; Outokesh, M.; Adeli, R.; Mohamrnadi, A. Preparation and characteristics of epoxy/clay/B4C nanocomposite at high concentration of boron carbide for neutron shielding application. Radiat. Phys. Chem. 2017, 141, 223–228. [Google Scholar] [CrossRef]
- Hu, G.; Shi, G.; Hu, H.S.; Yang, Q.Z.; Yu, B.; Sun, W.Q. Development of gradient composite shielding material for shielding neutrons and gamma rays. Nucl. Eng. Technol. 2020, 52, 2387–2393. [Google Scholar] [CrossRef]
- Galehdari, N.A.; Kelkar, A.D. Effect of neutron radiation on the mechanical and thermophysical properties of nanoengineered polymer composites. J. Mater. Res. 2017, 141, 223–228. [Google Scholar] [CrossRef]



| Physical Properties | Value |
|---|---|
| Mw | 556 |
| Mn | 317 |
| Density (g/cm3) | 1.314 |
| Cross-linking density | 2179 |
| Viscosity (mPa·s@25 °C) | 1540 |
| Epoxy equivalent (g/mol) | 105 |
| Sm2O3-APTES Content (wt%) | T5 | T10 | T50 | Tmax |
|---|---|---|---|---|
| 0 | 347.7 | 361.4 | 431.5 | 693.7 |
| 5 | 325.2 | 357.1 | 514.4 | 659.9 |
| 10 | 309.6 | 354.5 | 506.7 | 644.9 |
| 15 | 327.3 | 360.8 | 519.7 | 646.2 |
| 20 | 364.5 | 371.1 | 581.3 | 662.4 |
| 30 | 367.5 | 374.4 | 578.3 | 623.7 |
| Atomic Abundance (%) | σA (b) | σA,w (b) | |
|---|---|---|---|
| 3.1 | 0.7 | 0.02 | |
| 15.1 | 57 | 8.61 | |
| 11.3 | 2.4 | 0.27 | |
| 13.9 | 42,080 | 5849.12 | |
| 7.4 | 104 | 7.70 | |
| 26.6 | 206 | 54.80 | |
| 22.6 | 8.4 | 1.90 |
| Shielding Materials | Decomposition Temperature (°C) | Tensile Strength (MPa) | Neutron Permeability (%) | Reference No. |
|---|---|---|---|---|
| B4C (5%)/epoxy resin | 218 (5% mass loss) | 32 | 56 (0.2 cm thickness) | [60] |
| Pb/B/GO/epoxy resin | 332 (5% mass loss) | / | / | [61] |
| Gd (3%)/epoxy resin | 342 (20% mass loss) | 46 | 66 (0.5 cm thickness) | [62] |
| Sm2O3-APTES (20%)/AFG-90H | 364 (5% mass loss) | 29 | 82 (0.2 cm thickness) | Our results |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Huang, Q.; Zhai, Y. Influences of Modified Sm2O3 on Thermal Stability, Mechanical and Neutron Shielding Properties of Aminophenol Trifunctional Epoxy Resin. Polymers 2022, 14, 638. https://doi.org/10.3390/polym14030638
Wang H, Huang Q, Zhai Y. Influences of Modified Sm2O3 on Thermal Stability, Mechanical and Neutron Shielding Properties of Aminophenol Trifunctional Epoxy Resin. Polymers. 2022; 14(3):638. https://doi.org/10.3390/polym14030638
Chicago/Turabian StyleWang, Hongqing, Qunying Huang, and Yutao Zhai. 2022. "Influences of Modified Sm2O3 on Thermal Stability, Mechanical and Neutron Shielding Properties of Aminophenol Trifunctional Epoxy Resin" Polymers 14, no. 3: 638. https://doi.org/10.3390/polym14030638
APA StyleWang, H., Huang, Q., & Zhai, Y. (2022). Influences of Modified Sm2O3 on Thermal Stability, Mechanical and Neutron Shielding Properties of Aminophenol Trifunctional Epoxy Resin. Polymers, 14(3), 638. https://doi.org/10.3390/polym14030638





