Compositions and Structural Geometries of Scaffolds Used in the Regeneration of Cleft Palates: A Review of the Literature
Abstract
1. Introduction
2. Etiology
2.1. Environmental or Extrinsic Factors
2.2. Genetic and Intrinsic Factors
- (1)
- Trisomy 13–14 or Patau’s syndrome. The pathology shows cardiac defects, mental retardation, impaired vision development, deafness, urogenital alterations and CP in 70–80% of cases [11];
- (2)
- Trisomy 17–18 or Edwards’ syndrome. Alterations in renal function, bone and cardiac malformations, mandibular micrognathia, mental retardation, joint hypermobility, and CP are present in 15–17% of cases [11];
- (3)
- Trisomy 21 or Down’s syndrome. There are abnormalities such as atrial dysplasia, mandibular prognathism and heart disease in 12% of cases, as well as mental retardation, macroglossia and CP in 6% of cases [11];
- (4)
- Treacher Collins syndrome. Autosomal dominant disease not linked to sex, which is characterized by hypoplasias that damage the structures of the first and second branchial arch, zygomatic process of the temporal bone, maxilla, zygomatic bone and middle-external ears. Linear coloboma in the outer third of the lower eyelid can also be observed in 50% of cases, as well as antimongoloid obliquity. Similarly, 30% of cases are associated with CP [15];
- (5)
- Van der Woude syndrome. Autosomal dominant disease not linked to sex, associated with deletion of chromosome 32 and characterized by CP, partial maxillary anodontia, pits in the lower lip (vermilion border) associated with minor salivary gland defects, ankyloglossia and alterations in the temporomandibular joint. In addition, other manifestations such as heart and lower limb malformations have been reported. This occurs in 1 in 100,000 births, while 2% of patients with CP are associated with this pathology [16];
- (6)
- Velocardiofacial syndrome or Shprintzen–Goldberg syndrome. Autosomal dominant pathology associated with deletion of chromosome 22 characterized by CP, mental retardation and cardiac abnormalities, as well as non-constant ophthalmological, immunological, endocrine and orthopedic alterations [17];
- (7)
- Robin sequence. Agglomerations of orofacial malformations characterized by glossoptosis, micrognathia and characteristic “u”-shaped CP. The complexity of the micrognathia can lead to upper airway obstructions [18].
3. Prevalence
- (a)
- Africans: 1:2500;
- (b)
- Caucasians: 1:1000;
- (c)
- Mexicans: 1:700;
- (d)
- Latin Americans: 1:650;
- (e)
- Asians: 1:500.
- (a)
- Cleft palate;
- (b)
- Submucosal cleft palate;
- (c)
- Velopharyngeal insufficiency;
- (d)
- Robin sequence.
- -
- Primary: When the anterior part of the palate (premaxilla) is involved;
- -
- Secondary: When the affected area is in the foramen.
4. Functional Aspects of the Bone Graft Healing Process and Bone Formation
5. Clinical Therapy
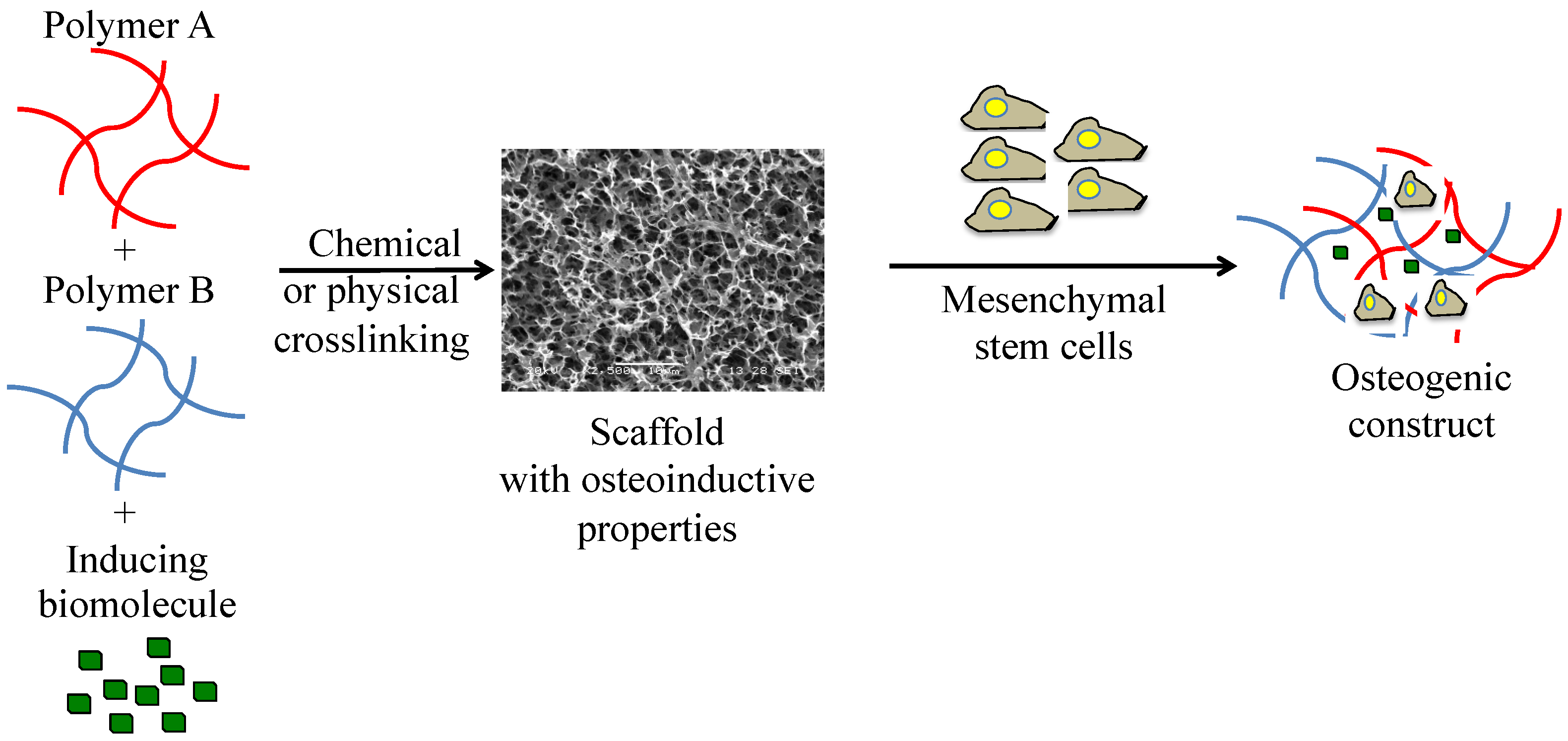
6. Biomaterials Applied in Bone Regeneration
6.1. Polymer-Based Bone Substitutes
6.2. Hydroxyapatite
6.3. Calcium Phosphate Cements
6.4. Other Biomaterials Used for Bone Regeneration
7. Biomaterials Applied in the Regeneration of the Cleft Palate
8. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Becker, D.B.; Coalson, R.S.; Sachanandani, N.S.; Fair, D.; Lugar, H.M.; Kirchner, L.E.; Schlaggar, B.L.; Kane, A.A. Functional neuroanatomy of lexical processing in children with cleft lip and palate. Plast. Reconstr. Surg. 2008, 122, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Montaño López, A.; Rincón Rodríguez, H.; Landa Solís, C.I. Revista odontológica mexicana nasoalveolar bone graft integration range in patients with cleft lip and palate sequels. Medigraphic 2012, 16, 18–30. Available online: http://www.medigraphic.org.mx/ (accessed on 17 February 2021).
- Chigurupati, R. Cleft Lip and Palate: Timing and approaches to reconstruction. In Current Therapy in Oral and Maxillofacial Surgery; Elsevier Inc.: St. Louis, MS, USA, 2012; pp. 726–750. ISBN 9781416025276. [Google Scholar]
- Gunzburg, R.; Szpalski, M.; Passuti, N.; Aebi, M. The Use of Bone Substitutes in Spine Surgery: A State of the Art Review. Available online: https://link.springer.com/book/9783540426875 (accessed on 23 February 2021).
- Saikia, K.C.; Bhattacharya, T.D.; Bhuyan, S.K.; Talukdar, D.J.; Saikia, S.P.; Jitesh, P. Calcium phosphate ceramics as bone graft substitutes in filling bone tumor defects. Indian J. Orthop. 2008, 42, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, C.T.; El-Amin, S.F. Xenotransplantation in orthopaedic surgery. J. Am. Acad. Orthop. Surg. 2008, 16, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Evaniew, N.; Tan, V.; Parasu, N.; Jurriaans, E.; Finlay, K.; Deheshi, B.; Ghert, M. Use of a calcium sulfate-calcium phosphate synthetic bone graft composite in the surgical management of primary bone tumors. Orthopedics 2013, 36, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, J.S.; Cornell, C.N.; Hoang, B.H.; Hsu, W.; Watson, J.T.; Watters, W.C.; Turkelson, C.M.; Wies, J.L.; Anderson, S. Bone void fillers. J. Am. Acad. Orthop. Surg. 2010, 18, 576–579. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, T.; Lin, Y.; Cai, X. Vascularization in Craniofacial Bone Tissue Engineering. J. Dent. Res. 2018, 97, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Mercado-Pagán, Á.E.; Stahl, A.M.; Shanjani, Y.; Yang, Y. Vascularization in Bone Tissue Engineering Constructs. Ann. Biomed. Eng. 2015, 43, 718–729. [Google Scholar] [CrossRef]
- Kazemi, A.; Stearns, J.W.; Fonseca, R.J. Secondary grafting in the alveolar cleft patient. Oral Maxillofac. Surg. Clin. N. Am. 2002, 14, 477–490. [Google Scholar] [CrossRef]
- Wyszynski, D.F.; Beaty, T.H. Review of the role of potential teratogens in the origin of human nonsyndromic oral clefts. Teratology 1996, 53, 309–317. [Google Scholar] [CrossRef]
- Shkoukani, M.A.; Lawrence, L.A.; Liebertz, D.J.; Svider, P.F. Cleft palate: A clinical review. Birth Defects Res. Part C Embryo Today Rev. 2014, 102, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.M. Etiology and Pathogenesis of Orofacial Clefting. Oral Maxillofac. Surg. Clin. N. Am. 2000, 12, 379–397. [Google Scholar] [CrossRef]
- Bergland, O.; Semb, G.; Aabyholm, F.E. Elimination of the residual alveolar cleft by secondary bone grafting and subsequent orthodontic treatment. Cleft Palate J. 1986, 23, 175–205. [Google Scholar] [PubMed]
- Reinoso Quezada, S.J.; Moscoso Mesías, M. Case report Síndrome de Van der Woude. Rev. Fac. Odontol. 2020, 22, 119–129. [Google Scholar] [CrossRef]
- Kernahan, D.A. The striped Y—a symbolic classification for cleft lip and palate. Plast. Reconstr. Surg. 1971, 47, 469–470. [Google Scholar] [CrossRef]
- Brusati, R.; Garattini, G. The Early Secondary Gingivoperiosteoplasty. Oral Maxillofac. Surg. Clin. N. Am. 2000, 12, 443–453. [Google Scholar] [CrossRef]
- Marx, R.E. Philosophy and Particulars of Autogenous Bone Grafting. Oral Maxillofac. Surg. Clin. N. Am. 1993, 5, 599–612. [Google Scholar] [CrossRef]
- Opitz, C.; Meier, B.; Stoll, C.; Subklew, D. Radiographic evaluation of the transplant bone height in patients with clefts of the lip/alveolus/palate after secondary bone grafting. J. Orofac. Orthop. 1999, 60, 383–391. [Google Scholar] [CrossRef]
- Parada, C.; Chai, Y. Roles of BMP Signaling Pathway in Lip and Palate Development. Front. Oral Biol. 2012, 16, 60–70. [Google Scholar] [CrossRef]
- Dixon, M.J.; Marazita, M.L.; Beaty, T.H.; Murray, J.C. Cleft lip and palate: Understanding genetic and environmental influences. Nat. Rev. Genet. 2011, 12, 167–178. [Google Scholar] [CrossRef]
- Bauer, T.W.; Muschler, G.F. Bone Graft Materials: An Overview of the Basic Science. Clin. Orthop. Relat. Res. 2000, 371, 10–27. [Google Scholar] [CrossRef]
- de Grado, G.F.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 1–18. [Google Scholar] [CrossRef]
- Janicki, P.; Schmidmaier, G. What should be the characteristics of the ideal bone graft substitute? Combining scaffolds with growth factors and/or stem cells. Injury 2011, 42, S77–S88. [Google Scholar] [CrossRef] [PubMed]
- Titsinides, S.; Agrogiannis, G.; Karatzas, T. Bone grafting materials in dentoalveolar reconstruction: A comprehensive review. Jpn. Dent. Sci. Rev. 2019, 55, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Roden, R.D. Principles of Bone Grafting. Oral Maxillofac. Surg. Clin. N. Am. 2010, 22, 295–300. [Google Scholar] [CrossRef]
- Guo, J.; Li, C.; Zhang, Q.; Wu, G.; Deacon, S.A.; Chen, J.; Hu, H.; Zou, S.; Ye, Q. Secondary bone grafting for alveolar cleft in children with cleft lip or cleft lip and palate. Cochrane Database Syst. Rev. 2011, 6, CD008050. [Google Scholar] [CrossRef]
- Berger, M.; Probst, F.; Schwartz, C.; Cornelsen, M.; Seitz, H.; Ehrenfeld, M.; Otto, S. A concept for scaffold-based tissue engineering in alveolar cleft osteoplasty. J. Cranio Maxillofac. Surg. 2015, 43, 830–836. [Google Scholar] [CrossRef]
- Al-Ahmady, H.H.; Abd Elazeem, A.F.; Bellah Ahmed, N.E.M.; Shawkat, W.M.; Elmasry, M.; Abdelrahman, M.A.; Abderazik, M.A. Combining autologous bone marrow mononuclear cells seeded on collagen sponge with Nano Hydroxyapatite, and platelet-rich fibrin: Reporting a novel strategy for alveolar cleft bone regeneration. J. Cranio-Maxillofac. Surg. 2018, 46, 1593–1600. [Google Scholar] [CrossRef]
- Wagner, Q.; Offner, D.; Idoux-Gillet, Y.; Saleem, I.; Somavarapu, S.; Schwinté, P.; Benkirane-Jessel, N.; Keller, L. Advanced nanostructured medical device combining mesenchymal cells and VEGF nanoparticles for enhanced engineered tissue vascularization. Nanomedicine 2016, 11, 2419–2430. [Google Scholar] [CrossRef]
- Eap, S.; Ferrand, A.; Mendoza Palomares, C.; Hébraud, A.; Stoltz, J.F.; Mainard, D.; Schlatter, G.; Benkirane-Jessel, N. Electrospun nanofibrous 3D scaffold for bone tissue engineering. Bio-Med. Mater. Eng. 2012, 22, 137–141. [Google Scholar] [CrossRef]
- Porter, J.R.; Henson, A.; Popat, K.C. Biodegradable poly(ε-caprolactone) nanowires for bone tissue engineering applications. Biomaterials 2009, 30, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Slotosch, A.; Thibaudeau, L.; Taubenberger, A.; Loessner, D.; Vaquette, C.; Dalton, P.D.; Hutmacher, D.W. Design and Fabrication of Tubular Scaffolds via Direct Writing in a Melt Electrospinning Mode. Biointerphases 2012, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Valadez-González, A.; Rosales-Ibáñez, R.; Rodríguez-Navarrete, A.; Villamar-Duque, T.E.; Cano-Brown, J.; Carrillo-Escalante, H.J.; Ortiz-Fernández, A.; Hernández-Sánchez, F. Tailoring surface properties of carbon nanofibers via oxidation and its influence on dental pulp stem cell viability of PCL/CNF composites. Polym. Bull. 2021, 78, 695–711. [Google Scholar] [CrossRef]
- Rosales-Ibáñez, R.; Cubo-Mateo, N.; Rodríguez-Navarrete, A.; González-González, A.M.; Villamar-Duque, T.E.; Flores-Sánchez, L.O.; Rodríguez-Lorenzo, L.M. Assessment of a PCL-3D printing-dental pulp stem cells triplet for bone engineering: An in vitro study. Polymers 2021, 13, 1154. [Google Scholar] [CrossRef]
- Almansoori, A.A.; Kwon, O.-J.; Nam, J.-H.; Seo, Y.-K.; Song, H.-R.; Lee, J.-H. Mesenchymal stem cells and platelet-rich plasma-impregnated polycaprolactone-β tricalcium phosphate bio-scaffold enhanced bone regeneration around dental implants. Int. J. Implant Dent. 2021, 7, 1–8. [Google Scholar] [CrossRef]
- D’Antò, V.; Raucci, M.G.; Guarino, V.; Martina, S.; Valletta, R.; Ambrosio, L. Behaviour of human mesenchymal stem cells on chemically synthesized HA-PCL scaffolds for hard tissue regeneration. J. Tissue Eng. Regen. Med. 2016, 10, E147–E154. [Google Scholar] [CrossRef]
- Eap, S.; Keller, L.; Schiav, J.; Huck, O.; Jacomine, L.; Fioretti, F.; Gauthier, C.; Sebastian, V.; Schwinté, P.; Benkirane-Jessel, N. A living thick nanofibrous implant bifunctionalized with active growth factor and stem cells for bone regeneration. Int. J. Nanomed. 2015, 10, 1061–1075. [Google Scholar] [CrossRef]
- Ferrand, A.; Eap, S.; Richert, L.; Lemoine, S.; Kalaskar, D.; Demoustier-Champagne, S.; Atmani, H.; Mély, Y.; Fioretti, F.; Schlatter, G.; et al. Osteogenetic properties of electrospun nanofibrous PCL scaffolds equipped with chitosan-based nanoreservoirs of growth factors. Macromol. Biosci. 2014, 14, 45–55. [Google Scholar] [CrossRef]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef]
- Nandi, S.K.; Roy, S.; Mukherjee, P.; Kundu, B.; Basu, D. Orthopaedic applications of bone graft & graft substitutes: A review. Indian J. Med. Res. 2010, 132, 15–30. [Google Scholar]
- Hernigou, P.; Ma, W. Open wedge tibial osteotomy with acrylic bone cement as bone substitute. Knee 2001, 8, 103–110. [Google Scholar] [CrossRef]
- Laurencin, C.; Khan, Y.; El-Amin, S.F. Bone graft substitutes. Expert Rev. Med. Devices 2006, 3, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alidadi, S.; Bigham-Sadegh, A.; Moshiri, A. Healing potentials of polymethylmethacrylate bone cement combined with platelet gel in the critical-sized radial bone defect of rats. PLoS ONE 2018, 13, e0194751. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Nandi, S.K.; Kundu, B.; Datta, S.; De, D.K.; Roy, S.K.; Basu, D. In Vivo response of porous hydroxyapatite and β-tricalcium phosphate prepared by aqueous solution combustion method and comparison with bioglass scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 86, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.K.; Kundu, B.; Ghosh, S.K.; De, D.K.; Basu, D. Efficacy of nano-hydroxyapatite prepared by an aqueous solution combustion technique in healing bone defects of goat. J. Vet. Sci. 2008, 9, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, A.; Koshino, T.; Saito, T.; Takagi, T. Osseous tissue reaction around hydroxyapatite block implanted into proximal metaphysis of tibia of rat with collagen-induced arthritis. Biomaterials 2000, 21, 483–487. [Google Scholar] [CrossRef]
- Daculsi, G. Biphasic calcium phosphate concept applied to artificial bone, implant coating and injectable bone substitute. Biomaterials 1998, 19, 1473–1478. [Google Scholar] [CrossRef]
- Johnson, K.D.; Frierson, K.E.; Keller, T.S.; Cook, C.; Scheinberg, R.; Zerwekh, J.; Meyers, L.; Sciadini, M.F. Porous ceramics as bone graft substitutes in long bone defects: A biomechanical, histological, and radiographic analysis. J. Orthop. Res. 1996, 14, 351–369. [Google Scholar] [CrossRef]
- Spivak, J.M.; Hasharoni, A. Use of hydroxyapatite in spine surgery. Eur. Spine J. 2001, 10, S197–S204. [Google Scholar] [CrossRef]
- Nudelman, F.; Lausch, A.J.; Sommerdijk, N.A.J.M.; Sone, E.D. In Vitro models of collagen biomineralization. J. Struct. Biol. 2013, 183, 258–269. [Google Scholar] [CrossRef]
- Strauss, F.; Kuchler, U.; Kobatake, R.; Heimel, P.; Tangl, S.; Gruber, R. Acid bone lysates reduce bone regeneration in rat calvaria defects. J. Biomed. Mater. Res. Part A 2021, 109, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Baumann, M.J.; McCabe, L.R. Osteoblasts respond to hydroxyapatite surfaces with immediate changes in gene expression. J. Biomed. Mater. Res. Part A 2004, 71, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Chatzipetros, E.; Damaskos, S.; Tosios, K.I.; Christopoulos, P.; Donta, C.; Kalogirou, E.-M.; Yfanti, Z.; Tsiourvas, D.; Papavasiliou, A.; Tsiklakis, K. The effect of nano-hydroxyapatite/chitosan scaffolds on rat calvarial defects for bone regeneration. Int. J. Implant Dent. 2021, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kashiwazaki, H.; Kishiya, Y.; Matsuda, A.; Yamaguchi, K.; Iizuka, T.; Tanaka, J.; Inoue, N. Fabrication of porous chitosan/hydroxyapatite nanocomposites: Their mechanical and biological properties. Biomed. Mater. Eng. 2009, 19, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Nájera-Romero, G.V.; Yar, M.; Rehman, I.U. Heparinized chitosan/hydroxyapatite scaffolds stimulate angiogenesis. Funct. Compos. Mater. 2020, 1, 1–15. [Google Scholar] [CrossRef]
- Chiodelli, P.; Bugatti, A.; Urbinati, C.; Rusnati, M. Heparin/Heparan Sulfate Proteoglycans Glycomic Interactome in Angiogenesis: Biological Implications and Therapeutical Use. Molecules 2015, 20, 6342–6388. [Google Scholar] [CrossRef]
- Liang, C.; Luo, Y.; Yang, G.; Xia, D.; Liu, L.; Zhang, X.; Wang, H. Graphene Oxide Hybridized nHAC/PLGA Scaffolds Facilitate the Proliferation of MC3T3-E1 Cells. Nanoscale Res. Lett. 2018, 13, 1–10. [Google Scholar] [CrossRef]
- Kwon, G.-W.; Gupta, K.C.; Jung, K.-H.; Kang, I.-K. Lamination of microfibrous PLGA fabric by electrospinning a layer of collagen-hydroxyapatite composite nanofibers for bone tissue engineering. Biomater. Res. 2017, 21, 1–12. [Google Scholar] [CrossRef]
- Cetina-Diaz, S.M.; Chan-Chan, L.H.; Vargas-Coronado, R.F.; Cervantes-Uc, J.M.; Quintana-Owen, P.; Paakinaho, K.; Kellomaki, M.; Di Silvio, L.; Deb, S.; Cauich-Rodríguez, J.V. Physicochemical characterization of segmented polyurethanes prepared with glutamine or ascorbic acid as chain extenders and their hydroxyapatite composites. J. Mater. Chem. B 2014, 2, 1966–1976. [Google Scholar] [CrossRef]
- Luo, K.; Wang, L.; Wang, Y.; Zhou, S.; Zhang, P.; Li, J. Porous 3D hydroxyapatite/polyurethane composite scaffold for bone tissue engineering and its in vitro degradation behavior. Ferroelectrics 2020, 566, 104–115. [Google Scholar] [CrossRef]
- Wang, C.; Cao, X.; Zhang, Y. A novel bioactive osteogenesis scaffold delivers ascorbic acid, β-glycerophosphate, and dexamethasone in vivo to promote bone regeneration. Oncotarget 2017, 8, 31612–31625. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Perez, F.J.; Vargas-Coronado, R.; Cervantes-Uc, J.M.; Cauich-Rodriguez, J.V.; Rosales-Ibañez, R.; Pavon-Palacio, J.J.; Torres-Hernandez, Y.; Rodriguez-Ortiz, J.A. Preparation and characterization of titanium—segmented polyurethane composites for bone tissue engineering. J. Biomater. Appl. 2018, 33, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.E. A new calcium phosphate water-setting. In Cements Research Progress; Brown, P.W., Ed.; American Ceramic Society: Westerville, OH, USA, 1986; pp. 352–379. [Google Scholar]
- Russell, T.A.; Leighton, R.K. Comparison of autogenous bone graft and endothermic calcium phosphate cement for defect augmentation in tibial plateau fractures. A multicenter, prospective, randomized study. J. Bone Jt. Surg. Ser. A 2008, 90, 2057–2061. [Google Scholar] [CrossRef] [PubMed]
- Burguera, E.F.; Xu, H.H.K.; Weir, M.D. Injectable and rapid-setting calcium phosphate bone cement with dicalcium phosphate dihydrate. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 77, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Afifi, A.M.; Gordon, C.R.; Pryor, L.S.; Sweeney, W.; Papay, F.A.; Zins, J.E. Calcium phosphate cements in skull reconstruction: A meta-analysis. Plast. Reconstr. Surg. 2010, 126, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 1–19. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- Du, G.-Y.; He, S.-W.; Sun, C.-X.; Mi, L.-D. Bone Morphogenic Protein-2 (rhBMP2)-Loaded Silk Fibroin Scaffolds to Enhance the Osteoinductivity in Bone Tissue Engineering. Nanoscale Res. Lett. 2017, 12, 1–7. [Google Scholar] [CrossRef][Green Version]
- Chen, J.-P.; Chang, G.-Y.; Chen, J.-K. Electrospun collagen/chitosan nanofibrous membrane as wound dressing. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313–314, 183–188. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
- Witte, F.; Feyerabend, F.; Maier, P.; Fischer, J.; Störmer, M.; Blawert, C.; Dietzel, W.; Hort, N. Biodegradable magnesium-hydroxyapatite metal matrix composites. Biomaterials 2007, 28, 2163–2174. [Google Scholar] [CrossRef] [PubMed]
- Huan, Z.G.; Leeflang, M.A.; Zhou, J.; Duszczyk, J. ZK30-bioactive glass composites for orthopedic applications: A comparative study on fabrication method and characteristics. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2011, 176, 1644–1652. [Google Scholar] [CrossRef]
- Huan, Z.; Zhou, J.; Duszczyk, J. Magnesium-based composites with improved in vitro surface biocompatibility. J. Mater. Sci. Mater. Med. 2010, 21, 3163–3169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rady, A.A.M.; Hamdy, S.M.; Abdel-Hamid, M.A.; Hegazy, M.G.A.; Fathy, S.A.; Mostafa, A.A. The role of VEGF and BMP-2 in stimulation of bone healing with using hybrid bio-composite scaffolds coated implants in animal model. Bull. Natl. Res. Cent. 2020, 44, 1–9. [Google Scholar] [CrossRef]
- Martín-del-Campo, M.; Rosales-Ibañez, R.; Rojo, L. Biomaterials for Cleft Lip and Palate Regeneration. Int. J. Mol. Sci. 2019, 20, 2176. [Google Scholar] [CrossRef]
- Martín-Piedra, M.A.; Alaminos, M.; Fernández-Valadés-gámez, R.; España-López, A.; Liceras-Liceras, E.; Sánchez-Montesinos, I.; Martínez-Plaza, A.; Sánchez-Quevedo, M.C.; Fernández-Valadés, R.; Garzón, I. Development of a multilayered palate substitute in rabbits: A histochemical Ex Vivo and In Vivo analysis. Histochem. Cell Biol. 2017, 147, 377–388. [Google Scholar] [CrossRef]
- Janssen, N.G.; De Ruiter, A.P.; Van Hout, W.M.M.T.; Van Miegem, V.; Gawlitta, D.; De Groot, F.B.; Meijer, G.J.; Rosenberg, A.J.W.P.; Koole, R. Microstructured β-Tricalcium phosphate putty versus autologous bone for repair of alveolar clefts in a goat model. Cleft Palate-Craniofacial J. 2017, 54, 699–706. [Google Scholar] [CrossRef]
- Korn, P.; Ahlfeld, T.; Lahmeyer, F.; Kilian, D.; Sembdner, P.; Stelzer, R.; Pradel, W.; Franke, A.; Rauner, M.; Range, U.; et al. 3D Printing of Bone Grafts for Cleft Alveolar Osteoplasty—In vivo Evaluation in a Preclinical Model. Front. Bioeng. Biotechnol. 2020, 8, 217–232. [Google Scholar] [CrossRef]
- Amalraj, J.C.; Gangothri, M.; Babu, H. Reconstruction of Drug-induced Cleft Palate Using Bone Marrow Mesenchymal Stem Cell in Rodents. Ann. Maxillofac. Surg. 2017, 7, 82–88. [Google Scholar]
- Caballero, M.; Morse, J.C.; Halevi, A.E.; Emodi, O.; Pharaon, M.R.; Wood, J.S.; van Aalst, J.A. Juvenile Swine Surgical Alveolar Cleft Model to Test Novel Autologous Stem Cell Therapies. Tissue Eng. Part C Methods 2015, 21, 898–908. [Google Scholar] [CrossRef]
- Alkaabi, S.A.; Natsir Kalla, D.S.; Alsabri, G.A.; Fauzi, A.; Tajrin, A.; Müller, W.E.G.; Schröder, H.C.; Wang, X.G.; Forouzanfar, T.; Helder, M.N.; et al. Polyphosphate (PolyP) for alveolar cleft repair: Study protocol for a pilot randomized controlled trial. Trials 2021, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ahlfeld, T.; Lode, A.; Richter, R.F.; Pradel, W.; Franke, A.; Rauner, M.; Stadlinger, B.; Lauer, G.; Gelinsky, M.; Korn, P. Toward Biofabrication of Resorbable Implants Consisting of a Calcium Phosphate Cement and Fibrin—A Characterization In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 1218. [Google Scholar] [CrossRef]
- Kamal, M.; Andersson, L.; Tolba, R.; Al-Asfour, A.; Bartella, A.K.; Gremse, F.; Rosenhain, S.; Hölzle, F.; Kessler, P.; Lethaus, B. Bone regeneration using composite non-demineralized xenogenic dentin with beta-tricalcium phosphate in experimental alveolar cleft repair in a rabbit model. J. Transl. Med. 2017, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Naudot, M.; Davrou, J.; Djebara, A.-E.; Barre, A.; Lavagen, N.; Lardière, S.; Azdad, S.Z.; Zabijak, L.; Lack, S.; Devauchelle, B.; et al. Functional Validation of a New Alginate-based Hydrogel Scaffold Combined with Mesenchymal Stem Cells in a Rat Hard Palate Cleft Model. Plast. Reconstr. Surg. Glob. Open 2020, 8, 2743–2751. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, N.Z.; Talwar, R.; Shahin, M.; Unsworth, L.D.; Major, P.W.; Doschak, M.R. Cleft Palate Reconstruction Using Collagen and Nanofiber Scaffold Incorporating Bone Morphogenetic Protein in Rats. Tissue Eng. Part A 2015, 21, 85–95. [Google Scholar] [CrossRef]
- Li, W.; Fu, Y.; Jiang, B.; Lo, A.Y.; Ameer, G.A.; Barnett, C.; Wang, B. Polymer-integrated amnion scaffold significantly improves cleft palate repair. Acta Biomater. 2019, 92, 104–114. [Google Scholar] [CrossRef]
- Rizzo, M.I.; Tomao, L.; Tedesco, S.; Cajozzo, M.; Esposito, M.; De Stefanis, C.; Ferranti, A.M.; Mezzogori, D.; Palmieri, A.; Pozzato, G.; et al. Engineered mucoperiosteal scaffold for cleft palate regeneration towards the non-immunogenic transplantation. Sci. Rep. 2021, 11, 14570. [Google Scholar] [CrossRef]
- Reddy, G.S.P. Membrane Assisted Palatal Fistula Closure in a Cleft Palate Patient: A Novel Technique. J. Clin. Diagn. Res. 2016, 10, ZD22–ZD24. [Google Scholar] [CrossRef]
- Mossaad, A.; El Badry, T.; Abdelrahman, M.; Abd Elazeem, A.; Ghanem, W.; Hassan, S.; Adly, N.; Shawkat, W. Alveolar Cleft Reconstruction Using Different Grafting Techniques. Open Access Maced. J. Med. Sci. 2019, 7, 1369–1373. [Google Scholar] [CrossRef]
- Ahmed, A.; Gibson, C.; Ayliffe, P. Technical note Use of polydioxanone sheet to repair palatal fistulas in patients with cleft palate. Br. J. Oral Maxillofac. Surg. 2013, 51, e197–e198. [Google Scholar] [CrossRef]
- Rodriguez, I.A.; Madurantakam, P.A.; McCool, J.M.; Sell, S.A.; Yang, H.; Moon, P.C.; Bowlin, G.L. Mineralization potential of electrospun PDO-hydroxyapatite-fibrinogen blended scaffolds. Int. J. Biomater. 2012, 2012, 159484. [Google Scholar] [CrossRef] [PubMed]
- Puwanun, S.; Delaine-Smith, R.M.; Colley, H.E.; Yates, J.M.; MacNeil, S.; Reilly, G.C. A simple rocker-induced mechanical stimulus upregulates mineralization by human osteoprogenitor cells in fibrous scaffolds. J. Tissue Eng. Regen. Med. 2018, 12, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Sasayama, S.; Hara, T.; Tanaka, T.; Honda, Y.; Baba, S. Osteogenesis of Multipotent Progenitor Cells using the Epigallocatechin Gallate-Modified Gelatin Sponge Scaffold in the Rat Congenital Cleft-Jaw Model. Int. J. Mol. Sci. 2018, 19, 3803. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyna-Urrutia, V.A.; González-González, A.M.; Rosales-Ibáñez, R. Compositions and Structural Geometries of Scaffolds Used in the Regeneration of Cleft Palates: A Review of the Literature. Polymers 2022, 14, 547. https://doi.org/10.3390/polym14030547
Reyna-Urrutia VA, González-González AM, Rosales-Ibáñez R. Compositions and Structural Geometries of Scaffolds Used in the Regeneration of Cleft Palates: A Review of the Literature. Polymers. 2022; 14(3):547. https://doi.org/10.3390/polym14030547
Chicago/Turabian StyleReyna-Urrutia, Víctor A., Arely M. González-González, and Raúl Rosales-Ibáñez. 2022. "Compositions and Structural Geometries of Scaffolds Used in the Regeneration of Cleft Palates: A Review of the Literature" Polymers 14, no. 3: 547. https://doi.org/10.3390/polym14030547
APA StyleReyna-Urrutia, V. A., González-González, A. M., & Rosales-Ibáñez, R. (2022). Compositions and Structural Geometries of Scaffolds Used in the Regeneration of Cleft Palates: A Review of the Literature. Polymers, 14(3), 547. https://doi.org/10.3390/polym14030547







