The Pathways to Create Containers for Bacteriophage Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Their Cultivation
2.2. Sowing
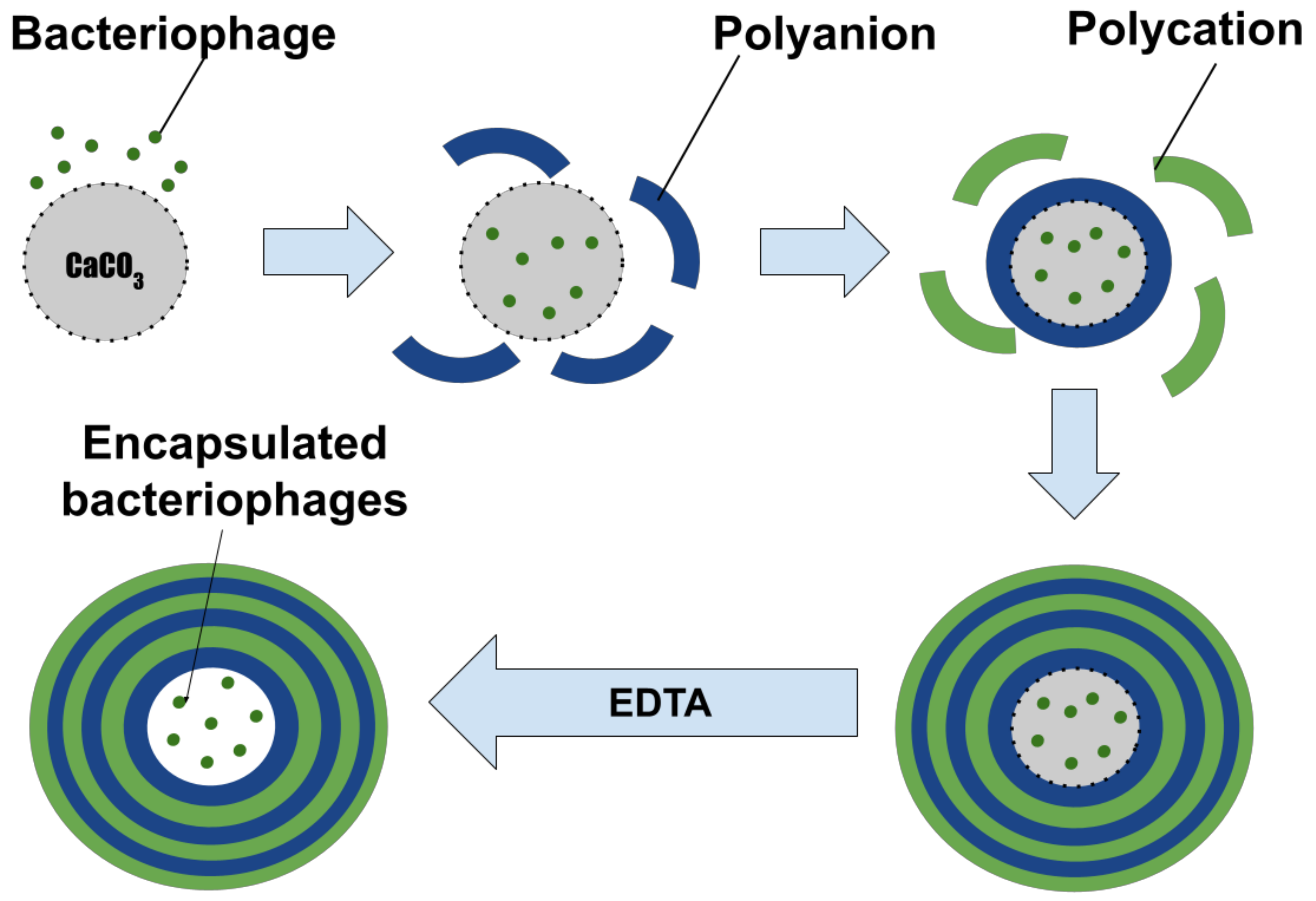
2.3. Preparation of CaCO3 Microspherolites
2.4. Preparation of Polyelectrolyte Microcapsules (PMCs)
2.5. Phage Labeling
2.6. Registration of the Bacteriophage Release from Polyelectrolyte Microcapsules and CaCO3 Microparticles
2.7. Fluorescence Microscopy
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Antibiotic/Antimicrobial Resistance. Available online: https://www.cdc.gov/drugresistance/about.html (accessed on 23 March 2021).
- Komolafe, O. Antibiotic resistance in bacteria—An emerging public health problem. Malawi Med. J. 2004, 15, 63–67. [Google Scholar] [CrossRef] [PubMed]
- D’Accolti, M.; Soffritti, I.; Piffanelli, M.; Bisi, M.; Mazzacane, S.; Caselli, E. Efficient removal of hospital pathogens from hard surfaces by a combined use of bacteriophages and probiotics: Potential as sanitizing agents. Infect. Drug Resist. 2018, 11, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Sohail, H.A.; Coffey, A.; Debrowska, K.; Meyer, I.M.; Middelboe, M.; Sohail, M.; Clokie, M.R.J. Bacteriophages: Emerging Applications in Medicine, Food, and Biotechnology. PHAGE 2020, 1, 75–82. [Google Scholar] [CrossRef]
- Alisky, J.; Iczkowski, K.; Rapoport, A.; Troitsky, N. Bacteriophages show promise as antimicrobial agents. J. Infect. 1998, 36, 5–15. [Google Scholar] [CrossRef]
- Brüssow, H. Phage therapy: The Escherichia coli experience. Microbiology 2005, 151, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Letarov, A.V.; Golomidova, A.K.; Tarasyan, K.K. Ecological basis for rational phage therapy. Acta Nat. 2010, 2, 60–72. [Google Scholar] [CrossRef]
- Peng, H.; Borg, R.E.; Dow, L.P.; Pruitt, B.L.; Chen, I.A. Controlled phage therapy by photothermal ablation of specific bacterial species using gold nanorods targeted by chimeric phages. Proc. Natl. Acad. Sci. USA 2020, 117, 1951–1961. [Google Scholar] [CrossRef]
- Kutter, E.; De Vos, D.; Gvasalia, G.; Alavidze, Z.; Gogokhia, L.; Kuhl, S.; Abedon, S. Phage Therapy in Clinical Practice: Treatment of Human Infections. Curr. Pharm. Biotechnol. 2010, 11, 69–86. [Google Scholar] [CrossRef]
- Stanford, K.; McAllister, T.A.; Niu, Y.D.; Stephens, T.P.; Mazzocco, A.; Waddell, T.E.; Johnson, R.P. Oral Delivery Systems for Encapsulated Bacteriophages Targeted at Escherichia coli O157:H7 in Feedlot Cattle. J. Food Prot. 2010, 73, 1304–1312. [Google Scholar] [CrossRef]
- Meurice, E.; Rguiti, E.; Brutel, A.; Hornez, J.; Leriche, A.; Descamps, M.; Bouchart, F. New antibacterial microporous CaP materials loaded with phages for prophylactic treatment in bone surgery. J. Mater. Sci. Mater. Med. 2012, 23, 2445–2452. [Google Scholar] [CrossRef]
- Hornez, J.C.; Bouchart, F.; Meurice, E.; Descamps, M.; Leriche, A. Synthesis and fabrication of porous calcium phosphate ceramics for antibacterial bone substitutes. MATEC Web Conf. 2013, 7, 10–12. [Google Scholar] [CrossRef]
- Cortés, P.; Cano-Sarabia, M.; Colom, J.; Otero, J.; Maspoch, D.; Llagostera, M. Nano/Micro formulations for bacteriophage delivery. Methods Mol. Biol. 2018, 1693, 271–283. [Google Scholar] [PubMed]
- Ma, Y.; Pacan, J.C.; Wang, Q.; Sabour, P.M.; Huang, X.; Xu, Y. Enhanced alginate microspheres as means of oral delivery of bacteriophage for reducing staphylococcus aureus intestinal carriage. Food Hydrocoll. 2012, 26, 434–440. [Google Scholar] [CrossRef]
- Nieth, A.; Verseux, C.; Römer, W. A Question of Attire: Dressing Up Bacteriophage Therapy for the Battle Against Antibiotic-Resistant Intracellular Bacteria. Springer Sci. Rev. 2015, 3, 1–11. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Pacan, J.C.; Wang, Q.; Xu, Y.; Huang, X.; Korenevsky, A.; Sabour, P.M. Microencapsulation of Bacteriophage Felix O1 into Chitosan-Alginate Microspheres for Oral Delivery. Appl. Environ. Microbiol. 2008, 74, 4799–4805. [Google Scholar] [CrossRef] [PubMed]
- Korehei, R.; Kadla, J.F. Encapsulation of T4 bacteriophage in electrospun poly(ethylene oxide)/cellulose diacetate fibers. Carbohydr. Polym. 2014, 100, 150–157. [Google Scholar] [CrossRef]
- Sturesson, C.; Wikingsson, L.D. Comparison of poly(acryl starch) and poly(lactide-co-glycolide) microspheres as drug delivery system for a rotavirus vaccine. J. Control. Release 2000, 68, 441–450. [Google Scholar] [CrossRef]
- Batalha, L.S.; Gontijo, M.T.P.; de Carvalho Teixeira, A.V.N.; Boggione, D.M.G.; Lopez, M.E.S.; Eller, M.R.; Mendonça, R.C.S. Encapsulation in alginate-polymers improves stability and allows controlled release of the UFV-AREG1 bacteriophage. Food Res. Int. 2021, 139, 109947. [Google Scholar] [CrossRef]
- Jamaledin, R.; Sartorius, R.; Di Natale, C.; Vecchione, R.; De Berardinis, P.; Netti, P.A. Recombinant Filamentous Bacteriophages Encapsulated in Biodegradable Polymeric Microparticles for Stimulation of Innate and Adaptive Immune Responses. Microorganisms 2020, 8, 650. [Google Scholar] [CrossRef]
- Sukhorukov, B.I.; Tikhonenko, S.A.; Saburova, E.A.; Dubrovskii, A.V.; Dybovskaya, Y.N.; Shabarchina, L.I. Protein-filled polyelectrolyte microcapsules in the design of enzymic microdiagnostics. Biophysics. 2007, 52, 575–581. [Google Scholar] [CrossRef]
- Donath, E.; Sukhorukov, G.B.; Caruso, F.; Davis, S.A.; Möhwald, H. Novel Hollow Polymer Shells by Colloid-Templated Assembly of Polyelectrolytes. Angew. Chem. Int. Ed. 1998, 37, 2201–2205. [Google Scholar] [CrossRef]
- Möhwald, H.; Donath, E.; Sukhorukov G., B. Multilayer Thin Films. Sequential Assembly of Nanocomposite Materials, 2nd ed.; Decher, G., Schlenoff, J.B., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2012; Chapter 34. [Google Scholar]
- Studer, D.; Palankar, R.; Bédard, M.; Winterhalter, M.; Springer, S. Retrieval of a Metabolite from Cells with Polyelectrolyte Microcapsules. Small 2010, 6, 2412–2419. [Google Scholar] [CrossRef] [PubMed]
- Del Mercato, L.L.; Abbasi, A.Z.; Ochs, M.; Parak, W.J. Multiplexed Sensing of Ions with Barcoded Polyelectrolyte Capsules. ACS Nano 2011, 5, 9668–9674. [Google Scholar] [CrossRef]
- Srivastava, R.; Jayant, R.D.; Chaudhary, A.; McShane, M.J. “Smart Tattoo” Glucose Biosensors and Effect of Coencapsulated Anti-Inflammatory Agents. J. Diabetes Sci. Technol. 2011, 5, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Sukhorukov, B.I.; Tikhonenko, S.A.; Saburova, E.A.; Dubrovsky, A.V.; Dybovskaya, Y.N.; Shabarchina, L.I. Encapsulation of enzymes in polyelectrolyte nano- and microcapsules in connection with the problem of microdiagnostics. Biophysics Oxf. 2007, 52, 1041–1048. [Google Scholar]
- Kim, A.L.; Musin, E.V.; Dubrovskii, A.V.; Tikhonenko, S.A. Determination of urea concentration using urease-containing polyelectrolyte microcapsules. Anal. Methods 2019, 11, 1585–1590. [Google Scholar] [CrossRef]
- Borodina, T.N.; Rumsh, L.D.; Kunizhev, S.M.; Sukhorukov, G.B.; Vorozhtsov, G.N.; Feldman, B.M.; Markvicheva, E.A. Polyelectrolyte microcapsules as the systems for delivery of biologically active substances. Biochemistry Suppl. Ser. B Biomed. Chem. 2008, 2, 88–93. [Google Scholar]
- De Koker, S.; Lambrecht, B.N.; Willart, M.A.; van Kooyk, Y.; Grooten, J.; Vervaet, C.; Remon, J.P.; De Geest, B.G. Designing polymeric particles for antigen delivery. Chem. Soc. Rev. 2011, 40, 320–339. [Google Scholar] [CrossRef]
- De Koker, S.; Hoogenboom, R.; De Geest, B.G. Polymeric multilayer capsules for drug delivery. Chem. Soc. Rev. 2012, 41, 2867. [Google Scholar] [CrossRef]
- Kochetkova, O.Y.; Kazakova, L.I.; Moshkov, D.A.; Vinokurov, M.G.; Shabarchina, L.I. Incorporation of proteins into polyelectrolyte microcapsules by coprecipitation and adsorption. Russ. J. Bioorg. Chem. 2013, 39, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Leporatti, S.; Donath, E.; Möhwald, H. Studies on the drug release properties of polysaccharide multilayers encapsulated ibuprofen microparticles. Langmuir 2001, 17, 5375–5380. [Google Scholar] [CrossRef]
- De Geest, B.G.; De Koker, S.; Sukhorukov, G.B.; Kreft, O.; Parak, W.J.; Skirtach, A.G.; Demeester, J.; De Smedt, S.C.; Hennink, W.E. Polyelectrolyte microcapsules for biomedical applications. Soft Matter 2009, 5, 282–291. [Google Scholar] [CrossRef]
- De Koker, S.; Naessens, T.; De Geest, B.G.; Bogaert, P.; Demeester, J.; De Smedt, S.; Grooten, J. Biodegradable Polyelectrolyte Microcapsules: Antigen Delivery Tools with Th17 Skewing Activity after Pulmonary Delivery. J. Immunol. 2010, 184, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.M.; Subramanian, A.; Ochs, C.J.; Dewavrin, J.-Y.; Beyer, S.; Trau, D.W. Edible polyelectrolyte microcapsules with water-soluble cargo assembled in organic phase. RSC Adv. 2014, 4, 35163–35166. [Google Scholar] [CrossRef]
- De Koker, S.; De Geest, B.G.; Cuvelier, C.; Ferdinande, L.; Deckers, W.; Hennink, W.E.; De Smedt, S.C.; Mertens, N. In vivo Cellular Uptake, Degradation, and Biocompatibility of Polyelectrolyte Microcapsules. Adv. Funct. Mater. 2007, 17, 3754–3763. [Google Scholar] [CrossRef]
- Koo, H.Y.; Lee, H.-J.; Kim, J.K.; Choi, W.S. UV-triggered encapsulation and release from polyelectrolyte microcapsules decorated with photoacid generators. J. Mater. Chem. 2010, 20, 3932. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, C.; Li, D.; Long, Y.; Song, K.; Tung, C.-H. Magnetic Compression of Polyelectrolyte Microcapsules for Controlled Release. Langmuir 2015, 31, 11195–11199. [Google Scholar] [CrossRef]
- Craig, M.; Altskär, A.; Nordstierna, L.; Holmberg, K. Bacteria-triggered degradation of nanofilm shells for release of antimicrobial agents. J. Mater. Chem. B 2016, 4, 672–682. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Q.J.; Gong, J.X.; Li, H.Q.; Zhang, J.F.; Liang, W.H.; Xu, J.; Huang, C.H. Preparation and Release Behavior of Polyelectrolyte Microcapsules. Adv. Mater. Res. 2012, 627, 765–769. [Google Scholar] [CrossRef]
- Musin, E.V.; Kim, A.L.; Dubrovskii, A.V.; Kudryashova, E.B.; Tikhonenko, S.A. Decapsulation of Dextran by Destruction of Polyelectrolyte Microcapsule Nanoscale Shell by Bacillus subtilis Bacteria. Nanomaterials 2019, 10, 12. [Google Scholar] [CrossRef]
- Shalitin, C.; Danon, D.; Katchalski, E. Inactivation of Escherichia coli bacteriophage T2 by poly-l-lysine. I. Nature of the inactivation process. Arch. Biochem. Biophys. 1962, 99, 494–507. [Google Scholar] [CrossRef]
- Shalitin, C.; Katchalski, E. Inactivation of Escherichia coli bacteriophage T2 by poly-l-lysine. II. Properties of the irreversibly inactivated phage. Arch. Biochem. Biophys. 1962, 99, 508–516. [Google Scholar] [CrossRef]
- Shima, S.; Fukuhara, Y.; Sakai, H. Inactivation of Bacteriophages by ε-Poly-l-lysine Produced by Streptomyces. Agric. Biol. Chem. 1982, 46, 1917–1919. [Google Scholar]
- Sela, M.; Katchalski, E. Biological Properties of Poly-α- Amino Acids. Adv. Protein Chem. 1959, 14, 391–478. [Google Scholar]
- Dubrovskii, A.V.; Shabarchina, L.I.; Kim, Y.A.; Sukhorukov, B.I. Influence of the temperature on polyelectrolyte microcapsules: Light scattering and confocal microscopy data. Russ. J. Phys. Chem. A 2006, 80, 1703–1707. [Google Scholar] [CrossRef]
- Khawaja, K.A.; Abbas, Z.; Rehman, S.U. Isolation and characterization of lytic phages TSE1-3 against Enterobacter cloacae. Open Life Sci. 2016, 11, 287–292. [Google Scholar] [CrossRef]
- Jończyk, E.; Kłak, M.; Międzybrodzki, R.; Górski, A. The influence of external factors on bacteriophages—Review. Folia Microbiol. 2011, 56, 191–200. [Google Scholar] [CrossRef]
- Taj, M.K.; Ling, J.X.; Bing, L.L.; Qi, Z.; Taj, I.; Hassani, T.M.; Samreen, Z.; Yunlin, W. Effect of dilution, temperature and pH on the lysis activity of t4 phage against E. coli BL21. J. Anim. Plant Sci. 2014, 24, 1252–1255. [Google Scholar]
- Pavlov, A.M.; Sukhorukov, G.B.; Gould, D.J. Location of molecules in layer-by-layer assembled microcapsules influences activity, cell delivery and susceptibility to enzyme degradation. J. Control. Release 2013, 172, 22–29. [Google Scholar] [CrossRef]
- Castillo, D.; Rørbo, N.; Jørgensen, J.; Lange, J.; Tan, D.; Kalatzis, P.G.; Svenningsen, S.L.; Middelboe, M. Phage defense mechanisms and their genomic and phenotypic implications in the fish pathogen Vibrio anguillarum. FEMS Microbiol. Ecol. 2019, 95, fiz004. [Google Scholar] [CrossRef] [PubMed]
- Roucourt, B.; Lavigne, R. The role of interactions between phage and bacterial proteins within the infected cell: A diverse and puzzling interactome. Environ. Microbiol. 2009, 11, 2789–2805. [Google Scholar] [CrossRef] [PubMed]
- Jabrane, T.; Dubé, M.; Mangin, P.J. Bacteriophage immobilization on paper surface: Effect of cationic pre-coat layer. In Proceedings of the PAPTAC 95th Annual Meeting, Montreal, QC, Canada, 3–4 February 2009. [Google Scholar]
- Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.d.L.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; Gropp, J.; et al. Safety and efficacy of l-arginine produced by fermentation with Escherichia coli NITE BP-02186 for all animal species. EFSA J. 2018, 16, 5276. [Google Scholar]
- Musin, E.V.; Kim, A.L.; Dubrovskii, A.V.; Kudryashova, E.B.; Ariskina, E.V.; Tikhonenko, S.A. The Influence of Polyanions and Polycations on Bacteriophage Activity. Polymers Basel 2021, 13, 914. [Google Scholar] [CrossRef]
- Smith, H.W.; Huggins, M.B.; Shaw, K.M. Factors Influencing the Survival and Multiplication of Bacteriophages in Calves and in Their Environment. Microbiology 1987, 133, 1127–1135. [Google Scholar] [CrossRef]
- Bahrom, H.; Goncharenko, A.A.; Fatkhutdinova, L.I.; Peltek, O.O.; Muslimov, A.R.; Koval, O.Y.; Eliseev, I.E.; Manchev, A.; Gorin, D.; Shishkin, I.I.; et al. Controllable Synthesis of Calcium Carbonate with Different Geometry: Comprehensive Analysis of Particle Formation, Cellular Uptake, and Biocompatibility. ACS Sustain. Chem. Eng. 2019, 7, 19142–19156. [Google Scholar] [CrossRef]
- Biradar, S.; Ravichandran, P.; Gopikrishnan, R.; Goornavar, V.; Hall, J.C.; Ramesh, V.; Baluchamy, S.; Jeffers, R.B.; Ramesh, G.T. Calcium Carbonate Nanoparticles: Synthesis, Characterization and Biocompatibility. J. Nanosci. Nanotechnol. 2011, 11, 6868–6874. [Google Scholar] [CrossRef]
- Choi, S.-J.; Lee, J.-A.; Kim, M.-K.; Kim, H.-M.; Lee, J.K.; Kim, Y.-R.; Oh, J.-M.; Jeong, J. The fate of calcium carbonate nanoparticles administered by oral route: Absorption and their interaction with biological matrices. Int. J. Nanomed. 2015, 10, 2273–2293. [Google Scholar] [CrossRef][Green Version]








| Capsule Type | Number of Plaques | |
|---|---|---|
| Dissolved core | (DS/PArg)3 | 0 |
| Undissolved core | (DS/PArg)3 | 3 ± 1 |
| Dissolved core | (DS/PArg)3 + PDADMAC | 0 |
| Dissolved core + added E. coli | (DS/PArg)3 | 0 |
| Undissolved core + added MgSO4 | (DS/PArg)3 | 0 |
| Dissolved core + added MgSO4 | (DS/PArg)3 | 0 |
| Phages | 65 ± 3 | |
| Type of Dissolved Capsule | Number of Plaques |
|---|---|
| (DS/PArg)3 | 6 ± 1 |
| (DS/PArg)3 + E. coli | 9 ± 2 |
| (DS/PArg)3 + 140 mM NaCl | 4 ± 1 |
| (DS/PArg)3 + 15 mM MgSO4 | 19 ± 2 |
| Phages | 642 ± 23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musin, E.V.; Kim, A.L.; Dubrovskii, A.V.; Ariskina, E.V.; Kudryashova, E.B.; Tikhonenko, S.A. The Pathways to Create Containers for Bacteriophage Delivery. Polymers 2022, 14, 613. https://doi.org/10.3390/polym14030613
Musin EV, Kim AL, Dubrovskii AV, Ariskina EV, Kudryashova EB, Tikhonenko SA. The Pathways to Create Containers for Bacteriophage Delivery. Polymers. 2022; 14(3):613. https://doi.org/10.3390/polym14030613
Chicago/Turabian StyleMusin, Egor V., Aleksandr L. Kim, Alexey V. Dubrovskii, Elena V. Ariskina, Ekaterina B. Kudryashova, and Sergey A. Tikhonenko. 2022. "The Pathways to Create Containers for Bacteriophage Delivery" Polymers 14, no. 3: 613. https://doi.org/10.3390/polym14030613
APA StyleMusin, E. V., Kim, A. L., Dubrovskii, A. V., Ariskina, E. V., Kudryashova, E. B., & Tikhonenko, S. A. (2022). The Pathways to Create Containers for Bacteriophage Delivery. Polymers, 14(3), 613. https://doi.org/10.3390/polym14030613






