Abstract
Functionalities of 3D printing filaments have gained much attention owing to their properties for various applications in the last few years. Innovative biocomposite 3D printing filaments based on polylactic acid (PLA) composited with ZnO nanoflowers at varying contents were successfully fabricated via a single-screw extrusion technique. The effects of the varying ZnO nanoflower contents on their chemical, thermal, mechanical, and antibacterial properties were investigated using Fourier transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC), and tensile testing, as well as qualitative and quantitative antibacterial tests, respectively. It was found that the ZnO nanoflowers did not express any chemical reactions with the PLA chains. The degrees of the crystallinity of the PLA/ZnO biocomposite filaments increased when compared with those of the neat PLA, and their properties slightly decreased when increasing the ZnO nanoflower contents. Additionally, the tensile strength of the PLA/ZnO biocomposite filaments gradually decreased when increasing the ZnO nanoflower contents. The antibacterial activity especially increased when increasing the ZnO nanoflower contents. Additionally, these 3D printing filaments performed better against Gram-positive (S. aureus) than Gram-negative (E. coli). This is probably due to the difference in the cell walls of the bacterial strains. The results indicated that these 3D printing filaments could be utilized for 3D printing and applied to medical fields.
1. Introduction
3D printing is a relatively new technology and has become popular in the last few years. This technology can process a wide variety of materials and produce fully functional components, which are fabricated in a bottom-up design without molding [1]. Additionally, it can be applied in many fields, including robotics, automobile components, firearms, medical science, space engineering, and so on. [2,3,4]. Filament types are important in fabricating 3D printing products, and commodity filaments have been produced from several materials, such as polylactic acid (PLA), polycaprolactone (PCL), polyurethane (PU), and acrylonitrile butadiene styrene (ABS). Recently, the biodegradable filaments for 3D printing have been of interest to researchers due to their vast variety of applications and advantages [5]; one advantage is that they contribute to a decrease in environmental pollution when compared with synthetic ones [6,7,8,9].
Presently, synthetic filaments for 3D printing are commonly fabricated from petroleum-based polymers, such as polystyrene (PS), polyetherimide (PEI), and polyethylene (PE) [10,11]. In addition, it was found that many additive fillers were loaded into melted polymers to fabricate 3D printing composite filaments, e.g., particles [12], cellulose nanofibers [13], nanomaterials [14], and fibers [15,16,17], in order to improve their mechanical and other properties. Among these fillers, nanoparticles are broadly studied, but few studies report their specialty functions, particularly, the effects of particles’ shapes on their composite properties, including an antibacterial property. Presently, biocomposite filaments (fabricated from biodegradable matrices reinforced with fillers) are gaining considerable interest as environmentally friendly materials and for their potential in specific applications. Previous studies have stated that biocomposite films prepared from PLA and metal-based nanoparticles, e.g., silver (Ag) [18], zinc oxide (ZnO) [19], and titanium dioxide (TiO2) [20], showed good thermal and antibacterial properties.
ZnO nanofibers, owing to their high surface-to-volume ratio, low density, interconnected porous structures, and low toxicity to humans, have gained renewed interest for their potential application in various fields [21,22,23,24,25]. Additionally, their nanoparticle shapes would affect their chemical properties. The flower-like shape of ZnO, called a ZnO nanoflower, is one of the most interesting shapes for characterization because it exhibits a large surface area property. A simple method for producing ZnO nanoflowers is the electrospinning method, which is able to produce at room temperature under ambient conditions. Additionally, their size, shape, and morphology can be defined by optimizing the conditions of the spinning processes [26].
Generally, the morphology or shape of fillers in biocomposites directly affects their properties, including chemical, thermal, mechanical, and antibacterial properties. To our best knowledge, there have been no studies about ZnO nanoflower-based biocomposites that include their properties. In this study, we presented a new paradigm in 3D printing technology with the fabrication of biocomposite 3D printing filaments based on PLA and ZnO nanoflowers in order to improve their antibacterial properties as a novel biomaterial for biomedical applications. We investigated the effects of varying ZnO nanoflower contents on their chemical, thermal, mechanical, and antibacterial properties using Fourier transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC), and tensile tests, as well as qualitative and quantitative antibacterial tests, respectively.
2. Materials and Methods
2.1. Materials
Commercial-grade poly(lactic acid) (PLA) was obtained from NatureWorks LLC (Minnetonka, MN, USA) with the trade name of Ingeo 3100 HP (Mw = 140,000 g/mol). Zinc acetate was purchased from LOBA Chemie Co., Ltd., Bombay, India. Other chemical agents were of analytical-grade purity and used as received.
2.2. Preparation of 3D Printing Filaments
ZnO nanoflowers were successfully fabricated by electrospinning from a suspension of zinc acetate used as a precursor via a calcination process [26]. Then, 3D printing filaments of PLA loaded with varying ZnO nanoflower contents were prepared by an extrusion method as follows. PLA pellets were firstly dried at 80 °C for 12 h and then milled into powder form. Then, the powder was mixed with ZnO nanoflowers and incorporated utilizing a high-speed mixer. Subsequently, the dry mixture was fed into the hopper of a lab-scale single-screw extruder (LTE 26-44, Labtech Engineering, Samut Prakan, Thailand) with a screw diameter of 26 mm and a length–diameter ratio of 44. During the extrusion process, the fed temperature at the first zone of the extruder and die temperature were consecutively set to 165 °C and 180 °C. Screw rotation speed was constantly maintained at 20.75 rpm to match the traction system for producing the 3D filaments. In this paper, the 3D printing filament samples are referred to as PLA, PLA/ZnO-1, PLA/ZnO-3, and PLA/ZnO-5, corresponding to the neat PLA and the PLA composited with ZnO nanoflowers at contents of 1, 3 and 5 wt%, respectively.
2.3. Fourier Transform Infrared Spectroscopy
FTIR was performed to examine the presence of a chemical reaction between ZnO nanoflower molecules and PLA molecular chains. All 3D printing filaments were dried at 80 °C for 12 h to remove the moisture from the ambient air, then were cut into small pieces for FTIR investigation. An FTIR spectrometer (FTIR-4100, Jasco, Tokyo, Japan) in ATR mode and equipped with a diamond crystal was used for the tests. All the spectra were recorded in transmittance mode with 4 cm−1 resolution in the wavenumber range of 4000 cm−1–400 cm−1 under ambient conditions.
2.4. Differential Scanning Calorimetry
The thermal properties of these 3D printing filaments were characterized using a DSC technique (model DSC 200 F3, Netzsch, Selb, Wunsiedel, Germany). All the samples were in a sealed aluminum pan, and each sample used was approximately 5 mg. They were sequentially heated from 30 to 200 °C and cooled to 25 °C, and then reheated to 200 °C at the rate of 10 °C/min under a nitrogen flow of 50 mL/min. After that, the degree of crystallinity of the 3D printing filaments () was calculated by Equation (1) [27].
where, is the melting enthalpy for 100% crystalline PLA (93 J/g) [28], is the melting enthalpy for the 3D printing filaments, and w is the mass fraction of PLA in the biocomposites.
2.5. Tensile Testing
Tensile tests of all the 3D printing filaments were performed on a universal testing machine (model 5560, Instron, Norwood, MA, USA). A load cell of 25 kN was employed for testing all the samples. All the 3D printing filament specimens were fabricated into a rectangular shape (30 mm × 10 mm × 0.4 mm), according to the ASTM D638, via a 3D printer (model: RAISE3D N2). The conditions of fabrication were as follows: 0.1% of filled density with X-direction of printing, print speed of 40 mm/s, and printing and bed temperatures of 215 °C and 60 °C, respectively. Crosshead speed and gauge length were set at 5 mm/min and 25 mm, respectively. Five specimens of each sample were determined, and their results were averaged to obtain a mean value.
2.6. Antibacterial Testing
Antibacterial activities in both qualitative and quantitative tests were investigated in each 3D printing filament sample to evaluate their efficacies against Gram-negative (E. coli) and Gram-positive (S. aureus) bacterial strains, using the disk diffusion susceptibility and colony counting methods, respectively. The qualitative antibacterial activity of the samples was investigated as follows. The bacteria used were cultured in an LB medium and then incubated at 30 °C for 24 h. A 10−2 dilution of the incubated bacteria, approximately 108 CFU/mL, was transferred to an LB agar plate. Disc-shapes of 5.00 mm diameter were cut from the samples. The 3D printing filament sample of neat PLA was used as a control for comparing the potential antibacterial properties with the other samples. Next, the cut-out discs were placed on the LB agar plates spread with bacteria and incubated for 24 h at 37 °C. After the incubation period, the diameters of the bacterial inhibition zone were measured in mm with a transparent ruler. These experiments were performed in triplicate.
In addition, the potential quantitative antibacterial activity of the samples was performed as follows. The bacterial cells, both S. aureus and E. coli, were grown overnight in an LB broth medium at 30 °C. Then, the 10−2 dilution suspensions of grown bacteria were prepared, and the given content of the samples at the ratio of 1:1 by weight was added to the suspensions. The 3D printing filament sample from the neat PLA was also used as a control. The suspensions were shaken in a rotary shaker (model XY-80, Taitec, Koshigaya, Saitama, Japan) at the constant speed of 120 rpm for 3 h at 30 °C and were diluted 10-fold repeatedly. A 100 μL aliquot of the bacterial cell suspension was spread on the LB agar nutrient plates using a glass stick. The plates were incubated at 37 °C for 24 h. Then, the number of viable bacterial cells on the plates was counted and then calculated to the initial number of bacterial cells before dilution. The antibacterial efficacy or the percent reduction of bacteria was also calculated by Equation (2):
where R is the percent reduction in the number of viable bacteria, A is the number of bacterial cells in the presence of the 3D printing filaments from the neat PLA (CFU/mL), and B is the number of bacterial cells in the presence of those filaments from PLA/ZnO biocomposites (CFU/mL). All of the tests were also repeated in triplicate.
3. Results and Discussion
3.1. Feasible Preparation of 3D Printing Filaments
The 3D printing filaments were successfully fabricated by a single-screw extrusion process, as depicted in Figure 1. From the optical images, the results indicate that the single-screw extrusion could produce the 3D printing filaments, with roughly uniform diameters from all four samples. The images of the four filaments also appear to be similar, but their color shades became more white-turbid with increasing ZnO nanoflowers, as shown in Figure 1. It is well known that the diameter of 3D printing filaments will affect a production process’s ability. Therefore, we measured their diameters and found that the average diameters of 3D printing filaments fabricated from PLA incorporated with ZnO nanoflowers exhibited slightly larger than those from the neat PLA filament. However, the diameters of the PLA/ZnO filaments decreased when increasing the ZnO nanoflower contents, as depicted in Figure 2. This is probably due to the distribution of nanoflowers within the PLA matrix. These results indicated that ZnO nanoflowers affected the mechanical and thermal properties. Therefore, it is worthwhile to further examine the effects of ZnO nanoflower contents on these properties.
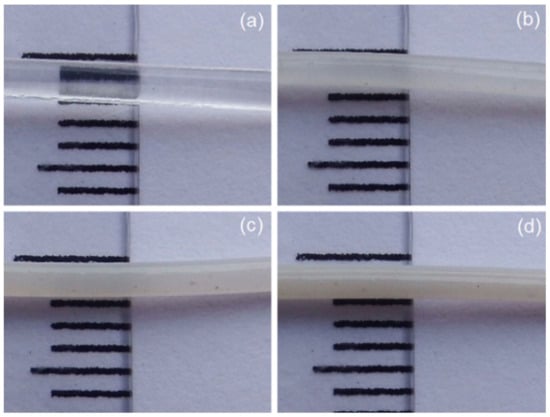
Figure 1.
Optical images of 3D printing filaments from (a) neat PLA and PLA composited with ZnO nanoflowers at contents of 1 wt% (b), 3 wt% (c) and 5 wt% (d), respectively.
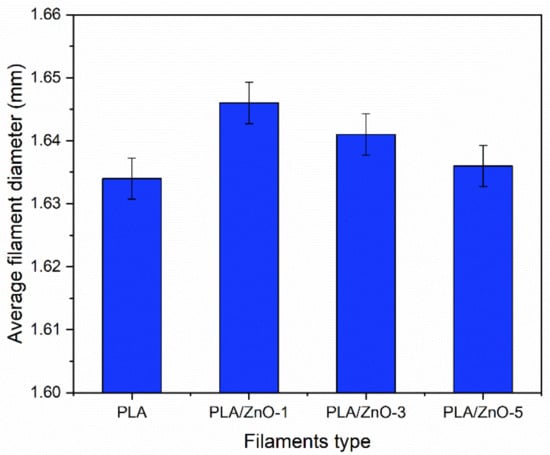
Figure 2.
Average filament diameters from neat PLA and PLA composited with ZnO nanoflowers at varying contents.
3.2. Fourier Transform Infrared Spectroscopy
Compositions and chemical structures of all the 3D printing filaments were verified by FTIR. We compared the neat ZnO nanoflowers and the PLA/ZnO filaments, as illustrated in Figure 3. FTIR spectra of ZnO nanoflowers showed that a strong absorption peak appeared at approximately 544 cm−1, thereby signifying the presence of Zn-O bonding [29,30,31]. In the case of the neat PLA, the peak displayed at around 2921 cm−1, signifying the presence of CH stretching. Absorption peaks also appeared at around 1600, 1187 and 1072 cm−1, relating to the vibration of -C=O stretching and the ester -C-O-C- symmetric stretching and asymmetric stretching bands, respectively [32]. The FTIR spectra of 3D printing filaments from PLA composited with ZnO nanoflowers showed absorption characteristic peaks of both ZnO and PLA molecules with essentially no additional peaks. These results demonstrate that no chemical reaction occurred between ZnO nanoflowers and PLA chains. Additionally, the peak around 3300 cm−1 becomes stronger and broader with increasing ZnO nanoflowers, indicating the formation of increasing interactions of hydrogen bonds between ZnO nanoflowers and PLA chains.
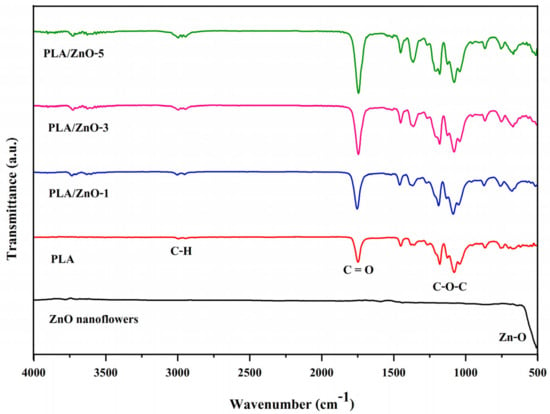
Figure 3.
FTIR spectra of ZnO nanoflowers, neat PLA, and PLA/ZnO biocomposite filaments.
3.3. Thermal Property Analysis
Differential scanning calorimetry (DSC) was performed in order to detect glass transitions and melting temperatures of all the PLA/ZnO biocomposite filaments. DSC thermograms of neat PLA and PLA composited with varying contents of ZnO nanoflowers are shown in Figure 4, and the results are collected in Table 1. The effect of ZnO nanoflowers on the thermal properties of the samples was characterized by the glass transition temperature (Tg) and the melting temperature (Tm) together with the degree of crystallinity (χc). The DSC curves of 3D printing filaments displayed endothermic Tg regions and endothermic Tm peaks. This shows that the endothermic Tg peak is related to PLA chain relaxation [33]. Table 1 shows that the neat PLA filament has lower Tg and Tm values when compared with the filaments of PLA composited with ZnO nanoflowers. However, the content of ZnO nanoflowers within a PLA matrix slightly affected the thermal properties of the 3D printing filaments in both Tg and Tm values, as shown in Table 1.
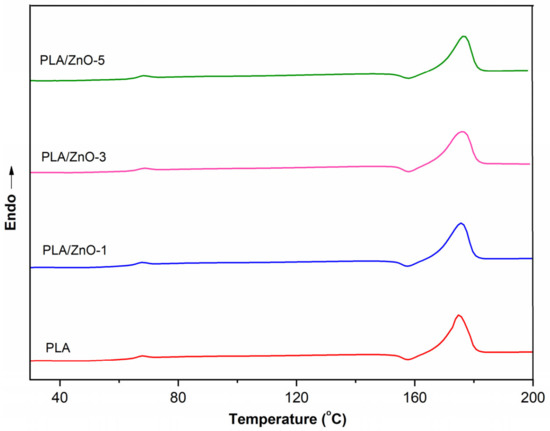
Figure 4.
DSC thermograms of neat PLA and PLA/ZnO biocomposite filaments.

Table 1.
Thermal properties of 3D printing filaments from neat PLA and PLA composited with ZnO nanoflowers at varying contents.
Moreover, the Tm value of PLA/ZnO-5 was shifted a bit towards higher temperatures compared with PLA/ZnO-3 and PLA/ZnO-1, respectively, as shown in Figure 4. This behavior was due to the distribution of ZnO nanoflowers in the PLA matrix, relating to the degree of crystallization via the nucleating efficiency process [34]. However, the ZnO nanoflowers content did not show a significant influence on the thermal properties of the filaments of PLA composited with ZnO nanoflowers. There were no significant changes in the thermal properties of the PLA/ZnO biocomposite filaments. This means that ZnO nanoflowers have little influence on the intermolecular interactions or chain flexibility of PLA polymer chains, in agreement with the result of Müller and his colleagues [35]. However, we found that the degrees of the crystallinity of the PLA/ZnO biocomposite filaments increased when compared with those of the neat PLA. Additionally, they slightly decreased when increasing the ZnO nanoflower contents. This means that the PLA/ZnO biocomposite filaments became amorphous and were more liable to undergo higher ZnO nanoflower contents, which might be from the dispersibility of nanofillers within the matrix of PLA.
3.4. Tensile Testing
All the 3D printing filaments were fabricated into specimens for tensile testing, according to the ASTM D638-14 type IV [36], as shown in Figure 5. The stress-strain curves for all specimens were obtained and are shown in Figure 6. From these curves, the tensile strength, modulus, and elongation at break were obtained. The mechanical properties of the PLA/ZnO biocomposite filaments are presented in Figure 7 and Table 2. The tensile strength of composite materials generally relies on the stress transfer capacity between fillers and matrices, which are significantly affected by their interfacial adhesion [37,38]. We found that the tensile strength of the PLA/ZnO biocomposite filaments gradually decreased with increasing ZnO nanoflower contents (Figure 6a). The PLA/ZnO-5 encountered a decrease in tensile strength because of the higher ZnO nanoflower contents, caused by filler agglomeration during the melt extrusion process, resulting in poor interfacial adhesion of particles and matrix [39,40]. From Table 2, the modulus strength of the PLA/ZnO biocomposite filaments decreased when the ZnO nanoflower contents increased. We found that PLA/ZnO-1 exhibited the highest elongation at break among the four specimens (53.46% higher than that of PLA/ZnO-5 (Figure 6c)). This result is in agreement with the result of the thermal properties, relating to the degree of distribution of nanodomain within polymer matrices [41].
Figure 5.
Optical images of ASTM D638-14 type IV tensile test specimens of (a) neat PLA, (b) PLA/ZnO-1, (c) PLA/ZnO-3, and (d) PLA/ZnO-5, respectively.
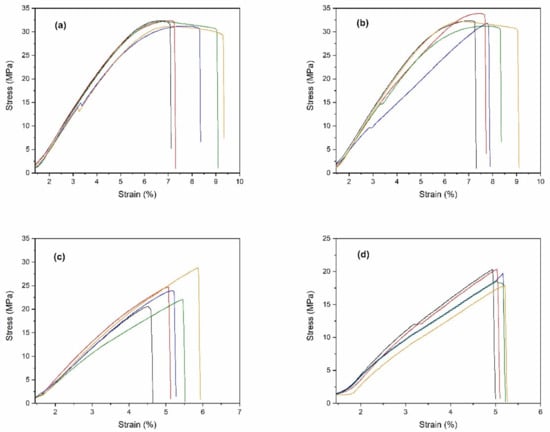
Figure 6.
Stress-strain curves of: (a) neat PLA, (b) PLA/ZnO-1, (c) PLA/ZnO-3, and (d) PLA/ZnO-5, respectively. Each curve indicates the sample number.
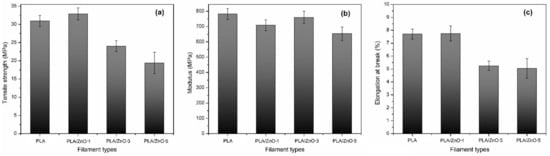
Figure 7.
Effect of varying ZnO nanoflower contents on the mechanical properties: tensile strength (a), modulus strength (b), and elongation at break (c), of PLA and PLA/ZnO biocomposite filaments.

Table 2.
Mechanical properties of 3D printing filaments from neat PLA and PLA composited with ZnO nanoflowers at varying contents.
3.5. Antibacterial Testing
As a potential application, the neat PLA and PLA/ZnO biocomposite filaments were tested as a bactericidal action. Antibacterial activities were performed by agar well-diffusion and colony unit counting methods against Gram-positive (S. aureus) and Gram-negative (E. coli) pathogenic bacteria, as depicted in Figure 8, and the detailed results of the antibacterial efficacies are shown in Table 3. From the experimental results, the sizes of the inhibition zones were varied with respect to the type of bacterial strain. This is probably due to the differences in the bacterial surface characteristics. It is well known that Gram-positive and Gram-negative bacteria are different when observing their cell walls. Gram-positive bacteria have a thick, multi-layered peptidoglycan (negatively charged), no lipopolysaccharide, and the presence of teichoic acid. In contrast, Gram-negative bacteria have a thin, single-layered peptidoglycan, containing high lipopolysaccharides and an absence of teichoic acid. All these different cell surface compositions result in explicit differences in the sensitivity of bacteria to ZnO nanoflowers [42,43,44].
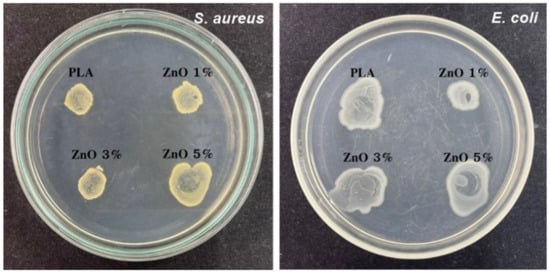
Figure 8.
Inhibition zones of antibacterial activities of neat PLA and PLA/ZnO biocomposite filaments.

Table 3.
Antibacterial efficacies of PLA/ZnO composite filaments.
More precisely, the results showed that the percent antibacterial efficacies that the colony unit counting method depicted depended on the ZnO nanoflower contents within the PLA matrix. The percent antibacterial efficacies enhanced when increasing the ZnO nanoflower contents in both Gram-positive and Gram-negative bacteria, as shown in Figure 9. Additionally, it was observed that the numbers of viable bacteria cells of S. aureus exhibited lower than those of E. coli at the given ZnO nanoflower contents. These findings are in good agreement with the reported studies [45,46,47]. It is well known that the antibacterial activity of ZnO nanoflowers is dependent on their contents, and that a higher content of ZnO nanoflowers shows higher bactericidal activity owing to their higher surface-to-volume ratios for reacting with bacterium cells [42,48,49].
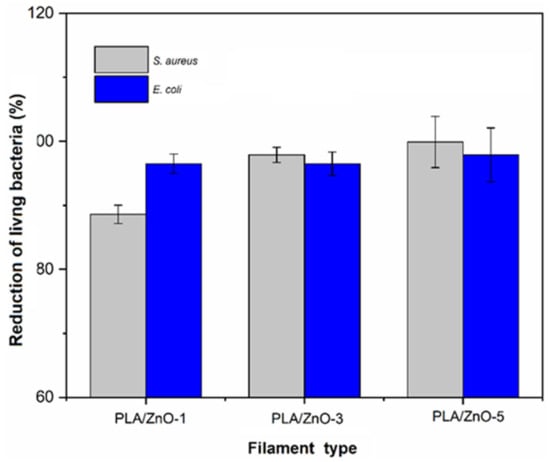
Figure 9.
Percent antimicrobial efficacies for S. aureus and E. coli of PVA/ZnO composite filaments.
4. Conclusions
The antibacterial functionality of PLA-composited ZnO nanoflowers was assessed at the various concentrations, and 3D printing filaments were successfully fabricated via a single-screw extrusion technique. We found that the contents of ZnO nanoflowers incorporated with the PLA matrix did not significantly affect the thermal and surface-morphological properties. The Tg and Tm values of the 3D printing filaments from PLA composited with ZnO nanoflowers shifted a bit towards higher temperatures when compared with those from the neat PLA. However, they encountered a decrease in tensile strength because of higher ZnO nanoflower contents, caused by filler agglomeration during the melted extrusion process, which then resulted in poor interfacial adhesion of ZnO nanoflowers and the PLA matrix. Investigating an antibacterial property of 3D printing filaments is of the utmost importance. We found that the antibacterial efficacy directly depended on the ZnO nanoflower contents. The antibacterial activities increased with increasing ZnO nanoflower contents, and these 3D printing filaments were better against Gram-positive than Gram-negative bacteria because of differences in their cell walls. We tentatively suggest that our results indicate the possibility that the ZnO nanoflower could be released from the PLA filaments. Further investigations into this possibility are currently underway. Additionally, the functional 3D print with the proposed composition will be studied, and we intend to address this phenomenon in detail in a future publication. Finally, FTIR spectra indicated that the ZnO nanoflowers were merely distributed in the PLA matrix without any chemical bonding of ZnO molecules with PLA chains, but entirely dispersed in the PLA matrix. This study showed that these biocomposite 3D printing filaments based on PLA and ZnO nanoflowers can be applied in biomedical fields.
Author Contributions
Conceptualization, T.J. and C.-F.H.; methodology and investigation, O.J., A.K., A.L. and N.S.; physical tests and analysis, A.P., P.P., R.M. and T.J.; writing—original draft, T.J. and O.J.; writing—review and editing, A.W. and C.-F.H. All authors have read and agreed to the published version of the manuscript.
Funding
The present work was financially co-supported by the Program Management Unit: Competitiveness (PMUC), the Office of National Higher Education Science Research and Innovation Policy Council, Thailand, grant number C10F630034, and the KU-NCHU joint research project, grant number 00042021. We also acknowledge the Faculty of Science at Sriracha, Kasetsart University for providing funding and convenient laboratories.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that there have been no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Leigh, S.J.; Bradley, R.J.; Purssell, C.P.; Billson, D.R.; Hutchins, D.A. A simple, low-cost conductive composite material for 3D printing of electronic sensors. PLoS ONE 2012, 7, e49365. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Snider, B.; Clarke, T.; Kozutsky, S.; Lacki, M.; Hosseini, A. Large-scale 3D printers for additive manufacturing: Design considerations and challenges. Int. J. Adv. Manuf. Technol. 2019, 104, 3679–3693. [Google Scholar] [CrossRef]
- Kroll, E.; Artzi, D. Enhancing aerospace engineering students’ learning with 3D printing wind-tunnel models. Rapid Prototyp. J. 2011, 17, 393–402. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Guo, Y.; Chang, C.C.; Halada, G.; Cuiffo, M.A.; Xue, Y.; Zuo, X.; Pack, S.; Zhang, L.; He, S.; Weil, E.; et al. Engineering flame retardant biodegradable polymer nanocomposites and their application in 3D printing. Polym. Degrad. Stab. 2017, 137, 205–215. [Google Scholar] [CrossRef]
- Han, Y.M.; Chen, H.H.; Huang, C.F. Polymerization and degradation of aliphatic polyesters synthesized by atom transfer radical polyaddition. Polym. Chem. 2015, 6, 4565–4574. [Google Scholar] [CrossRef]
- Huang, Y.S.; Chen, J.K.; Kuo, S.W.; Hsieh, Y.A.; Yamamoto, S.; Nakanishi, J.; Huang, C.F. Synthesis of poly(N-vinylpyrrolidone)-based polymer bottlebrushes by ATRPA and RAFT polymerization: Toward drug delivery application. Polymers 2019, 11, 1079. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.C.; Chou, L.C.; Huang, C.F. Iron-catalysed atom transfer radical polyaddition for the synthesis and modification of novel aliphatic polyesters displaying lower critical solution temperature and pH-dependent release behaviors. Polym. Chem. 2019, 10, 3912–3921. [Google Scholar] [CrossRef]
- Lin, S.T.; Wang, C.C.; Chang, C.J.; Nakamura, Y.; Lin, K.Y.A.; Huang, C.F. Progress in the preparation of functional and (bio)degradable polymers via living polymerizations. Int. J. Mol. Sci. 2020, 21, 9581. [Google Scholar] [CrossRef]
- Anderson, I. Mechanical properties of specimens 3D printed with virgin and recycled polylactic acid. 3D Print. Addit. Manuf. 2017, 4, 110–115. [Google Scholar] [CrossRef]
- Mikula, K.; Skrzypczak, D.; Izydorczyk, G.; Warchoł, J.; Moustakas, K.; Chojnacka, K.; Witek-Krowiak, A. 3D printing filament as a second life of waste plastics-a review. Environ. Sci. Pollut. Res. 2021, 28, 12321–12333. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Müller, M.B.; Gilmore, K.J.; Wallace, G.G.; Li, D. Mechanically strong, electrically conductive, and biocompatible graphene paper. Adv. Mater. 2008, 20, 3557–3561. [Google Scholar] [CrossRef]
- Chen, R.D.; Huang, C.F.; Hsu, S.H. Composites of waterborne polyurethane and cellulose nanofibers for 3Dprinting and bioapplications. Carbohyd. Polym. 2019, 212, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Wang, J.; Senthil, T.; Wu, L. Mechanical and thermal properties of ABS/montmorillonite nanocomposites for fused deposition modeling 3D printing. Mater. Des. 2016, 102, 276–283. [Google Scholar] [CrossRef]
- Jamnongkan, T.; Wattanakornsiri, A.; Pansila, P.; Migliaresi, C.; Kaewpirom, S. Effect of poly(vinyl alcohol)/chitosan ratio on electrospun-nanofiber morphologies. Adv. Mat. Res. 2012, 463–464, 734–738. [Google Scholar] [CrossRef]
- Christ, S.; Schnabel, M.; Vorndran, E.; Groll, J.; Gbureck, U. Fiber reinforcement during 3D printing. Mater. Lett. 2015, 139, 165–168. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Liu, S. Rapid prototyping of continuous carbon fiber reinforced polylactic acid composites by 3D printing. J. Mater. Process. Technol. 2016, 238, 218–225. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Q.; Wang, Y.; Yu, H.; Chen, X.; Jing, X. Biodegradable electrospun poly(L-lactide) fibers containing antibacterial silver nanoparticles. Eur. Polym. J. 2006, 42, 2081–2087. [Google Scholar] [CrossRef]
- Kim, I.; Viswanathan, K.; Kasi, G.; Sadeghi, K.; Thanakkasaranee, S.; Seo, J. Poly(lactic acid)/ZnO bionanocomposite films with positively charged ZnO as potential antimicrobial food packaging materials. Polymers 2019, 11, 1427. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, N.; Hayashi, T. Preparation and characterization of poly(L-lactic acid)/TiO2 nanoparticle nanocomposite films with high transparency and efficient photodegradability. Polym. Degrad. Stab. 2007, 92, 1255–1264. [Google Scholar] [CrossRef]
- Imran, M.; Haider, S.; Ahmad, K.; Mahmood, A.; Al-masry, W.A. Fabrication and characterization of zinc oxide nanofibers for renewable energy applications. Arab. J. Chem. 2017, 10, S1067–S1072. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Dong, Z.; Jenness, N.J.; Clark, R.L. In-situ formation of Cu metal crystals within nanostructured ZnO electrospun fibers. Mater. Lett. 2011, 65, 2683–2685. [Google Scholar] [CrossRef]
- Guo, J.; Song, Y.; Chen, D.; Jiao, X. Fabrication of ZnO nanofibers by electrospinning and electrical properties of a single nanofiber. J. Dispers. Sci. Technol. 2010, 31, 684–689. [Google Scholar] [CrossRef]
- Fontecha-Umaña, F.; Ríos-Castillo, A.G.; Ripolles-Avila, C.; Rodríguez-Jerez, J.J. Antimicrobial activity and prevention of bacterial biofilm formation of silver and zinc oxide nanoparticle-containing polyester surfaces at various concentrations for use. Foods 2020, 9, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayyif, S.M.I.; Mohammed, H.B.; Curuțiu, C.; Bîrcă, A.C.; Grumezescu, A.M.; Vasile, B.S.; Ditu, L.M.; Lazăr, V.; Chifiriuc, M.C.; Mihăescu, G.; et al. ZnO nanoparticles-modified dressings to inhibit wound pathogens. Materials 2021, 14, 3084. [Google Scholar] [CrossRef] [PubMed]
- Khankhuean, A.; Kuratsameethong, W.; Santibenchakul, S.; Laobuthee, A.; Sugimoto, M.; Srisawat, N.; Jamnongkan, T. Oriented ZnO nanoflowers obtained after calcination of electrospinning poly(vinyl alcohol)/zinc oxide/zinc acetate composite mats. S. Afr. J. Chem. Eng. 2021, 37, 179–185. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Frone, A.N.; Chiulan, I.; Gabor, R.A.; Spataru, I.C.; Căşărică, A. Biocomposites from polylactic acid and bacterial cellulose nanofibers obtained by mechanical treatment. BioResources 2017, 12, 662–672. [Google Scholar] [CrossRef]
- Dong, J.; Li, M.; Zhou, L.; Lee, S.; Mei, C.; Xu, X.; Wu, Q. The influence of grafted cellulose nanofibers and postextrusion annealing treatment on selected properties of poly(lactic acid) filaments for 3D printing. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 847–855. [Google Scholar] [CrossRef]
- Handore, K.; Bhavsar, S.; Horne, A.; Chhattise, P.; Mohite, K.; Ambekar, J.; Pande, N.; Chabukswar, V. Novel green route of synthesis of ZnO nanoparticles by using natural biodegradable polymer and its application as a catalyst for oxidation of aldehydes. J. Macromol. Sci. A 2014, 51, 941–947. [Google Scholar] [CrossRef]
- Thirugnanam, T. Effect of polymers (PEG and PVP) on sol-gel synthesis of microsized zinc oxide. J. Nanomater. 2013, 2013, 362175. [Google Scholar] [CrossRef]
- Quadri, T.W.; Olasunkanmi, L.O.; Fayemi, O.E.; Solomon, M.M.; Ebenso, E.E. Zinc oxide nanocomposites of selected polymers: Synthesis, characterization, and corrosion inhibition studies on mild steel in HCl solution. ACS Omega 2017, 2, 8421–8437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riba, J.R.; Cailloux, J.; Cantero, R.; Canals, T.; Maspoch, M.L. Multivariable methods applied to FTIR: A powerful technique to highlight architectural changes in poly(lactic acid). Polym. Test. 2018, 65, 264–269. [Google Scholar] [CrossRef]
- Greco, A.; Ferrari, F. Thermal behavior of PLA plasticized by commercial and cardanol-derived plasticizers and the effect on the mechanical properties. J. Therm. Anal. Calorim. 2021, 146, 131–141. [Google Scholar] [CrossRef]
- Ortenzi, M.A.; Basilissi, L.; Farina, H.; Di Silvestro, G.; Piergiovanni, L.; Mascheroni, E. Evaluation of crystallinity and gas barrier properties of films obtained from PLA nanocomposites synthesized via “in situ” polymerization of l-lactide with silane-modified nanosilica and montmorillonite. Eur. Polym. J. 2015, 66, 478–491. [Google Scholar] [CrossRef]
- Müller, A.J.; Ávila, M.; Saenz, G.; Salazar, J. CHAPTER 3. Crystallization of PLA-based Materials. In Polymer Chemistry Series; Jiménez, A., Peltzer, M., Ruseckaite, R., Eds.; Royal Society of Chemistry: Cambridge, UK, 2014; pp. 66–98. ISBN 978-1-84973-879-8. [Google Scholar]
- ASTM D638-14; Standard Test Methods for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2010.
- Fu, S.Y.; Feng, X.Q.; Lauke, B.; Mai, Y.W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Wu, C.L.; Zhang, M.Q.; Rong, M.Z.; Friedrich, K. Silica nanoparticles filled polypropylene: Effects of particle surface treatment, matrix ductility and particle species on mechanical performance of the composites. Compos. Sci. Technol. 2005, 65, 635–645. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Chow, L.C.; Yang, M.; Mitchell, J.W. Effects of inorganic fillers on the thermal and mechanical properties of poly(lactic acid). Int. J. Polym. Sci. 2014, 2014, 827028. [Google Scholar] [CrossRef] [PubMed]
- El-Fattah, A.A.; Youssef, H.; Gepreel, M.A.H.; Abbas, R.; Kandil, S. Surface morphology and mechanical properties of polyether ether ketone (PEEK) nanocomposites reinforced by nano-sized silica (SiO2) for prosthodontics and restorative dentistry. Polymers 2021, 13, 3006. [Google Scholar] [CrossRef]
- Kotsilkova, R.; Petrova-Doycheva, I.; Menseidov, D.; Ivanov, E.; Paddubskaya, A.; Kuzhir, P. Exploring thermal annealing and graphene-carbon nanotube additives to enhance crystallinity, thermal, electrical and tensile properties of aged poly(lactic) acid-based filament for 3D printing. Compos. Sci. Technol. 2019, 181, 107712. [Google Scholar] [CrossRef]
- Rutherford, D.; Jíra, J.; Kolářová, K.; Matolínová, I.; Mičová, J.; Remeš, Z.; Rezek, B. Growth inhibition of Gram-positive and Gram-negative bacteria by zinc oxide hedgehog particles. Int. J. Nanomed. 2021, 16, 3541–3554. [Google Scholar] [CrossRef]
- Tayel, A.A.; El-Tras, W.F.; Moussa, S.; El-Baz, A.F.; Mahrous, H.; Salem, M.F.; Brimer, L. Antibacterial action of zinc oxide nanoparticles against foodborne pathogens. J. Food Saf. 2011, 31, 211–218. [Google Scholar] [CrossRef]
- Jamnongkan, T.; Sukumaran, S.K.; Sugimoto, M.; Hara, T.; Takatsuka, Y.; Koyama, K. Towards novel wound dressings: Antibacterial properties of zinc oxide nanoparticles and electrospun fiber mats of zinc oxide nanoparticle/poly (vinyl alcohol) hybrids. J. Polym. Eng. 2015, 35, 575–586. [Google Scholar] [CrossRef]
- Siddique, S.; Shah, Z.H.; Shahid, S.; Yasmin, F. Preparation, characterization and antibacterial activity of ZnO nanoparticles on broad spectrum of microorganisms. Acta Chim. Slov. 2013, 60, 660–665. [Google Scholar] [PubMed]
- Yusof, N.A.A.; Zain, N.M.; Pauzi, N. Synthesis of ZnO nanoparticles with chitosan as stabilizing agent and their antibacterial properties against Gram-positive and Gram-negative bacteria. Int. J. Biol. Macromol. 2019, 124, 1132–1136. [Google Scholar] [CrossRef]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murthy, H.C.A. A Review on enhancing the antibacterial activity of ZnO: Mechanisms and microscopic investigation. Nanoscale Res. Lett. 2020, 15, 190. [Google Scholar] [CrossRef]
- Kavitha, T.; Gopalan, A.I.; Lee, K.P.; Park, S.Y. Glucose sensing, photocatalytic and antibacterial properties of graphene–ZnO nanoparticle hybrids. Carbon 2012, 50, 2994–3000. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanopart. Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).