Thermal Degradation Kinetics Analysis of Polymer Composite Electrolyte Membranes of PEVOH and PBT Nano Fiber
Abstract
:1. Introduction
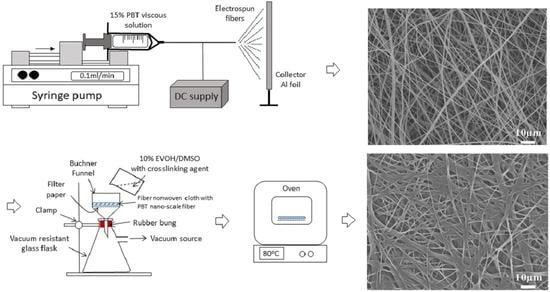
2. Materials and Methods
3. Results and Discussion
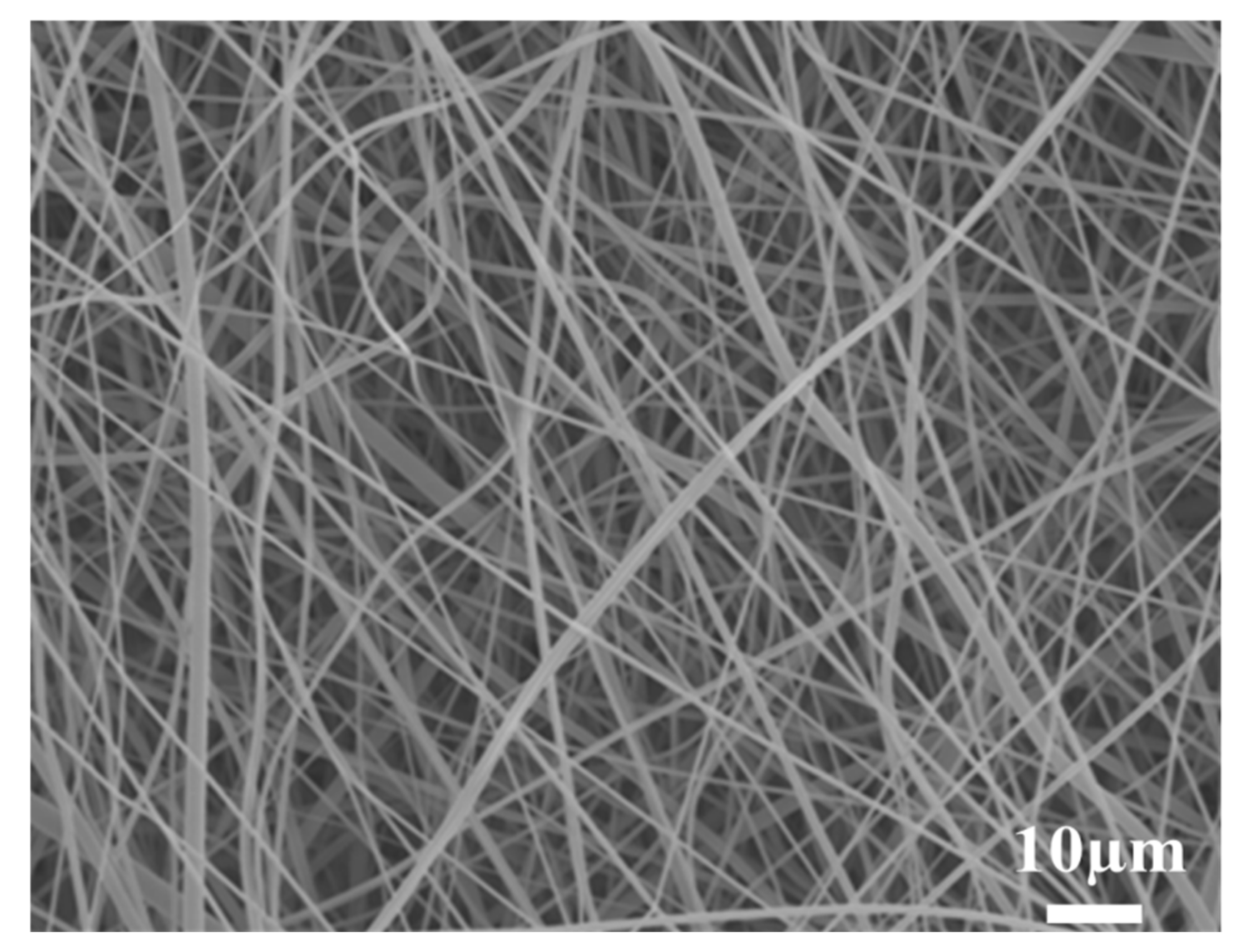
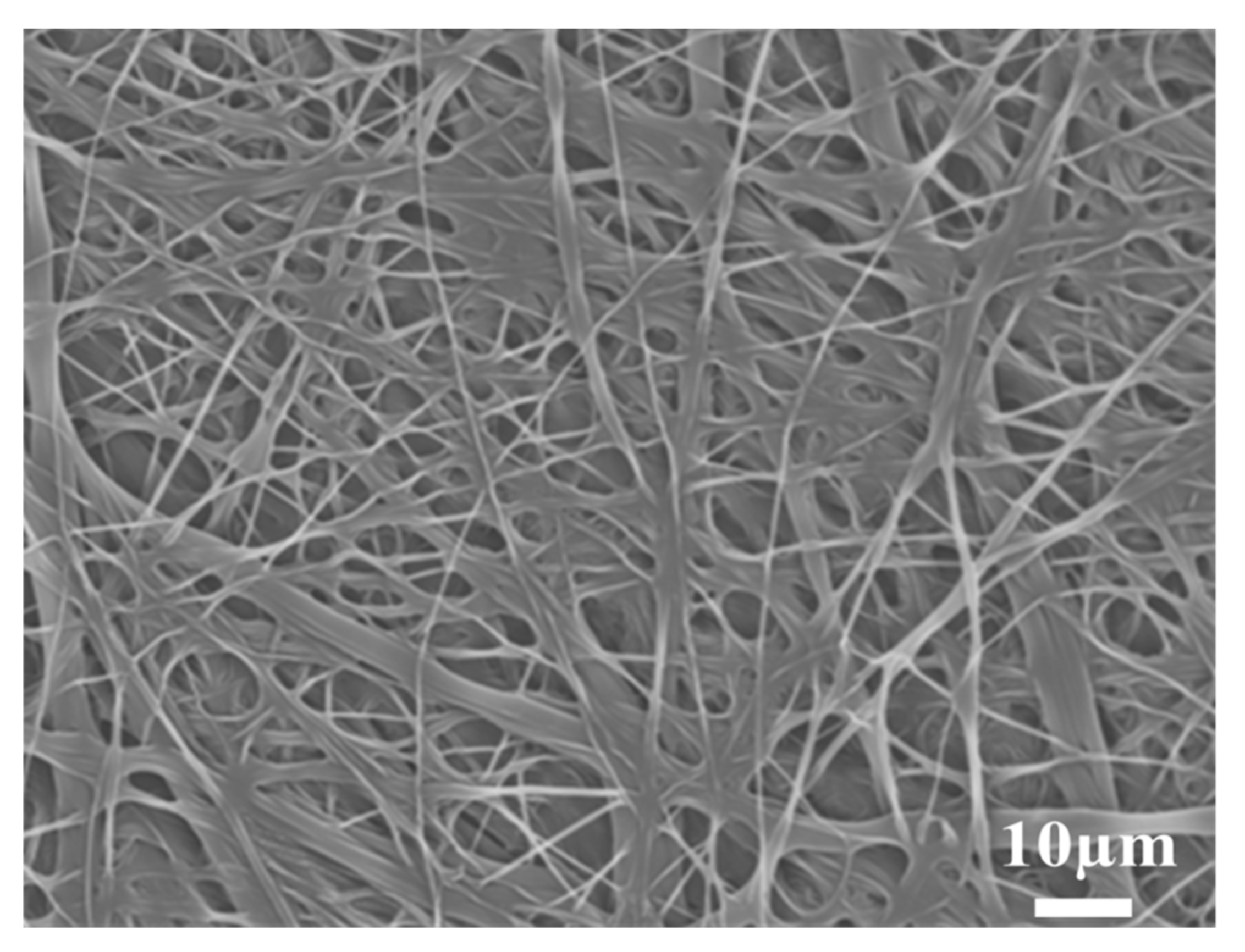
3.1. Surface Morphology
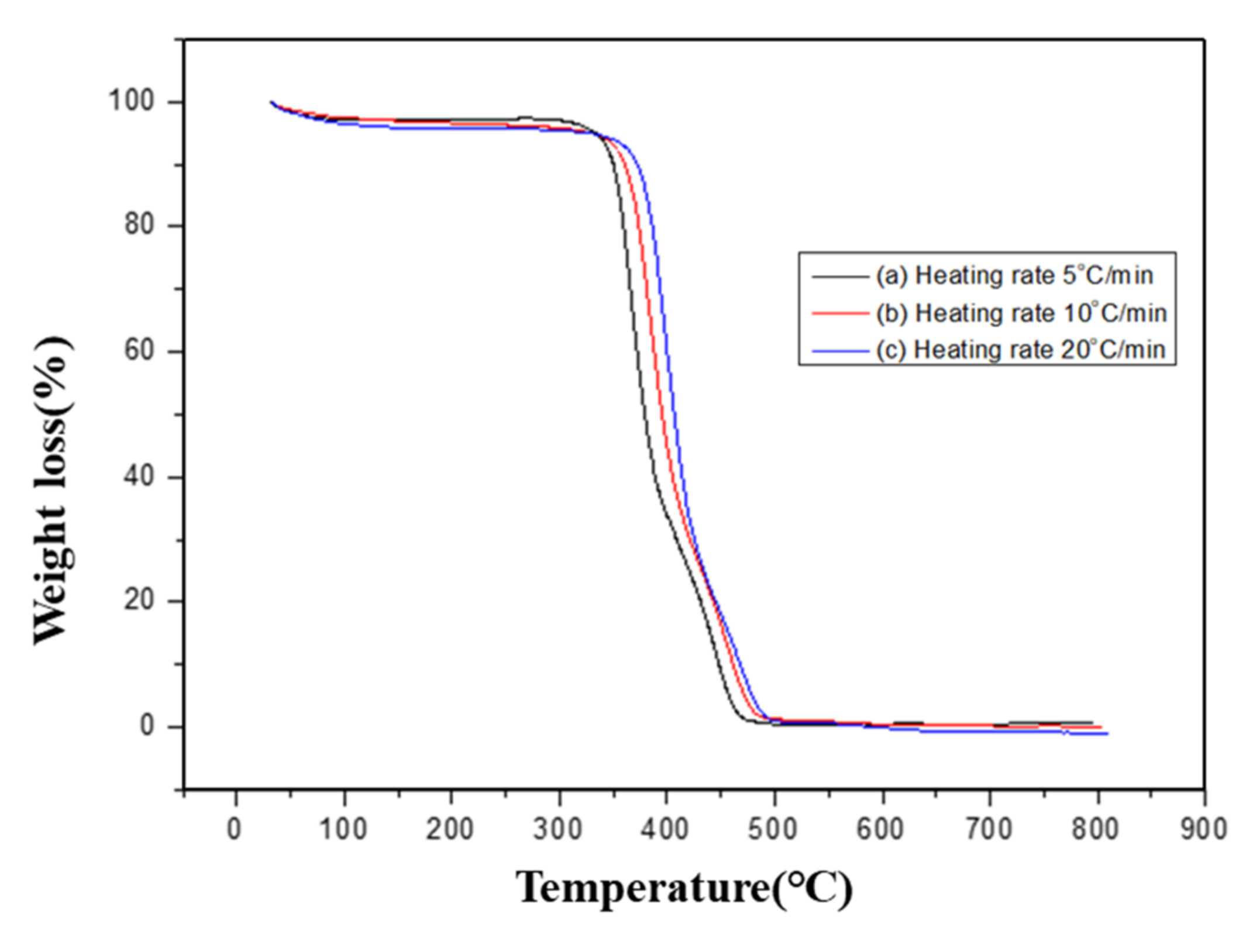
3.2. Thermal Degradation Stability
3.3. Differential Scanning Calorimetry (DSC) Analysis
3.4. X-Ray Diffraction (XRD) Analysis
3.5. Ionic Conductivity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barbosa, J.C.; Goncalves, R.; Costa, C.M.; Lanceros-Mendez, S. Recent advances on materials for lithium-ion batteries. Energies 2021, 14, 3154. [Google Scholar] [CrossRef]
- Taillades, G.; Hachemi, I.; Pers, P.; Dailly, J.; Marrony, M. Synthesis and characterizations of barium zirconate–alkali carbonate composite electrolytes for intermediate temperature fuel cells. J. Compos. Sci. 2021, 5, 183. [Google Scholar] [CrossRef]
- Zhan, H.; Wu, M.; Wang, R.; Wu, S.; Li, H.; Tian, T.; Tang, H. Excellent performances of composite polymer electrolytes with porous vinyl-functionalized SiO2 nanoparticles for lithium metal batteries. Polymers 2021, 13, 2468. [Google Scholar] [CrossRef] [PubMed]
- Sazali, N.; Salleh, W.N.W.; Jamaludin, A.S.; Razali, M.N.M. New perspectives on fuel cell technology: A brief review. Membranes 2020, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Waidha, A.I.; Vanita, V.; Clemens, O. PEO infiltration of porous garnet-type lithium-conducting solid electrolyte thin films. Ceramics 2021, 4, 421–436. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Nofal, M.M.; Karim, W.O.; Brevik, I.; Brza, M.A.; Abdulwahid, R.T.; Al-Zangana, S.; Kadir, M.F.Z. Structural, Impedance and electrochemical characteristics of electrical double layer capacitor devices based on chitosan: Dextran biopolymer blend electrolytes. Polymers 2020, 12, 1411. [Google Scholar] [CrossRef]
- Harun, N.A.M.; Shaari, N.; Zaiman, N.F.H.N. A review of alternative polymer electrolyte membrane for fuel cell application based on sulfonated poly(ether ether ketone). Int. J. Energy Res. 2021, 45, 19671–19708. [Google Scholar] [CrossRef]
- Niu, C.; Liu, J.; Chen, G.; Liu, C.; Qian, T.; Zhang, J.; Cao, B.; Shang, W.; Chen, Y.; Han, J. Anion-regulated solid polymer electrolyte enhances the stable deposition of lithium ion for lithium metal batteries. J. Power Source 2019, 417, 70–75. [Google Scholar] [CrossRef]
- Mackanic, D.G.; Yan, X.; Zhang, Q.; Matsuhisa, N.; Yu, Z.; Jiang, Y.; Manika, T.; Lopez, J.; Yan, H.; Liu, K.; et al. Decoupling of mechanical properties and ionic conductivity in supramolecular lithium ion conductors. Nat. Commun. 2019, 10, 5384. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Tong, R.A.; Zhang, J.; Chen, L.; Wang, C.A. Blending poly(ethylene oxide) and Li6.4La3Zr1.4Ta0.6O12 by Haake rheomixer without any solvent: A low-cost manufacture method for mass production of composite polymer electrolyte. J. Power Source 2020, 451, 227797. [Google Scholar] [CrossRef]
- Vinodh, R.; Atchudan, R.; Kim, H.J.; Yi, M. Recent advancements in polysulfone based membranes for fuel cell (PEMFCs, DMFCs and AMFCs) applications: A critical review. Polymers 2022, 14, 300. [Google Scholar] [CrossRef]
- Mery, A.; Rousselot, S.; Lepage, D.; Dolle, M. A critical review for an accurate electrochemical stability window measurement of solid polymer and composite electrolytes. Materials 2021, 14, 3840. [Google Scholar] [CrossRef]
- Raju, M.M.; Altayran, F.; Johnson, M.; Wang, D.; Zhang, Q. Crystal structure and preparation of Li7La3Zr2O12 (LLZO) solid-state electrolyte and doping impacts on the conductivity: An overview. Electrochem 2021, 2, 390–414. [Google Scholar] [CrossRef]
- Song, J.J.; Wang, Y.Y.; Wan, C.C. Review of gel-type polymer electrolytes for lithium-ion batteries. J. Power Source 1999, 77, 183–197. [Google Scholar] [CrossRef]
- Yurova, P.A.; Malakhova, V.R.; Gerasimova, E.V.; Stenina, I.A.; Yaroslavtsev, Q. Nafion/surface modified ceria hybrid membranes for proton exchange fuel cell application. Polymers 2021, 13, 2513. [Google Scholar] [CrossRef]
- Jeong, H.S.; Hong, S.C.; Lee, S.Y. Effect of microporous structure on thermal shrinkage and electrochemical performance of Al2O3/poly(vinylidene fluoride-hexafluoropropylene) composite separators for lithium-ion batteries. J. Membr. Sci. 2010, 364, 177–182. [Google Scholar] [CrossRef]
- Liang, Y.H.; Wang, C.C.; Chen, C.Y. Conductivity and characterization of plasticized polymer electrolyte based on (polyacrylonitrile-b-polyethylene glycol) copolymer. J. Power Source 2007, 172, 886–892. [Google Scholar] [CrossRef]
- Rao, M.; Liu, J.; Li, W.; Liang, Y.; Zhou, D. Preparation and performance analysis of PE-supported P(AN-co-MMA) gel polymer electrolyte for lithium ion battery application. J. Membr. Sci. 2008, 322, 314–319. [Google Scholar] [CrossRef]
- Oh, B.; Amine, K. Evaluation of macromonomer-based gel polymer electrolyte for high-power applications. Solid State Ion. 2004, 175, 785–788. [Google Scholar] [CrossRef]
- Liao, Y.; Sun, C.; Hu, S.; Li, W. Anti-thermal shrinkage nanoparticles/polymer and ionic liquid based gel polymer electrolyte for lithium ion battery. Electrochim. Acta 2013, 89, 461–468. [Google Scholar] [CrossRef]
- Li, H.; Wei, Z. Impacts of modified graphite oxide on crystallization, thermal and mechanical properties of polybutylene terephthalate. Polymers 2021, 13, 2431. [Google Scholar] [CrossRef] [PubMed]
- Dobrota, D.; Lazar, S.V. Ultrasonic welding of PBT-GF30 (70% polybutylene terephthalate + 30% fiber glass) and expanded polytetrafluoroethylene (e-PTFE). Polymers 2021, 13, 298. [Google Scholar] [CrossRef] [PubMed]
- Lemma, S.M.; Boi, C.; Carbonell, R.G. Nonwoven ion-exchange membranes with high protein binding capacity for bioseparations. Membranes 2021, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Sazali, N.; Salleh, W.N.W.; Jamaludin, A.S.; Razali, M.N.M. Iminodiacetic acid (IDA) cation-exchange nonwoven membranes for efficient capture of antibodies and antibody fragments. Membranes 2021, 11, 530. [Google Scholar]
- Xiao, B.; Wang, W.; Zhang, X.; Long, G.; Fan, J.; Chen, H.; Deng, L. A novel fractal solution for permeability and Kozeny-Carman constant of fibrous porous media made up of solid particles and porous fibers. Powder Technol. 2019, 349, 92–98. [Google Scholar] [CrossRef]
- Liang, M.; Fu, C.; Xiao, B.; Luo, L.; Wang, Z. A fractal study for the effective electrolyte diffudion through charged porous media. Int. J. Heat Mass Transf. 2019, 137, 365–371. [Google Scholar] [CrossRef]
- Dai, M.; Jin, S.; Nugen, S.R. Water-soluble electrospun nanofibers as a method for on-chip reagent storage. Biosensors 2012, 2, 388–395. [Google Scholar] [CrossRef] [Green Version]
- Kohn, S.; Wehlage, D.; Junger, I.J.; Ehrmann, A. Electrospinning a dye-sensitized solar cell. Catalysts 2019, 9, 975. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Chen, X.; Chen, H.; Liu, X.; Li, J.; Luo, J.; He, A.; Han, C.C.; Liu, Y.; Xu, S. Molecular interaction, chain conformation, and rheological modification during electrospinning of hyaluronic acid aqueous solution. Membranes 2020, 10, 217. [Google Scholar] [CrossRef]
- Xiao, W.; Song, J.; Huang, L.; Yang, Z.; Qiao, Q. PVA-ZrO2 multilayer composite separator with enhanced electrolyte property and mechanical strength for lithium-ion batteries. Ceram. Int. 2020, 46, 29212–29221. [Google Scholar] [CrossRef]
- Aragon-Gutierrez, A.; Rosa, E.; Gallur, M.; Lopez, D.; Hernandez-Munoz, P.; Gavara, R. Melt-processed bioactive EVOH films incorporated with ferulic acid. Polymers 2021, 13, 68. [Google Scholar] [CrossRef]
- Melendez, B.; Torres-Giner, S.; Zavagna, L.; Sammon, C.; Cabedo, L.; Prieto, C.; Lagaron, J.M. Development and characterization of electrospun fiber-based poly(ethylene-co-vinyl alcohol) films of application interest as high-gas-barrier interlayers in food packaging. Polymers 2021, 13, 2061. [Google Scholar] [CrossRef]
- Mori, M.; Stropnik, R.; Sekavcnik, M.; Lotric, A. Criticality and life-cycle assessment of materials used in fuel-cell and hydrogen technologies. Sustainability 2021, 13, 3565. [Google Scholar] [CrossRef]
- Lu, Q.; Fang, J.; Yang, J.; Miao, R.; Wang, J.; Nuli, Y. Novel cross-linked copolymer gel electrolyte supported by hydrophilic polytetrafluoroethylene for rechargeable lithium batteries. J. Membr. Sci. 2014, 449, 176–183. [Google Scholar] [CrossRef]
- Bianchi, O.; Oliveira, R.V.B.; Fiorio, R.; Martins, J.D.N.; Zattera, A.J.; Canto, L.B. Assessment of Avrami, Ozawa and Avrami–Ozawa equations for determination of EVA crosslinking kinetics from DSC measurements. Polym. Test. 2008, 27, 722–729. [Google Scholar] [CrossRef]
- Cui, H.W.; Suganuma, K.; Uchida, H. Using the Ozawa method to study the thermally initiated curing kinetics of vinyl ester resin. RSC Adv. 2015, 5, 2677–2683. [Google Scholar] [CrossRef]
- Meng, J.; Pan, Y.; Ran, Z.; Li, Y.; Jiang, J.; Wang, Q.; Jiang, J. Thermal hazard and decomposition kinetics of 1-butyl-2,3-dimethylimidazolium nitrate via TGA/DSC and FTIR. J. Loss Prev. Process Ind. 2021, 72, 104562. [Google Scholar] [CrossRef]
- Canakci, D. Thermal stability, degradation kinetic and structural characterization of novel aromatic amide compounds. J. Mol. Struct. 2020, 1205, 127645. [Google Scholar] [CrossRef]
- Zhang, X. Applications of kinetic methods in thermal analysis: A review. Eng. Sci. 2021, 14, 1–13. [Google Scholar] [CrossRef]
- Pal, A.K.; Kativar, V. Theoretical and analyzed data related to thermal degradation kinetics of poly(L-lactic acid)/chitosan-grafted-ologo L-lactic acid (PLA/CH-g-OLLA) bionanocomposite films. Data Brief 2017, 10, 304–311. [Google Scholar] [CrossRef]
- Wu, G.M.; Lin, S.J.; Yang, C.C. High performance composite solid polymer electrolyte systems for electrochemical cells. J. Power Source 2013, 244, 287–293. [Google Scholar] [CrossRef]

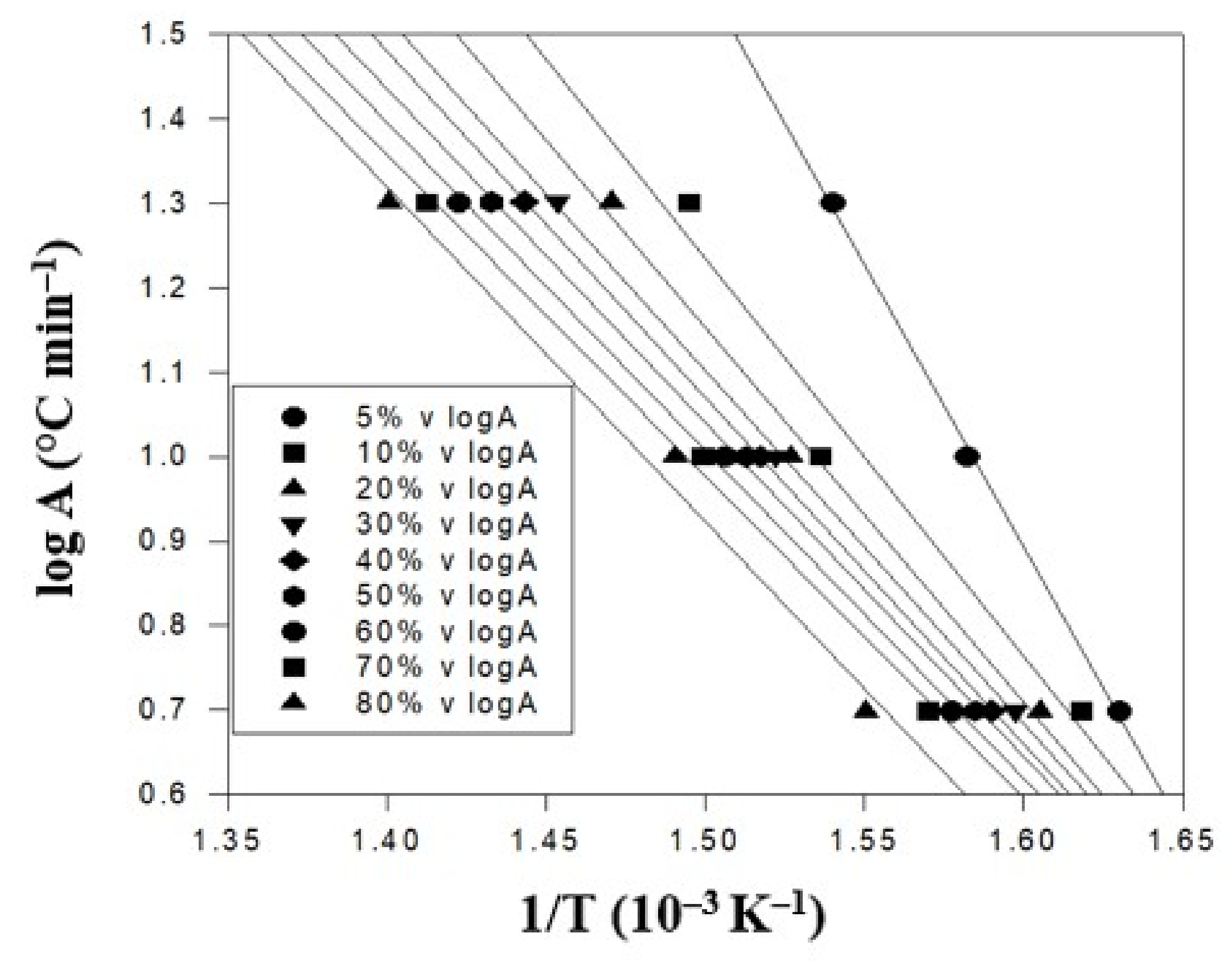
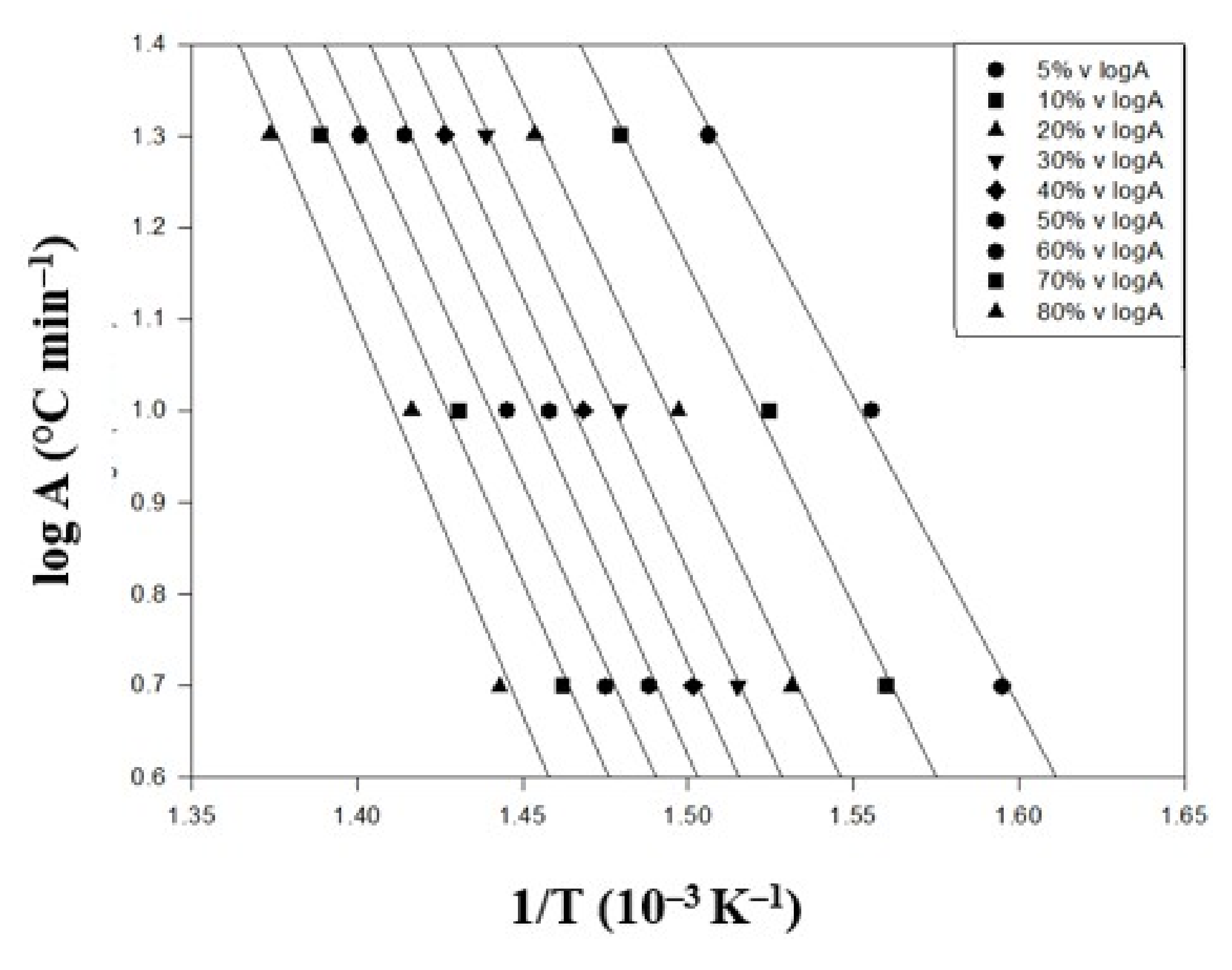



| Conversion (%) | Heating Rate (°C/min) | Ea (kJ/mol) | Slope | R2 | ||
|---|---|---|---|---|---|---|
| 5 | 10 | 20 | ||||
| T (°C) | ||||||
| 5 | 339 | 359 | 376 | 211 | 11.58 | 0.999 |
| 10 | 345 | 378 | 396 | 151 | 8.30 | 0.965 |
| 20 | 350 | 382 | 407 | 142 | 7.80 | 0.991 |
| 30 | 353 | 384 | 415 | 134 | 7.37 | 0.999 |
| 40 | 356 | 386 | 420 | 131 | 7.22 | 0.999 |
| 50 | 358 | 388 | 425 | 127 | 6.97 | 0.999 |
| 60 | 361 | 391 | 430 | 124 | 6.83 | 0.998 |
| 70 | 364 | 394 | 435 | 122 | 6.69 | 0.996 |
| 80 | 372 | 398 | 441 | 125 | 6.87 | 0.997 |
| Conversion (%) | Heating Rate (°C/min) | Ea (kJ/mol) | Slope | R2 | ||
|---|---|---|---|---|---|---|
| 5 | 10 | 20 | ||||
| T (°C) | ||||||
| 5 | 354 | 370 | 391 | 209 | 11.47 | 0.996 |
| 10 | 368 | 383 | 403 | 224 | 12.29 | 0.995 |
| 20 | 380 | 395 | 415 | 227 | 12.49 | 0.995 |
| 30 | 387 | 403 | 422 | 230 | 12.65 | 0.999 |
| 40 | 393 | 408 | 428 | 232 | 12.72 | 0.995 |
| 50 | 399 | 413 | 434 | 232 | 12.76 | 0.990 |
| 60 | 405 | 419 | 441 | 228 | 12.51 | 0.990 |
| 70 | 411 | 426 | 447 | 231 | 12.68 | 0.993 |
| 80 | 420 | 433 | 455 | 237 | 13.03 | 0.982 |
| Conversion (%) | Heating Rate (°C/min) | Ea (kJ/mol) | Slope | R2 | ||
|---|---|---|---|---|---|---|
| 5 | 10 | 20 | ||||
| T (°C) | ||||||
| 5 | 350 | 363 | 379 | 259 | 14.21 | 0.998 |
| 10 | 372 | 384 | 398 | 296 | 16.26 | 0.999 |
| 20 | 385 | 397 | 410 | 312 | 17.15 | 0.999 |
| 30 | 392 | 404 | 418 | 304 | 16.69 | 0.999 |
| 40 | 398 | 409 | 424 | 305 | 16.76 | 0.994 |
| 50 | 403 | 415 | 430 | 297 | 16.34 | 0.997 |
| 60 | 409 | 420 | 435 | 310 | 17.01 | 0.994 |
| 70 | 416 | 427 | 442 | 313 | 17.17 | 0.994 |
| 80 | 425 | 435 | 449 | 340 | 18.69 | 0.993 |
| Triallylamine Content (%) | Time (h) | L (cm) | Rb (Ω) | Area (cm2) | σ (S cm−1) |
|---|---|---|---|---|---|
| 0.0 | 24 | 0.0097 | 15.78 | 1.29 | 4.77 × 10−4 |
| 0.2 | 24 | 0.0106 | 4.67 | 1.29 | 1.76 × 10−3 |
| 1.5 | 24 | 0.0105 | 9.74 | 1.29 | 8.36 × 10−4 |
| 0.0 | 48 | 0.0100 | 1.88 | 1.29 | 4.12 × 10−3 |
| 0.2 | 48 | 0.0108 | 1.97 | 1.29 | 4.25 × 10−3 |
| 1.5 | 48 | 0.0095 | 1.46 | 1.29 | 5.04 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-J.; Wu, G. Thermal Degradation Kinetics Analysis of Polymer Composite Electrolyte Membranes of PEVOH and PBT Nano Fiber. Polymers 2022, 14, 537. https://doi.org/10.3390/polym14030537
Lin S-J, Wu G. Thermal Degradation Kinetics Analysis of Polymer Composite Electrolyte Membranes of PEVOH and PBT Nano Fiber. Polymers. 2022; 14(3):537. https://doi.org/10.3390/polym14030537
Chicago/Turabian StyleLin, Sheng-Jen, and Gwomei Wu. 2022. "Thermal Degradation Kinetics Analysis of Polymer Composite Electrolyte Membranes of PEVOH and PBT Nano Fiber" Polymers 14, no. 3: 537. https://doi.org/10.3390/polym14030537
APA StyleLin, S.-J., & Wu, G. (2022). Thermal Degradation Kinetics Analysis of Polymer Composite Electrolyte Membranes of PEVOH and PBT Nano Fiber. Polymers, 14(3), 537. https://doi.org/10.3390/polym14030537