Unraveling of Advances in 3D-Printed Polymer-Based Bone Scaffolds
Abstract
1. Introduction
2. Process, Advantages, and Disadvantages of 3D Printing Technology
2.1. Laser-Assisted Printing
2.1.1. SLA
2.1.2. SLS
2.1.3. LAB
2.2. Non-Laser-Assisted Printing
2.2.1. Extrusion Bioprinting
2.2.2. Inkjet Bioprinting
2.2.3. FDM
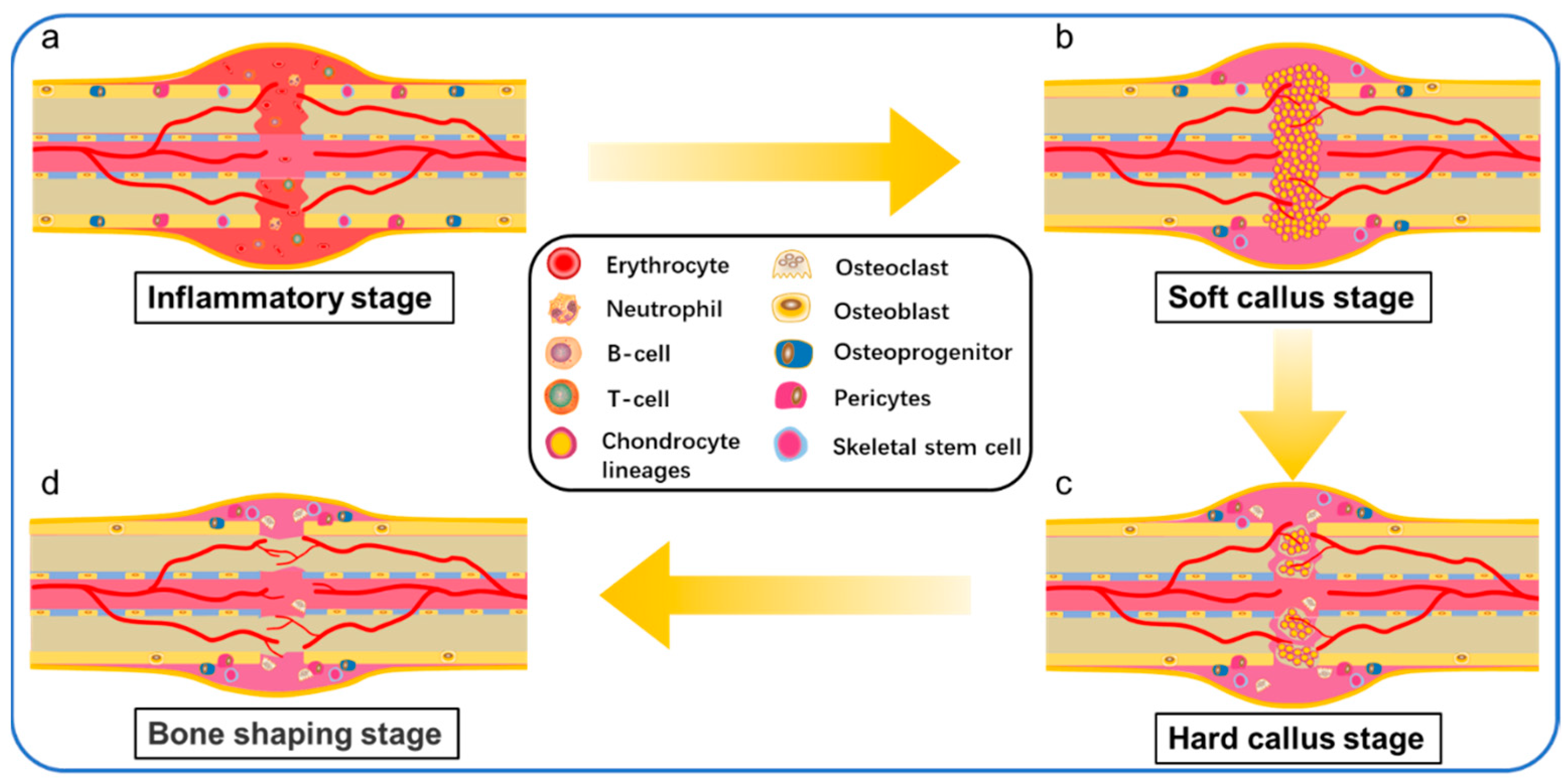
3. The Process of Bone Healing
4. Properties of Ideal Bone Scaffolds
4.1. Biocompatibility
4.2. Biodegradability
4.3. Mechanical Properties
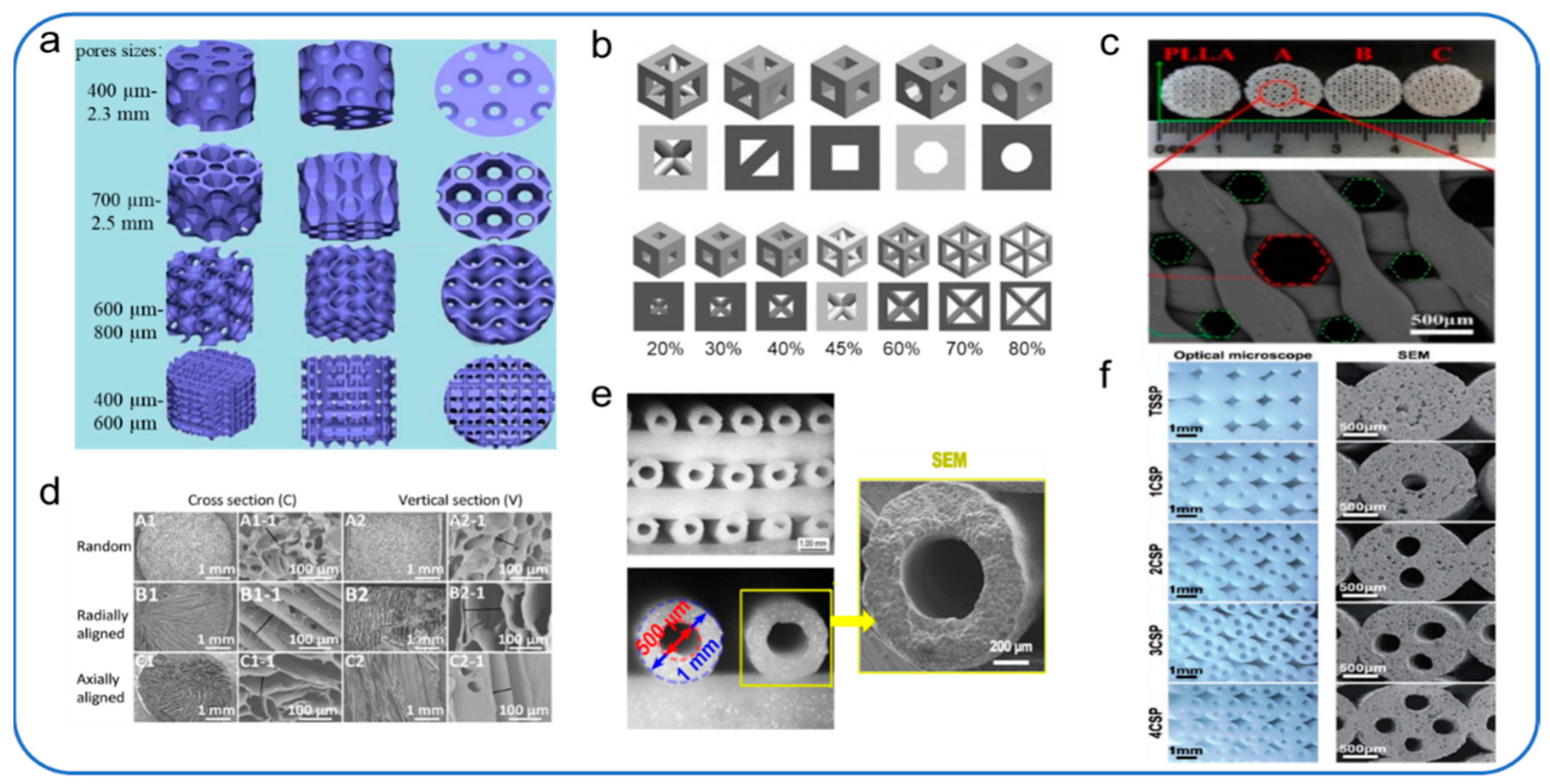
4.4. Microstructures
4.4.1. Porosity
4.4.2. Pore Size
4.4.3. Pore Structure
5. Polymer Material of 3D-Printed Bone Scaffolds
5.1. Natural Polymers and Mixtures Based on Natural Polymers
5.1.1. Chitin and Chitosan
5.1.2. Alginate
5.1.3. Collagen
5.1.4. Gelatin
5.1.5. Hyaluronic Acid
5.1.6. Cellulose
5.2. Synthetic Polymers and Mixtures Based on Synthetic Polymers
5.2.1. Acrylonitrile Butadiene Styrene
5.2.2. Polylactic Acid
5.2.3. Polycaprolactone
5.2.4. Polycarbonate
5.2.5. Polyetheretherketone
5.2.6. Polypropylene
5.2.7. Polyamide
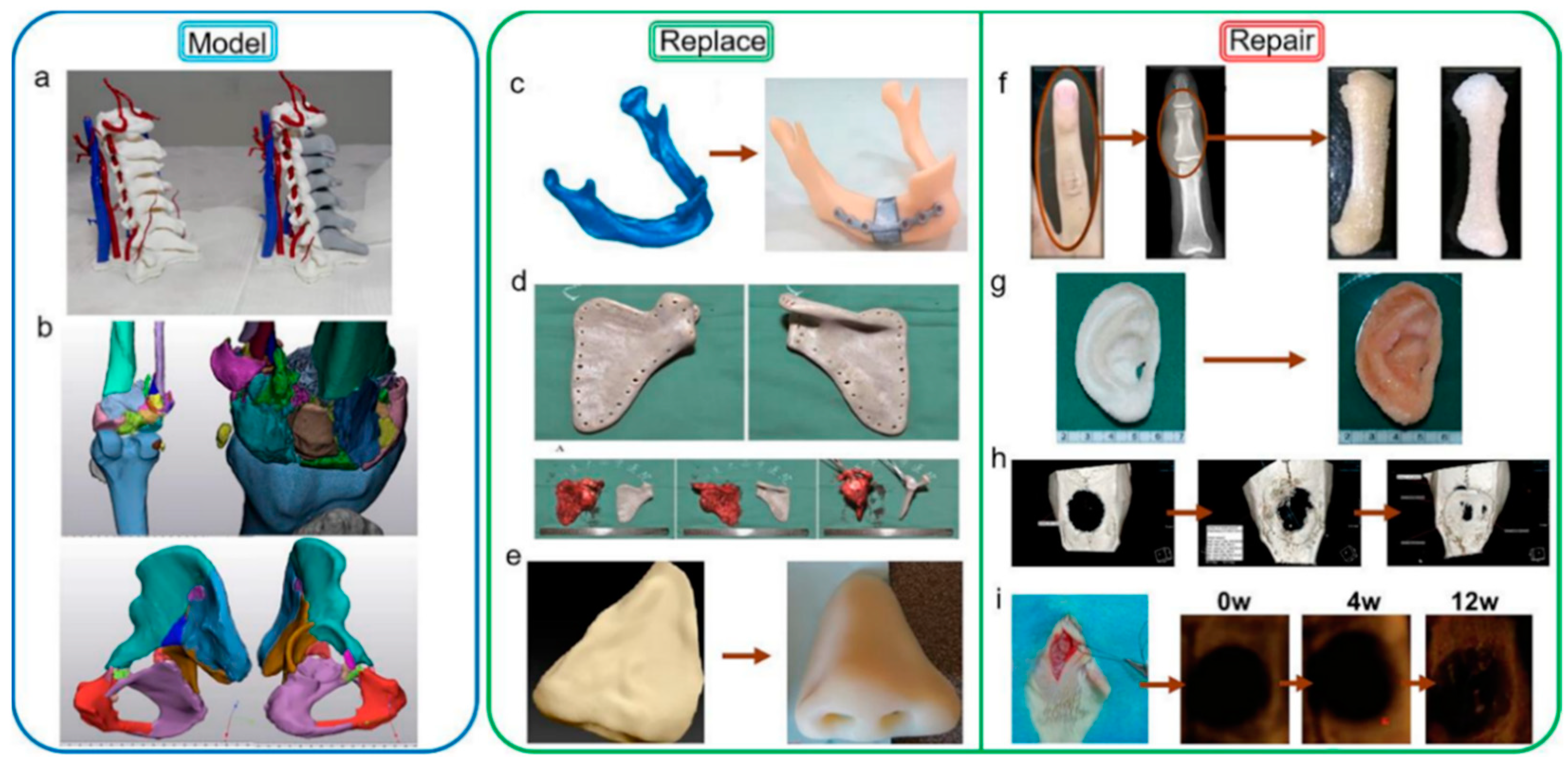
6. 3D-Printing for Medical Applications
6.1. Bone Model
6.2. Bone Replacement

6.3. Bone Repair
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Tuan Rahim, T.N.A.; Abdullah, A.; Md Akil, H.; Mohamad, D.; Rajion, Z. The improvement of mechanical and thermal properties of polyamide 12 3D printed parts by fused deposition modelling. Express Polym. Lett. 2017, 11, 963–982. [Google Scholar] [CrossRef]
- Bao, X.; Zhu, L.; Huang, X.; Tang, D.; He, D.; Shi, J.; Xu, G. 3D biomimetic artificial bone scaffolds with dual-cytokines spatiotemporal delivery for large weight-bearing bone defect repair. Sci. Rep. 2017, 7, 7814. [Google Scholar] [CrossRef]
- Biggemann, J.; Pezoldt, M.; Stumpf, M.; Greil, P.; Fey, T. Modular ceramic scaffolds for individual implants. Acta Biomater. 2018, 80, 390–400. [Google Scholar] [CrossRef]
- Pape, H.C.; Evans, A.; Kobbe, P. Autologous Bone Graft: Properties and Techniques. J. Orthop. Trauma 2010, 24, 36–40. [Google Scholar] [CrossRef]
- Larsen, M.; Pelzer, M.; Friedrich, P.F.; Wood, C.M.; Bishop, A.T. Living bone allotransplants survive by surgical angiogenesis alone: Development of a novel method of composite tissue allotransplantation. J. Bone Jt. Surg. Am. 2011, 93, 261–273. [Google Scholar] [CrossRef][Green Version]
- Wee, J.; Thevendran, G. The role of orthobiologics in foot and ankle surgery. EFORT Open Rev. 2017, 2, 272–280. [Google Scholar] [CrossRef]
- Lee, S.C.; Jung, K.A.; Nam, C.H.; Jung, S.H.; Hwang, S.H. The Short-term Follow-up Results of Open Wedge High Tibial Osteotomy with Using an Aescula Open Wedge Plate and an Allogenic Bone Graft: The Minimum 1-Year Follow-up Results. CIOS 2010, 2, 47–54. [Google Scholar] [CrossRef]
- Buza, J.A., 3rd; Einhorn, T. Bone healing in 2016. Clin. Cases Miner. Bone Metab. 2016, 13, 101–105. [Google Scholar] [CrossRef]
- Yuan, H.; Fernandes, H.; Habibovic, P.; de Boer, J.; Barradas, A.M.C.; de Ruiter, A.; Walsh, W.R.; van Blitterswijk, C.A.; de Bruijn, J.D. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc. Natl. Acad. Sci. USA 2010, 107, 13614–13619. [Google Scholar] [CrossRef]
- Reichert, J.C.; Cipitria, A.; Epari, D.R.; Saifzadeh, S.; Krishnakanth, P.; Berner, A.; Woodruff, M.A.; Schell, H.; Mehta, M.; Schuetz, M.A.; et al. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Sci. Transl. Med. 2012, 4, 141ra193. [Google Scholar] [CrossRef]
- Bez, M.; Sheyn, D.; Tawackoli, W.; Avalos, P.; Shapiro, G.; Giaconi, J.C.; Da, X.; David, S.B.; Gavrity, J.; Awad, H.A.; et al. In situ bone tissue engineering via ultrasound-mediated gene delivery to endogenous progenitor cells in mini-pigs. Sci. Transl. Med. 2017, 9, 390. [Google Scholar] [CrossRef]
- Ito, H.; Koefoed, M.; Tiyapatanaputi, P.; Gromov, K.; Goater, J.J.; Carmouche, J.; Zhang, X.; Rubery, P.T.; Rabinowitz, J.; Samulski, R.J.; et al. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat. Med. 2005, 11, 291–297. [Google Scholar] [CrossRef]
- Nagarajan, S.; Belaid, H.; Radhakrishnan, S.; Teyssier, C.; Balme, S.; Miele, P.; Cornu, D.; Subbaraya, N.K.; Cavaillès, V.; Bechelany, M. Sacrificial mold-assisted 3D printing of stable biocompatible gelatin scaffolds. Bioprinting 2021, 22, 140. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Wang, G.; Zhu, P.; Gao, C. Recent Progress on 3D-Printed Polylactic Acid and Its Applications in Bone Repair. Adv. Eng. Mater. 2019, 22, 4. [Google Scholar] [CrossRef]
- Mishra, R.; Varshney, R.; Das, N.; Sircar, D.; Roy, P. Synthesis and characterization of gelatin-PVP polymer composite scaffold for potential application in bone tissue engineering. Eur. Polym. J. 2019, 119, 155–168. [Google Scholar] [CrossRef]
- Haaparanta, A.M.; Jarvinen, E.; Cengiz, I.F.; Ella, V.; Kokkonen, H.T.; Kiviranta, I.; Kellomaki, M. Preparation and characterization of collagen/PLA, chitosan/PLA, and collagen/chitosan/PLA hybrid scaffolds for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2014, 25, 1129–1136. [Google Scholar] [CrossRef]
- Garot, C.; Bettega, G.; Picart, C. Additive Manufacturing of Material Scaffolds for Bone Regeneration: Toward Application in the Clinics. Adv. Funct. Mater. 2021, 31, 2006967. [Google Scholar] [CrossRef]
- Yin, G.-B.; Zhang, Y.-Z.; Wang, S.-D.; Shi, D.-B.; Dong, Z.-H.; Fu, W.-G. Study of the electrospun PLA/silk fibroin-gelatin composite nanofibrous scaffold for tissue engineering. J. Biomed. Mater. Res. Part A 2010, 93, 158–163. [Google Scholar] [CrossRef]
- Ng, J.; Spiller, K.; Bernhard, J.; Vunjak-Novakovic, G. Biomimetic Approaches for Bone Tissue Engineering. Tissue Eng. Part B Rev. 2016, 23, 480–493. [Google Scholar] [CrossRef]
- Tertuliano, O.; Greer, J. The nanocomposite nature of bone drives its strength and damage resistance. Nat. Mater. 2016, 15, 1195–1202. [Google Scholar] [CrossRef]
- Wegst, U.G.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2014, 14, 23–36. [Google Scholar] [CrossRef]
- Shahrousvand, M.; Ghollasi, M.; Zarchi AA, K.; Salimi, A. Osteogenic differentiation of hMSCs on semi-interpenetrating polymer networks of polyurethane/poly(2-hydroxyethyl methacrylate)/cellulose nanowhisker scaffolds. Int. J. Biol. Macromol. 2019, 138, 262–271. [Google Scholar] [CrossRef]
- Chen, S.; McCarthy, A.; John, J.V.; Su, Y.; Xie, J. Converting 2D Nanofiber Membranes to 3D Hierarchical Assemblies with Structural and Compositional Gradients Regulates Cell Behavior. Adv. Mater. 2020, 32, 2003754. [Google Scholar] [CrossRef]
- Cunniffe, G.M.; Díaz-Payno, P.J.; Sheehy, E.J.; Critchley, S.E.; Almeida, H.V.; Pitacco, P.; Carroll, S.F.; Mahon, O.R.; Dunne, A.; Levingstone, T.J.; et al. Tissue-specific extracellular matrix scaffolds for the regeneration of spatially complex musculoskeletal tissues. Biomaterials 2018, 188, 63–73. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Zamparini, F.; Degli Esposti, M.; Chiellini, F.; Fava, F.; Fabbri, P.; Taddei, P.; Prati, C. Highly porous polycaprolactone scaffolds doped with calcium silicate and dicalcium phosphate dihydrate designed for bone regeneration. Mater. Sci. Eng. C 2019, 102, 341–361. [Google Scholar] [CrossRef]
- Pavia, F.C.; Conoscenti, G.; Greco, S.; La Carrubba, V.; Ghersi, G.; Brucato, V. Preparation, characterization and in vitro test of composites poly-lactic acid/hydroxyapatite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2018, 119, 945–953. [Google Scholar] [CrossRef]
- Cheng, G.; Yin, C.; Tu, H.; Jiang, S.; Wang, Q.; Zhou, X.; Xing, X.; Xie, C.; Shi, X.; Du, Y.; et al. Controlled Co-delivery of Growth Factors through Layer-by-Layer Assembly of Core-Shell Nanofibers for Improving Bone Regeneration. ACS Nano 2019, 13, 6372–6382. [Google Scholar] [CrossRef]
- Horner, C.B.; Maldonado, M.; Tai, Y.; Rony, R.M.I.K.; Nam, J. Spatially Regulated Multiphenotypic Differentiation of Stem Cells in 3D via Engineered Mechanical Gradient. ACS Appl. Mater. Interfaces 2019, 11, 45479–45488. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, K.; Wang, K.; Pei, X.; Dong, Z.; Hong, Y.; Zhang, X. Combination of fused deposition modeling and gas foaming technique to fabricated hierarchical macro/microporous polymer scaffolds. Mater. Des. 2016, 109, 415–424. [Google Scholar] [CrossRef]
- Mao, D.; Li, Q.; Bai, N.; Dong, H.; Li, D. Porous stable poly(lactic acid)/ethyl cellulose/hydroxyapatite composite scaffolds prepared by a combined method for bone regeneration. Carbohydr. Polym. 2017, 180, 104–111. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A. Current status and challenges of Additive manufacturing in orthopaedics: An overview. J. Clin. Orthop. Trauma 2019, 10, 380–386. [Google Scholar] [CrossRef]
- Melchels, F.P.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D printing of polymer matrix composites: A review and prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Lai, W.; Wang, Y.; Fu, H.; He, J. Hydroxyapatite/polyetheretherketone nanocomposites for selective laser sintering: Thermal and mechanical performances. e-Polymers 2020, 20, 542–549. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, J.; Yan, M.; Tian, X. Poly ether ether ketone and its composite powder prepared by thermally induced phase separation for high temperature selective laser sintering. Mater. Des. 2021, 201, 109510. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Hasan, A.; Kaarela, O.; Byambaa, B.; Sheikhi, A.; Gaharwar, A.K.; Khademhosseini, A. Advancing Frontiers in Bone Bioprinting. Adv. Healthc. Mater. 2019, 8, 1801048. [Google Scholar] [CrossRef]
- Hakobyan, D.; Kerouredan, O.; Remy, M.; Dusserre, N.; Medina, C.; Devillard, R.; Fricain, J.-C.; Oliveira, H. Laser-assisted bioprinting for bone repair. In 3D Bioprinting; Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.S.; Heinrich, M.A.; De Ferrari, F.; Jang, H.L.; Bakht, S.M.; Alvarez, M.M.; Yang, J.; Li, Y.-C.; de Santiago, G.T.; et al. Rapid Continuous Multimaterial Extrusion Bioprinting. Adv. Mater. 2016, 29, 3. [Google Scholar] [CrossRef]
- Miri, A.K.; Mirzaee, I.; Hassan, S.; Oskui, S.M.; Nieto, D.; Khademhosseini, A.; Zhang, Y.S. Effective bioprinting resolution in tissue model fabrication. Lab Chip 2019, 19, 2019–2037. [Google Scholar] [CrossRef]
- Emmermacher, J.; Spura, D.; Cziommer, J.; Kilian, D.; Wollborn, T.; Fritsching, U.; Steingroewer, J.; Walther, T.; Gelinsky, M.; Lode, A. Engineering considerations on extrusion-based bioprinting: Interactions of material behaviour, mechanical forces and cells in the printing needle. Biofabrication 2020, 12, 2. [Google Scholar] [CrossRef]
- Genova, T.; Roato, I.; Carossa, M.; Motta, C.; Cavagnetto, D.; Mussano, F. Advances on Bone Substitutes through 3D Bioprinting. Int. J. Mol. Sci. 2020, 21, 7012. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.H.; Cui, B.; Zheng, T.F.; Wang, C.H. Ultrasonic manipulation of cells for alleviating the clogging of extrusion-based bioprinting nozzles. J. Phys. Conf. Ser. 2021, 1798, 012009. [Google Scholar] [CrossRef]
- Takagi, D.; Lin, W.; Matsumoto, T.; Yaginuma, H.; Hemmi, N.; Hatada, S.; Seo, M. High-precision 3D inkjet technology for live cell bioprinting. Int. J. Bioprint. 2019, 5, 208. [Google Scholar] [CrossRef] [PubMed]
- Saunders, R.; Derby, B. Inkjet printing biomaterials for tissue engineering: Bioprinting. Int. Mater. Rev. 2014, 59, 430–448. [Google Scholar] [CrossRef]
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet Bioprinting of Biomaterials. Chem. Rev. 2020, 120, 10793–10833. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Yonezawa, T.; Hubbell, K.; Dai, G.; Cui, X. Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol. J. 2015, 10, 1568–1577. [Google Scholar] [CrossRef]
- Jayanth, N.; Senthil, P.; Prakash, C. Effect of chemical treatment on tensile strength and surface roughness of 3D-printed ABS using the FDM process. Virtual Phys. Prototyp. 2018, 13, 155–163. [Google Scholar] [CrossRef]
- Wang, P.; Zou, B.; Xiao, H.; Ding, S.; Huang, C. Effects of printing parameters of fused deposition modeling on mechanical properties, surface quality, and microstructure of PEEK. J. Mater. Process. Technol. 2019, 271, 62–74. [Google Scholar] [CrossRef]
- Kruth, J.-P.; Leu, M.C.; Nakagawa, T. Progress in Additive Manufacturing and Rapid Prototyping. CIRP Ann. 1998, 47, 525–540. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 469–504. [Google Scholar] [CrossRef]
- Huang, S.; Jin, M.; Su, N.; Chen, L. New insights on the reparative cells in bone regeneration and repair. Biol. Rev. 2020, 96, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Unger, R.E.; Dohle, E.; Kirkpatrick, C.J. Improving vascularization of engineered bone through the generation of pro-angiogenic effects in co-culture systems. Adv. Drug Deliv. Rev. 2015, 94, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, T.; Nakayama, N.; Haniu, H.; Aoki, K.; Okamoto, M.; Nomura, H.; Tanaka, M.; Sobajima, A.; Yoshida, K.; Kamanaka, T.; et al. Titanium Fiber Plates for Bone Tissue Repair. Adv. Mater. 2017, 30, 1703608. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Feng, C.; Chang, J.; Wu, C. 3D-printed Bioceramic Scaffolds: From Bone Tissue Engineering to Tumor Therapy. Acta Biomater. 2018, 79, 37–59. [Google Scholar] [CrossRef]
- Lee, G.; Carrillo, M.; McKittrick, J.; Martin, D.G.; Olevsky, E.A. Fabrication of ceramic bone scaffolds by solvent jetting 3D printing and sintering: Towards load-bearing applications. Addit. Manuf. 2020, 33, 101107. [Google Scholar] [CrossRef]
- Ran, J.; Hu, J.; Sun, G.; Chen, S.; Jiang, P.; Shen, X.; Tong, H. A novel chitosan-tussah silk fibroin/nano-hydroxyapatite composite bone scaffold platform with tunable mechanical strength in a wide range. Int. J. Biol. Macromol. 2016, 93, 87–97. [Google Scholar] [CrossRef]
- Maji, K.; Dasgupta, S.; Kundu, B.; Bissoyi, A. Development of gelatin-chitosan-hydroxyapatite based bioactive bone scaffold with controlled pore size and mechanical strength. J. Biomater. Sci. Polym. Ed. 2015, 26, 1190–1209. [Google Scholar] [CrossRef]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef]
- Ren, X.; Liu, Q.; Zheng, S.; Zhu, J.; Qi, Z.; Fu, C.; Yang, X.; Zhao, Y. Synergistic delivery of bFGF and BMP-2 from poly(l-lactic-co-glycolic acid)/graphene oxide/hydroxyapatite nanofibre scaffolds for bone tissue engineering applications. RSC Adv. 2018, 8, 31911–31923. [Google Scholar] [CrossRef]
- Zhang, X.; Lou, Q.; Wang, L.; Min, S.; Zhao, M.; Quan, C. Immobilization of BMP-2-derived peptides on 3D-printed porous scaffolds for enhanced osteogenesis. Biomed. Mater. 2019, 15, 015002. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2018, 84, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Sun, M.; Leng, X.; Hu, X.; Ao, Y. Recent Progress in 3D Printing of Elastic and High-Strength Hydrogels for the Treatment of Osteochondral and Cartilage Diseases. Front. Bioeng. Biotechnol. 2020, 8, 604814. [Google Scholar] [CrossRef] [PubMed]
- Abdal-Hay, A.; Raveendran, N.T.; Fournier, B.; Ivanovski, S. Fabrication of biocompatible and bioabsorbable polycaprolactone/magnesium hydroxide 3D printed scaffolds: Degradation and in vitro osteoblasts interactions. Compos. Part B Eng. 2020, 197, 108158. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Di Luca, A.; Longoni, A.; Criscenti, G.; Mota, C.; Van Blitterswijk, C.; Moroni, L. Toward mimicking the bone structure: Design of novel hierarchical scaffolds with a tailored radial porosity gradient. Biofabrication 2016, 8, 045007. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, Z.; Liu, L.; Wang, W.; Leng, J.; Liu, Y. Porous bone tissue scaffold concept based on shape memory PLA/Fe3O4. Compos. Sci. Technol. 2020, 203, 108563. [Google Scholar] [CrossRef]
- Prochor, P.; Gryko, A. Numerical Analysis of the Influence of Porosity and Pore Geometry on Functionality of Scaffolds Designated for Orthopedic Regenerative Medicine. Materials 2020, 14, 109. [Google Scholar] [CrossRef]
- Liu, K.; Li, W.; Chen, S.; Wen, W.; Lu, L.; Liu, M.; Zhou, C.; Luo, B. The design, fabrication and evaluation of 3D printed gHNTs/gMgO whiskers/PLLA composite scaffold with honeycomb microstructure for bone tissue engineering. Compos. Part B Eng. 2020, 192, 108001. [Google Scholar] [CrossRef]
- Feng, X.; Xu, P.; Shen, T.; Zhang, Y.; Ye, J.; Gao, C. Influence of pore architectures of silk fibroin/collagen composite scaffolds on the regeneration of osteochondral defects in vivo. J. Mater. Chem. B 2020, 8, 391–405. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, C.; Yang, G.; Li, G.; Ding, X.; Wang, S.; Dou, Y.; Zhang, Z.; Chang, J.; Wu, C.; et al. 3D-printed scaffolds with synergistic effect of hollow-pipe structure and bioactive ions for vascularized bone regeneration. Biomaterials 2017, 135, 85–95. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, W.; Deng, C.; Li, G.; Chang, J.; Zhang, Z.; Jiang, X.; Wu, C. 3D Printing of Lotus Root-Like Biomimetic Materials for Cell Delivery and Tissue Regeneration. Adv. Sci. 2017, 4, 700401. [Google Scholar] [CrossRef] [PubMed]
- Heintz, K.A.; Bregenzer, M.E.; Mantle, J.L.; Lee, K.H.; West, J.L.; Slater, J.H. Fabrication of 3D Biomimetic Microfluidic Networks in Hydrogels. Adv. Healthc. Mater. 2016, 5, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, W.; Tang, X.; Liu, X. Osteogenic induction of bone marrow mesenchymal cells on electrospun polycaprolactone/chitosan nanofibrous membrane. Dent. Mater. J. 2017, 36, 325–332. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luo, Y.; Lode, A.; Akkineni, A.R.; Gelinsky, M. Concentrated gelatin/alginate composites for fabrication of predesigned scaffolds with a favorable cell response by 3D plotting. RSC Adv. 2015, 5, 43480–43488. [Google Scholar] [CrossRef]
- Chen, M.; Le, D.Q.; Baatrup, A.; Nygaard, J.V.; Hein, S.; Bjerre, L.; Kassem, M.; Zou, X.; Bunger, C. Self-assembled composite matrix in a hierarchical 3-D scaffold for bone tissue engineering. Acta Biomater. 2011, 7, 2244–2255. [Google Scholar] [CrossRef]
- Rosenzweig, D.H.; Carelli, E.; Steffen, T.; Jarzem, P.; Haglund, L. 3D-Printed ABS and PLA Scaffolds for Cartilage and Nucleus Pulposus Tissue Regeneration. Int. J. Mol. Sci. 2015, 16, 15118–15135. [Google Scholar] [CrossRef]
- Nuseir, A.; Hatamleh, M.M.; Alnazzawi, A.; Al-Rabab’ah, M.; Kamel, B.; Jaradat, E. Direct 3D Printing of Flexible Nasal Prosthesis: Optimized Digital Workflow from Scan to Fit. J. Prosthodont. 2019, 28, 10–14. [Google Scholar] [CrossRef]
- Lee, J.W.; Kang, K.S.; Lee, S.H.; Kim, J.-Y.; Lee, B.-K.; Cho, D.-W. Bone regeneration using a microstereo-lithography-produced customized poly(propylene fumarate)/diethyl fumarate photopolymer 3D scaffold incorpo-rating BMP-2 loaded PLGA microspheres. Biomaterials 2011, 32, 744–752. [Google Scholar] [CrossRef]
- Sun, A.X.; Numpaisal, P.-O.; Gottardi, R.; Shen, H.; Yang, G.; Tuan, R.S. Cell and Biomimetic Scaffold-Based Approaches for Cartilage Regeneration. Oper. Tech. Orthop. 2016, 26, 135–146. [Google Scholar] [CrossRef]
- Gaikwad, V.; Ghose, A.; Cholake, S.; Rawal, A.; Iwato, M.; Sahajwalla, V. Transformation of E-Waste Plastics into Sustainable Filaments for 3D Printing. ACS Sustain. Chem. Eng. 2018, 6, 14432–14440. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, J.E.; Park, J.H.; Lyu, M.-Y.; Park, K.; Koo, M.S.; Jin, S.C.; Kim, K.Y.; Son, Y. FDM 3D printing of environmental friendly and high strength bio-based PC Filaments for baby toys. Elastomers Compos. 2017, 52, 99–104. [Google Scholar] [CrossRef]
- Lee, C.-H.; Takagi, H.; Okamoto, H.; Kato, M. Preparation and mechanical properties of a copolycarbonate composed of bio-based isosorbide and bisphenol A. Polym. J. 2015, 47, 639–643. [Google Scholar] [CrossRef]
- Fenouillot, F.; Rousseau, A.; Colomines, G.; Saint-Loup, R.; Pascault, J.P. Polymers from renewable 1,4:3,6-dianhydrohexitols (isosorbide, isomannide and isoidide): A review. Prog. Polym. Sci. 2010, 35, 578–622. [Google Scholar] [CrossRef]
- Cheng, K.-J.; Liu, Y.-F.; Wang, R.; Zhang, J.-X.; Jiang, X.-F.; Dong, X.-T.; Xu, X. Topological optimization of 3D printed bone analog with PEKK for surgical mandibular reconstruction. J. Mech. Behav. Biomed. Mater. 2020, 107, 103758. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Zhang, J.; Zheng, J.; Wang, L.; Li, D.; Liu, S. 3D-printed PEEK implant for mandibular defects repair—A new method. J. Mech. Behav. Biomed. Mater. 2021, 116, 104335. [Google Scholar] [CrossRef]
- Oliveira, T.A.; Oliveira, R.R.; Barbosa, R.; Azevedo, J.B.; Alves, T.S. Effect of reprocessing cycles on the degradation of PP/PBAT-thermoplastic starch blends. Carbohydr. Polym. 2017, 168, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Savu, I.D.; Savu, S.V.; Simion, D.; Sîrbu, N.-A.; Ciornei, M.; Ratiu, S.A. PP in 3D Printing–Technical and Economic Aspects. Mater. Plast. 2019, 56, 931. [Google Scholar] [CrossRef]
- Kitson, P.J.; Glatzel, S.; Chen, W.; Lin, C.G.; Song, Y.F.; Cronin, L. 3D printing of versatile reactionware for chemical synthesis. Nat. Protoc. 2016, 11, 920–936. [Google Scholar] [CrossRef]
- Wang, L.; Gramlich, W.M.; Gardner, D.J.; Han, Y.; Tajvidi, M. Spray-dried cellulose nanofibril-reinforced polypropylene composites for extrusion-based additive manufacturing: Nonisothermal crystallization kinetics and thermal expansion. J. Compos. Sci. 2018, 2, 7. [Google Scholar] [CrossRef]
- Darsani, A.; Marwah, O.; Saude, N.; Adzila, S.; Ibrahim, M.; Sharif, S. Capability Behaviour of POFA Composite Filament for 3D Printing User. In Recent Trends in Manufacturing and Materials towards Industry 4.0; Springer: Berlin/Heidelberg, Germany, 2021; pp. 607–618. [Google Scholar] [CrossRef]
- Li, J.; Hsu, Y.; Luo, E.; Khadka, A.; Hu, J. Computer-Aided Design and Manufacturing and Rapid Prototyped Nanoscale Hydroxyapatite/Polyamide (n-HA/PA) Construction for Condylar Defect Caused by Mandibular Angle Ostectomy. Aesthet. Plast. Surg. 2011, 35, 636–640. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2016, 25, 854–866. [Google Scholar] [CrossRef]
- Chen, J.-K.; Shen, C.-R.; Liu, C.-L. N-acetylglucosamine: Production and applications. Mar. Drugs 2010, 8, 2493–2516. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Vinodhini, P.; Sudha, P.N.; Kim, S.-K. Chitin and chitosan composites for bone tissue regeneration. Adv. Food Nutr. Res. 2014, 73, 59–81. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, S.; Venkatesan, J.; Se-Kwon, K.; Joel, D.B.; Jayakumar, R. An overview of chitin or chitosan/nano ceramic composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1338–1353. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr. Polym. 2019, 230, 115658. [Google Scholar] [CrossRef]
- Wang, J.; Wei, J. Interpenetrating network hydrogels with high strength and transparency for potential use as external dressings. Mater. Sci. Eng. C 2017, 80, 460–467. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Y.; Qin, X.; Wa, Q. 3D printing of concentrated alginate/gelatin scaffolds with homogeneous nano apatite coating for bone tissue engineering. Mater. Des. 2018, 146, 12–19. [Google Scholar] [CrossRef]
- Bose, S.; Koski, C.; Vu, A.A. Additive manufacturing of natural biopolymers and composites for bone tissue engineering. Mater. Horiz. 2020, 7, 2011–2027. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef]
- Dai, H.; Li, X.; Du, J.; Ma, L.; Yu, Y.; Zhou, H.; Guo, T.; Zhang, Y. Effect of interaction between sorbitol and gelatin on gelatin properties and its mechanism under different citric acid concentrations. Food Hydrocoll. 2020, 101, 105557. [Google Scholar] [CrossRef]
- Shen, Z.-S.; Cui, X.; Hou, R.-X.; Li, Q.; Deng, H.-X.; Fu, J. Tough biodegradable chitosan-gelatin hydrogels via in situ precipitation for potential cartilage tissue engineering. RSC Adv. 2015, 5, 55640–55647. [Google Scholar] [CrossRef]
- Dreesmann, L.; Ahlers, M.; Schlosshauer, B. The pro-angiogenic characteristics of a cross-linked gelatin matrix. Biomaterials 2007, 28, 5536–5543. [Google Scholar] [CrossRef] [PubMed]
- Rohanizadeh, R.; Swain, M.V.; Mason, R.S. Gelatin sponges (Gelfoam®) as a scaffold for osteoblasts. J. Mater. Sci. Mater. Med. 2008, 19, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Yazdanian, M.; Tabesh, H.; Houshmand, B.; Tebyanian, H.; Soufdoost, R.S.; Tahmasebi, E.; Karami, A.; Ghullame, S. Fabrication and properties of βTCP/Zeolite/Gelatin scaffold as developed scaffold in bone regeneration: In vitro and in vivo studies. Biocybern. Biomed. Eng. 2020, 40, 1626–1637. [Google Scholar] [CrossRef]
- Yin, Y.; Ye, F.; Cui, J.; Zhang, F.; Li, X.; Yao, K. Preparation and characterization of macroporous chitosan-gelatin/β-tricalcium phosphate composite scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2003, 67, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Dai, H.; Wang, H.; Yu, Y.; Zhu, H.; Fu, Y.; Ma, L.; Peng, L.; Li, L.; Wang, Q.; et al. Preparation of high thermal stability gelatin emulsion and its application in 3D printing. Food Hydrocoll. 2021, 113, 106536. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.; Kim, G. Biomimetic gelatin/HA biocomposites with effective elastic properties and 3D-structural flexibility using a 3D-printing process. Addit. Manuf. 2020, 36, 101616. [Google Scholar] [CrossRef]
- Olde Damink, L.H.H.; Dijkstra, P.J.; Van Luyn, M.J.A.; Van Wachem, P.B.; Nieuwenhuis, P.; Feijen, J. Glutar-aldehyde as a crosslinking agent for collagen-based biomaterials. J. Mater. Sci. Mater. Med. 1995, 6, 460–472. [Google Scholar] [CrossRef]
- McAndrews, K.M.; Kim, M.J.; Lam, T.Y.; McGrail, D.J.; Dawson, M.R. Architectural and Mechanical Cues Direct Mesenchymal Stem Cell Interactions with Crosslinked Gelatin Scaffolds. Tissue Eng. Part A 2014, 20, 3252–3260. [Google Scholar] [CrossRef]
- Knudson, C.B. Hyaluronan and CD44: Strategic players for cell-matrix interactions during chondrogenesis and matrix assembly. Birth Defects Res. C Embryo Today 2003, 69, 174–196. [Google Scholar] [CrossRef]
- Canibano-Hernandez, A.; Saenz Del Burgo, L.; Espona-Noguera, A.; Orive, G.; Hernandez, R.M.; Ciriza, J.; Pedraz, J.L. Alginate Microcapsules Incorporating Hyaluronic Acid Recreate Closer in Vivo Environment for Mesenchymal Stem Cells. Mol. Pharm. 2017, 14, 2390–2399. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, M.; Weeks, A.; Jones, L.; Sheardown, H. Immobilized hyaluronic acid containing model silicone hydrogels reduce protein adsorption. J. Biomater. Sci. Polym. Ed. 2008, 19, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- Antich, C.; de Vicente, J.; Jiménez, G.; Chocarro, C.; Carrillo, E.; Montañez, E.; Gálvez-Martín, P.; Marchal, J.A. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 2020, 106, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, J.; Yao, Q.; Ji, C.; Liu, J.; Zhu, Q. 3D printing with cellulose materials. Cellulose 2018, 25, 4275–4301. [Google Scholar] [CrossRef]
- Gui, Z.; Zhu, H.; Gillette, E.; Han, X.; Rubloff, G.W.; Hu, L.; Lee, S.B. Natural Cellulose Fiber as Substrate for Supercapacitor. ACS Nano 2013, 7, 6037–6046. [Google Scholar] [CrossRef]
- Roy, D.; Semsarilar, M.; Guthrie, J.T.; Perrier, S. Cellulose modification by polymer grafting: A review. Chem. Soc. Rev. 2009, 38, 2046–2064. [Google Scholar] [CrossRef]
- Elanthikkal, S.; Gopalakrishnapanicker, U.; Varghese, S.; Guthrie, J.T. Cellulose microfibres produced from banana plant wastes: Isolation and characterization. Carbohydr. Polym. 2010, 80, 852–859. [Google Scholar] [CrossRef]
- Olsson, C.; Westm, G. Direct Dissolution of Cellulose: Background, Means and Applications. In Cellulose—Fundamental Aspects; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Medronho, B.; Lindman, B. Competing forces during cellulose dissolution: From solvents to mechanisms. Curr. Opin. Colloid Interface Sci. 2014, 19, 32–40. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Z.; Yang, Y.; Mikos, A.G.; Qiu, Z.; Song, T.; Cui, F.; Wang, X.; Zhang, C. A high-strength mineralized collagen bone scaffold for large-sized cranial bone defect repair in sheep. Regen. Biomater. 2018, 5, 283–292. [Google Scholar] [CrossRef]
- Luetchford, K.A.; Chaudhuri, J.B.; De Bank, P.A. Silk fibroin/gelatin microcarriers as scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2020, 106, 110116. [Google Scholar] [CrossRef]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludag, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Maroulakos, M.; Kamperos, G.; Tayebi, L.; Halazonetis, D.; Ren, Y. Applications of 3D printing on craniofacial bone repair: A systematic review. J. Dent. 2019, 80, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wojtyła, S.; Klama, P.; Baran, T. Is 3D printing safe? Analysis of the thermal treatment of thermoplastics: ABS, PLA, PET, and nylon. J. Occup. Environ. Hyg. 2017, 14, D80–D85. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Wang, J.; Senthil, T.; Wu, L. Mechanical and thermal properties of ABS/montmorillonite nano-composites for fused deposition modeling 3D printing. Mater. Des. 2016, 102, 276–283. [Google Scholar] [CrossRef]
- Fischer, F. Thermoplastics: The Best Choice for 3D Printing; White Paper; Stratasys Inc.: Eden Prairie, MN, USA, 2011. [Google Scholar]
- Napolitano, F.; Frizziero, L.; Santi, G.M.; Donnici, G.; Liverani, A.; Papaleo, P.; Giuseppetti, V. Description of the CAD-AM process for 3D bone printing: The case study of a flat foot. In Proceedings of the 5th NA International Conference on Industrial Engineering and Operations, Detroit, MI, USA, 10–14 August 2020; pp. 2258–2266. [Google Scholar]
- Popov, V.V.; Muller-Kamskii, G.; Kovalevsky, A.; Dzhenzhera, G.; Strokin, E.; Kolomiets, A.; Ramon, J. Design and 3D-printing of titanium bone implants: Brief review of approach and clinical cases. Biomed. Eng. Lett. 2018, 8, 337–344. [Google Scholar] [CrossRef]
- Bartikian, M.; Ferreira, A.; Gonçalves-Ferreira, A.; Neto, L.L. 3D printing anatomical models of head bones. Surg. Radiol. Anat. 2019, 41, 1205–1209. [Google Scholar] [CrossRef]
- Werz, S.M.; Zeichner, S.J.; Berg, B.-I.; Zeilhofer, H.-F.; Thieringer, F. 3D Printed Surgical Simulation Models as educational tool by maxillofacial surgeons. Eur. J. Dent. Educ. 2018, 22, e500–e505. [Google Scholar] [CrossRef]
- Griffith, L.G. Polymeric biomaterials. Acta Mater. 2000, 48, 263–277. [Google Scholar] [CrossRef]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic Acid Technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Madhavan Nampoothiri, K.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, D.Z.; Zhang, P.; Zhao, M.; Jafar, S. Mechanical Properties of Optimized Diamond Lattice Structure for Bone Scaffolds Fabricated via Selective Laser Melting. Materials 2018, 11, 374. [Google Scholar] [CrossRef] [PubMed]
- Coppola, B.; Cappetti, N.; Di Maio, L.; Scarfato, P.; Incarnato, L. 3D Printing of PLA/clay Nanocomposites: Influence of Printing Temperature on Printed Samples Properties. Materials 2018, 11, 1947. [Google Scholar] [CrossRef]
- Da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Schiller, C.; Epple, M. Carbonated calcium phosphates are suitable pH-stabilising fillers for biodegradable polyesters. Biomaterials 2003, 24, 2037–2043. [Google Scholar] [CrossRef]
- Liu, F.; Vyas, C.; Poologasundarampillai, G.; Pape, I.; Hinduja, S.; Mirihanage, W.; Bartolo, P. Structural Evolution of PCL during Melt Extrusion 3D Printing. Macromol. Mater. Eng. 2018, 303, 1700494. [Google Scholar] [CrossRef]
- Baker, S.C.; Rohman, G.; Southgate, J.; Cameron, N.R. The relationship between the mechanical properties and cell behaviour on PLGA and PCL scaffolds for bladder tissue engineering. Biomaterials 2009, 30, 1321–1328. [Google Scholar] [CrossRef]
- Eshraghi, S.; Das, S. Mechanical and microstructural properties of polycaprolactone scaffolds with one-dimensional, two-dimensional, and three-dimensional orthogonally oriented porous architectures produced by selective laser sintering. Acta Biomater. 2010, 6, 2467–2476. [Google Scholar] [CrossRef]
- Ponsart, S.; Coudane, J.; Vert, M. A Novel Route To Poly(ε-caprolactone)-Based Copolymers via Anionic Derivatization. Biomacromolecules 2000, 1, 275–281. [Google Scholar] [CrossRef]
- Park, S.H.; Park, D.S.; Shin, J.W.; Kang, Y.G.; Kim, H.K.; Yoon, T.R.; Shin, J.W. Scaffolds for bone tissue engi-neering fabricated from two different materials by the rapid prototyping technique: PCL versus PLGA. J. Mater. Sci. Mater. Med. 2012, 23, 2671–2678. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, J.E.; Lee, H.B.; Park, J.; Lee, N.-K.; Son, Y.; Park, S.-H. 3D printing of bio-based polycarbonate and its potential applications in ecofriendly indoor manufacturing. Addit. Manuf. 2020, 31, 100974. [Google Scholar] [CrossRef]
- Kurtz, S.M. Chemical and radiation stability of PEEK. In PEEK Biomaterials Handbook; Kurtz, S.M., Ed.; William Andrew Publishing: Oxford, UK, 2012; Chapter 6; pp. 75–79. [Google Scholar] [CrossRef]
- Stober, E.J.; Seferis, J.C.; Keenan, J.D. Characterization and exposure of polyetheretherketone (PEEK) to fluid environments. Polymer 1984, 25, 1845–1852. [Google Scholar] [CrossRef]
- Boinard, E.; Pethrick, R.A.; MacFarlane, C.J. The influence of thermal history on the dynamic mechanical and dielectric studies of polyetheretherketone exposed to water and brine. Polymer 2000, 41, 1063–1076. [Google Scholar] [CrossRef]
- Kwarteng, K.B.; Stark, C. Carbon fiber reinforced PEEK (APC-2/AS-4) composites for orthopaedic implants. SAMPE Q. 1990, 22, 10–14. [Google Scholar]
- Das, A.; Chatham, C.A.; Fallon, J.J.; Zawaski, C.E.; Gilmer, E.L.; Williams, C.B.; Bortner, M.J. Current understanding and challenges in high temperature additive manufacturing of engineering thermoplastic polymers. Addit. Manuf. 2020, 34, 101218. [Google Scholar] [CrossRef]
- Smith, J.A.; Li, S.; Mele, E.; Goulas, A.; Engstrom, D.; Silberschmidt, V.V. Printability and mechanical performance of biomedical PDMS-PEEK composites developed for material extrusion. J. Mech. Behav. Biomed. Mater. 2021, 115, 104291. [Google Scholar] [CrossRef]
- Dutta, A.; Mukherjee, K.; Dhara, S.; Gupta, S. Design of porous titanium scaffold for complete mandibular reconstruction: The influence of pore architecture parameters. Comput. Biol. Med. 2019, 108, 31–41. [Google Scholar] [CrossRef]
- McDonnell, J.M.; Nagassima Rodrigues dos Reis, K.; Ahern, D.P.; Mahon, J.; Butler, J.S. Are Carbon-fiber Implants More Efficacious Than Traditional Metallic Implants for Spine Tumor Surgery? Clin. Spine Surg. 2021, 34, 159–162. [Google Scholar] [CrossRef]
- Zarringhalam, H.; Hopkinson, N.; Kamperman, N.F.; de Vlieger, J.J. Effects of processing on microstructure and properties of SLS Nylon 12. Mater. Sci. Eng. A 2006, 435–436, 172–180. [Google Scholar] [CrossRef]
- Schmid, M.; Amado, A.; Wegener, K. Polymer powders for selective laser sintering (SLS). AIP Conf. Proc. 2015, 1664, 160009. [Google Scholar] [CrossRef]
- Abdullah, A.M.; Rahim, T.; Hamad, W.; Mohamad, D.; Akil, H.M.; Rajion, Z.A. Mechanical and cytotoxicity properties of hybrid ceramics filled polyamide 12 filament feedstock for craniofacial bone reconstruction via fused deposition modelling. Dent. Mater. 2018, 34, e309–e316. [Google Scholar] [CrossRef]
- O’Brien, E.K.; Wayne, D.B.; Barsness, K.A.; McGaghie, W.C.; Barsuk, J.H. Use of 3D printing for medical ed-ucation models in transplantation medicine: A critical review. Curr. Transplant. Rep. 2016, 3, 109–119. [Google Scholar] [CrossRef]
- Zein, N.N.; Hanouneh, I.A.; Bishop, P.D.; Samaan, M.; Eghtesad, B.; Quintini, C.; Miller, C.; Yerian, L.; Klatte, R. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transpl. 2013, 19, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Sodian, R.; Weber, S.; Markert, M.; Loeff, M.; Lueth, T.; Weis, F.C.; Daebritz, S.; Malec, E.; Schmitz, C.; Reichart, B. Pediatric cardiac transplantation: Three-dimensional printing of anatomic models for surgical planning of heart transplantation in patients with univentricular heart. J. Thorac. Cardiovasc. Surg. 2008, 136, 1098–1099. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Feng, X.; Fan, R.; Chen, S.; Song, J.; Gao, L.; Lu, Y. 3D printing of titanium-coated gradient composite lattices for lightweight mandibular prosthesis. Compos. Part B Eng. 2020, 193, 108057. [Google Scholar] [CrossRef]
- Liu, D.; Fu, J.; Fan, H.; Li, D.; Dong, E.; Xiao, X.; Wang, L.; Guo, Z. Application of 3D-printed PEEK scapula prosthesis in the treatment of scapular benign fibrous histiocytoma: A case report. J. Bone Oncol. 2018, 12, 78–82. [Google Scholar] [CrossRef]
- Gupta, A.; Prasad, A.; Mulchandani, N.; Shah, M.; Ravi Sankar, M.; Kumar, S.; Katiyar, V. Multifunctional nanohydroxyapatite-promoted toughened high-molecular-weight stereocomplex poly (lactic acid)-based bionano-composite for both 3D-printed orthopedic implants and high-temperature engineering applications. ACS Omega 2017, 2, 4039–4052. [Google Scholar] [CrossRef]
- Sun, Y.; You, Y.; Jiang, W.; Wang, B.; Wu, Q.; Dai, K. 3D bioprinting dual-factor releasing and gradient-structured constructs ready to implant for anisotropic cartilage regeneration. Sci. Adv. 2020, 6, eaay1422. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Wang, Z.; Qi, Y.; Li, L.; Zhang, P.; Chen, X.; Huang, Y. Composite PLA/PEG/nHA/Dexamethasone Scaffold Prepared by 3D Printing for Bone Regeneration. Macromol. Biosci. 2018, 18, e1800068. [Google Scholar] [CrossRef]
- Atay, A.; Peker, K.; Gunay, Y.; Ebrinc, S.; Karayazgan, B.; Uysal, O. Assessment of health-related quality of life in Turkish patients with facial prostheses. Health Qual. Life Outcomes 2013, 11, 11. [Google Scholar] [CrossRef]
- Nemli, S.K.; Aydin, C.; Yilmaz, H.; Bal, B.T.; Arici, Y.K. Quality of life of patients with implant-retained max-illofacial prostheses: A prospective and retrospective study. J. Prosthet. Dent. 2013, 109, 44–52. [Google Scholar] [CrossRef]
- Futran, N.D.; Mendez, E. Developments in reconstruction of midface and maxilla. Lancet Oncol. 2006, 7, 249–258. [Google Scholar] [CrossRef]
- Pushpakumar, S.B.; Barker, J.H.; Soni, C.V.; Joseph, H.; van Aalst, V.C.; Banis, J.C.; Frank, J. Clinical consid-erations in face transplantation. Burns 2010, 36, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-M.; Wang, H.-J.; Cheng, T.-Y.; Chang, K.-C.; Lin, F.-H. The rationale of mandible reconstruction in advanced oral cancer: Alloplastic material versus autogenous vascularized bone graft. Mater. Sci. Eng. C 2000, 13, 49–58. [Google Scholar] [CrossRef]
- Betz, R.R. Limitations of autograft and allograft: New synthetic solutions. Orthopedics 2002, 25, s561–s570. [Google Scholar] [CrossRef]
- Arabnejad, S.; Burnett Johnston, R.; Pura, J.A.; Singh, B.; Tanzer, M.; Pasini, D. High-strength porous bio-materials for bone replacement: A strategy to assess the interplay between cell morphology, mechanical properties, bone ingrowth and manufacturing constraints. Acta Biomater. 2016, 30, 345–356. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, J.; Li, J.; Li, M.M.; Huang, W.; Ow, A. Special considerations in virtual surgical planning for secondary accurate maxillary reconstruction with vascularised fibula osteomyocutaneous flap. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, 893–902. [Google Scholar] [CrossRef]
- Andrades, P.; Militsakh, O.; Hanasono, M.M.; Rieger, J.; Rosenthal, E.L. Current strategies in reconstruction of maxillectomy defects. Arch. Otolaryngol. Head Neck Surg. 2011, 137, 806–812. [Google Scholar] [CrossRef]
- Dong, Z.; Li, B.; Xie, R.; Wu, Q.; Zhang, L.; Bai, S. Comparative study of three kinds of fibula cutting guides in reshaping fibula for the reconstruction of mandible: An accuracy simulation study in vitro. J. Craniomaxillofac. Surg. 2017, 45, 1227–1235. [Google Scholar] [CrossRef]
- Price, D.L.; Sherris, D.A.; Kern, E.B. Computed tomography for constructing custom nasal septal buttons. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 1236–1239. [Google Scholar] [CrossRef][Green Version]
- Xu, Y.; Fan, F.; Kang, N.; Wang, S.; You, J.; Wang, H.; Zhang, B. Tissue Engineering of Human Nasal Alar Cartilage Precisely by Using Three-Dimensional Printing. Plast. Reconstr. Surg. 2015, 135, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Jiang, H.; Yin, Z.; Liu, Y.; Zhang, Q.; Zhang, C.; Pan, B.; Zhou, J.; Zhou, X.; Sun, H.; et al. In Vitro Regeneration of Patient-Specific Ear-Shaped Cartilage and Its First Clinical Application for Auricular Reconstruction. EBioMedicine 2018, 28, 287–302. [Google Scholar] [CrossRef] [PubMed]


| Polmer Type | Application | AM Technique | Advantages | Disadvantages | Ref |
|---|---|---|---|---|---|
| Chitin and chitosan | Bone repair | Extrusion Bioprinting | Osteogenic induction | Lack of mechanical strength | [74] |
| Biocompatibility | |||||
| Alginate | Bone repair | Extrusion Bioprinting | Biocompatibility | low cell adhesion and proliferation | [75] |
| Improve polymer viscosity | |||||
| Collagen | Bone repair | FDM | Similar to the natural extracellular environment | Weak mechanical properties | [75,76] |
| Extrusion Bioprinting | |||||
| Inkjet Bioprinting | Fast degradation rate | ||||
| Laser-Assisted Bioprinting | |||||
| Gelatin | Bone repair | Extrusion Bioprinting | Biocompatibility | Difficult to hot working | [35] |
| Inkjet Bioprinting | Low antigenicity | Poor mechanical strength | |||
| HA | Bone repair | FDM | Cell support | Poor mechanical strength | [76] |
| lubrication | |||||
| Cellulose | Bone repair | Extrusion Bioprinting | low cost | Unable to melt | [35] |
| High elastic | indissolvable | ||||
| ABS | Bone repair | FDM | low cost | Unable to autoclave | [77] |
| Bone model | SLA | Chemical resistance | Poor degradability | ||
| Good workability | |||||
| PLA | Bone repair | FDM | low cost | Poor tensile strength | [78,79,80] |
| Bone replacement | Biocompatibility | Harmful metabolite | |||
| Bone model | Degradable | ||||
| PCL | Bone repair | FDM | low cost | Poor mechanical strength | [76,80] |
| SLS | Degradable | Long degradation time | |||
| High elastic | |||||
| PC | Bone repair | FDM | high intensity | Easy to absorb moisture from the air | [81,82,83,84] |
| Non-biotoxicity | |||||
| High tensile strength | |||||
| PEEK | Bone replacement | FDM | Low water imbibition | Processing requires a higher temperature | [85,86] |
| High temperature resistance | |||||
| SLS | high transmittance | biologically inert surface | |||
| excellent mechanical properties | |||||
| PP | Bone repair | FDM | Lower density | Lower rigidity | [87,88,89,90,91] |
| Good workability | |||||
| Easy to curl | |||||
| PA | Bone replacement | FDM | Biocompatibility | lack of shape stability | [92] |
| Strong mechanical properties | |||||
| SLS | Wear Resistance | ||||
| Chemical stability |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Zhang, F.; Zhai, W.; Cheng, S.; Li, J.; Wang, Y. Unraveling of Advances in 3D-Printed Polymer-Based Bone Scaffolds. Polymers 2022, 14, 566. https://doi.org/10.3390/polym14030566
Xu Y, Zhang F, Zhai W, Cheng S, Li J, Wang Y. Unraveling of Advances in 3D-Printed Polymer-Based Bone Scaffolds. Polymers. 2022; 14(3):566. https://doi.org/10.3390/polym14030566
Chicago/Turabian StyleXu, Yuanhang, Feiyang Zhang, Weijie Zhai, Shujie Cheng, Jinghua Li, and Yi Wang. 2022. "Unraveling of Advances in 3D-Printed Polymer-Based Bone Scaffolds" Polymers 14, no. 3: 566. https://doi.org/10.3390/polym14030566
APA StyleXu, Y., Zhang, F., Zhai, W., Cheng, S., Li, J., & Wang, Y. (2022). Unraveling of Advances in 3D-Printed Polymer-Based Bone Scaffolds. Polymers, 14(3), 566. https://doi.org/10.3390/polym14030566





