Metronidazole Based Floating Bioadhesive Drug Delivery System for Potential Eradication of H. pylori: Preparation and In Vitro Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pre-Formulation Studies
2.2.1. Construction of Standard Calibration Curve
2.2.2. Metronidazole Solubility Analysis
2.2.3. Micromeritical Properties
- Angle of Repose
- θ = angle of repose
- h = pile height
- r = radius of the circle [24]
- b.
- Compressibility Index and Hausner’s Ratio
- m = powder mass either bulk or tapped
- v = bulk or tapped volume
- ρ tapped = tapped powder density
- ρ bulk = bulk powder density
2.2.4. Drug Excipients Compatibility Studies
2.3. Formulation Development and Optimization
- Tablet hardness more than 5.5 kg/cm2
- Tablet friability less than 0.8%
- Floating lag time not more than 120 s
- Buoyancy duration more than 6 h
2.4. Post-Compression Evaluation Parameters
2.4.1. Physical Appearance and Dimensional Specifications
2.4.2. Hardness and Friability of Floating Tablets
2.4.3. Uniformity of Weight Test for Floating Tablets
2.4.4. Drug Content Determination
2.4.5. Impact of pH
2.4.6. Impact of Agitational Speed
2.4.7. Impact of Osmotic Pressure
2.4.8. Floating Behavior Evaluation
- Swelling Index
- Wt = tablet weight at “t” time
- Wo = initial weight before immersion [34]
- b.
- In vitro Buoyancy/Floatability Study
- c.
- Tablet Density
- ρ = density; m = mass of the tablet in gram
- v = tablet volume in cm3
- r = radius of tablet in cm
- h = tablet crown thickness in cm
2.4.9. In Vitro Drug Release/Dissolution Test Studies
2.4.10. Dissolution Profile Kinetics
- Mt/M∞ = drug release fraction after “t” time
- K = rate constant; n = release exponent
- n = 0.5, it means drug release occurs by Quasi–Fickian diffusion mechanism
- n > 0.5, then release mechanism exhibited by drug will be non-Fickian, anomalous or case Ⅱ
- n = 1, it depicts case Ⅱ or zero order kinetics [38]
2.5. Statistical Analysis
3. Results
3.1. Standard Calibration Curve
3.2. Solubility Studies
3.3. Micromeritical Characteristics
3.4. Drug Excipients Compatibility Studies
3.5. Preparation of Osmotically Controlled Floating Tablets of Metronidazole
3.6. Metronidazole Floating Tablets Evaluation
3.6.1. Physical Appearance and Dimensional Specifications
3.6.2. Hardness of Floating Tablets
3.6.3. Friability of Floating Tablets
3.6.4. Weight Variation Study of Floating Tablets
3.6.5. Drug Content Determination/Content Uniformity Test
3.6.6. Floating Behavior Evaluation
3.6.7. Swelling Index Determination
3.6.8. Tablet Density
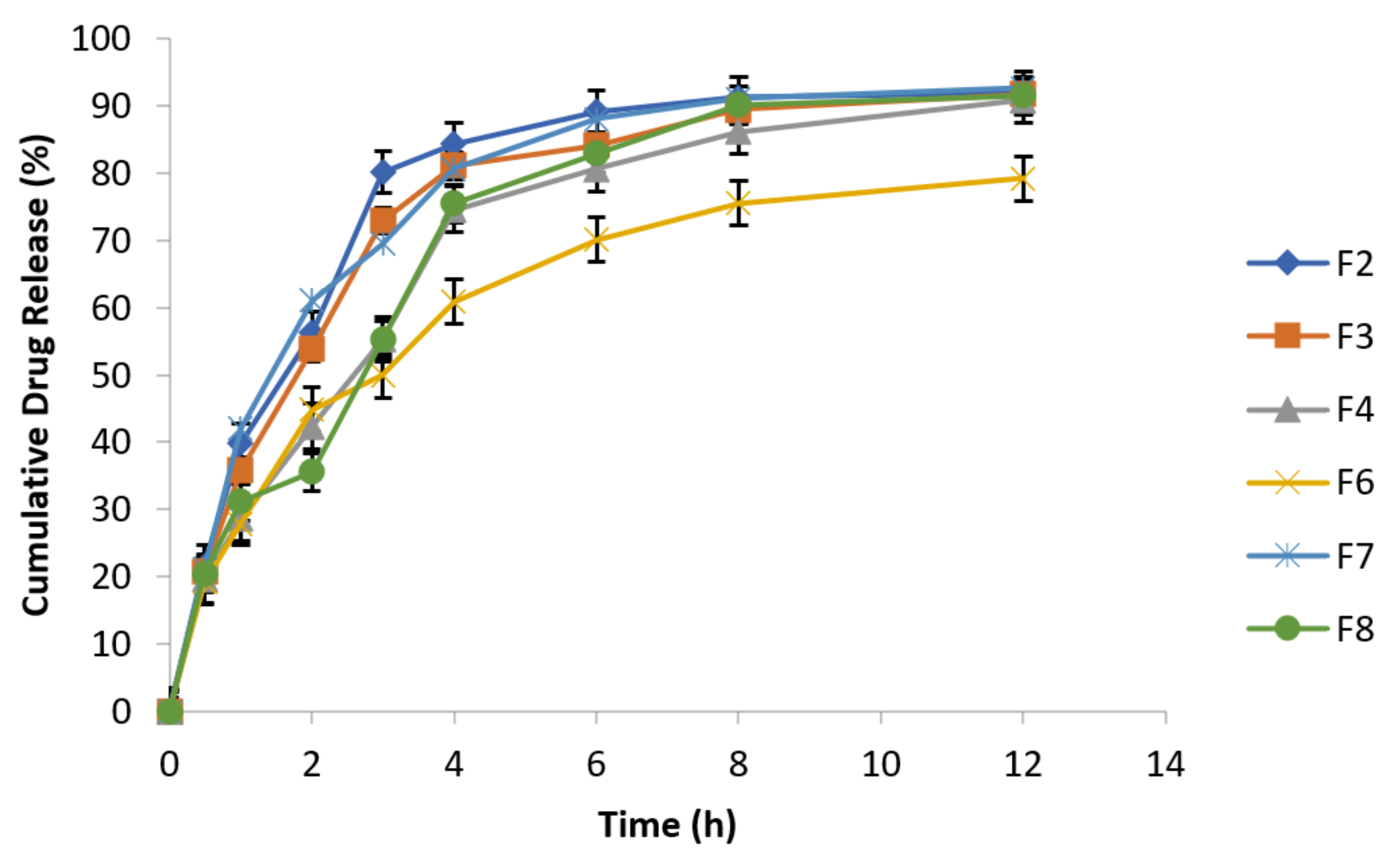
3.6.9. In Vitro Drug Release Behavior
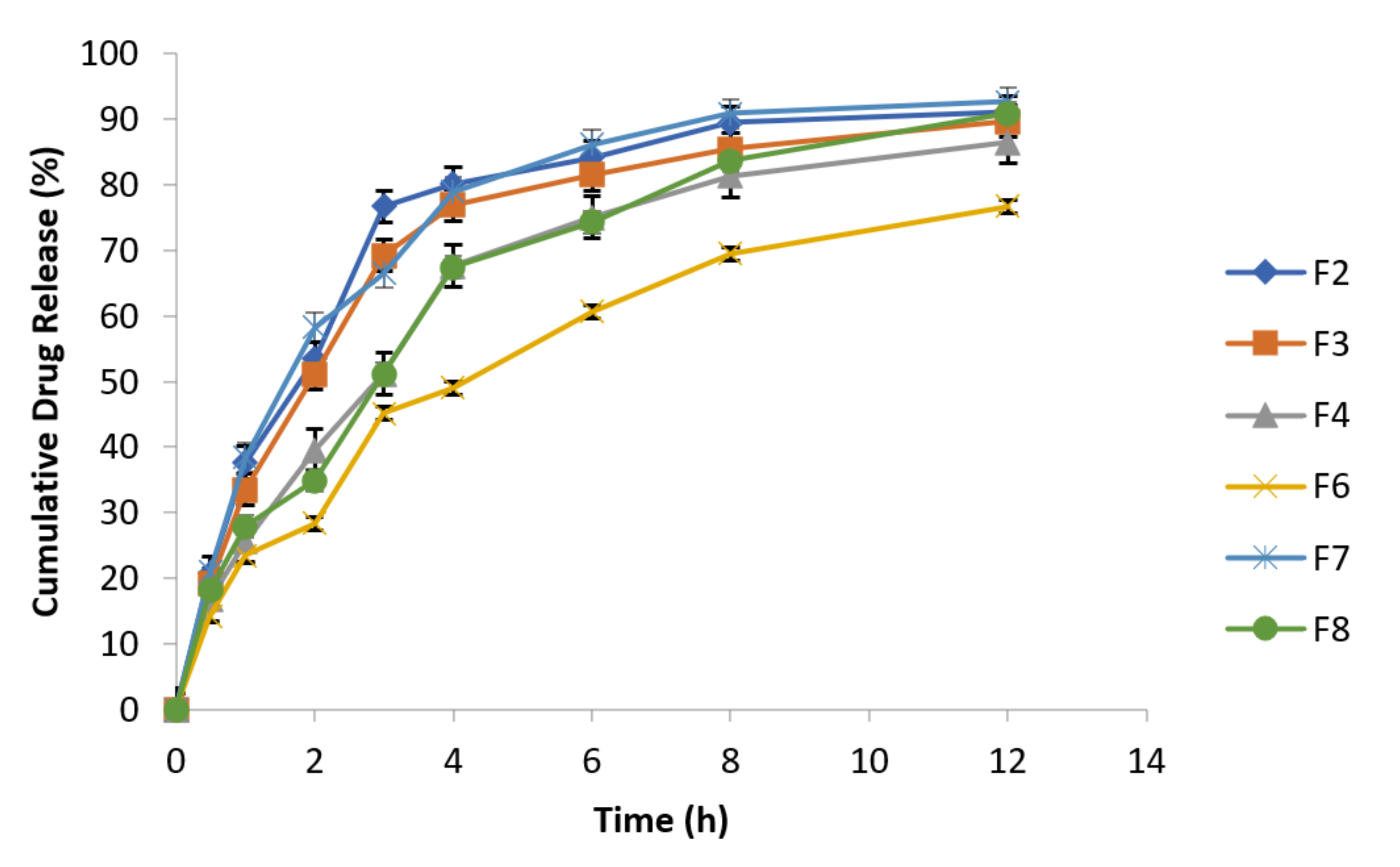
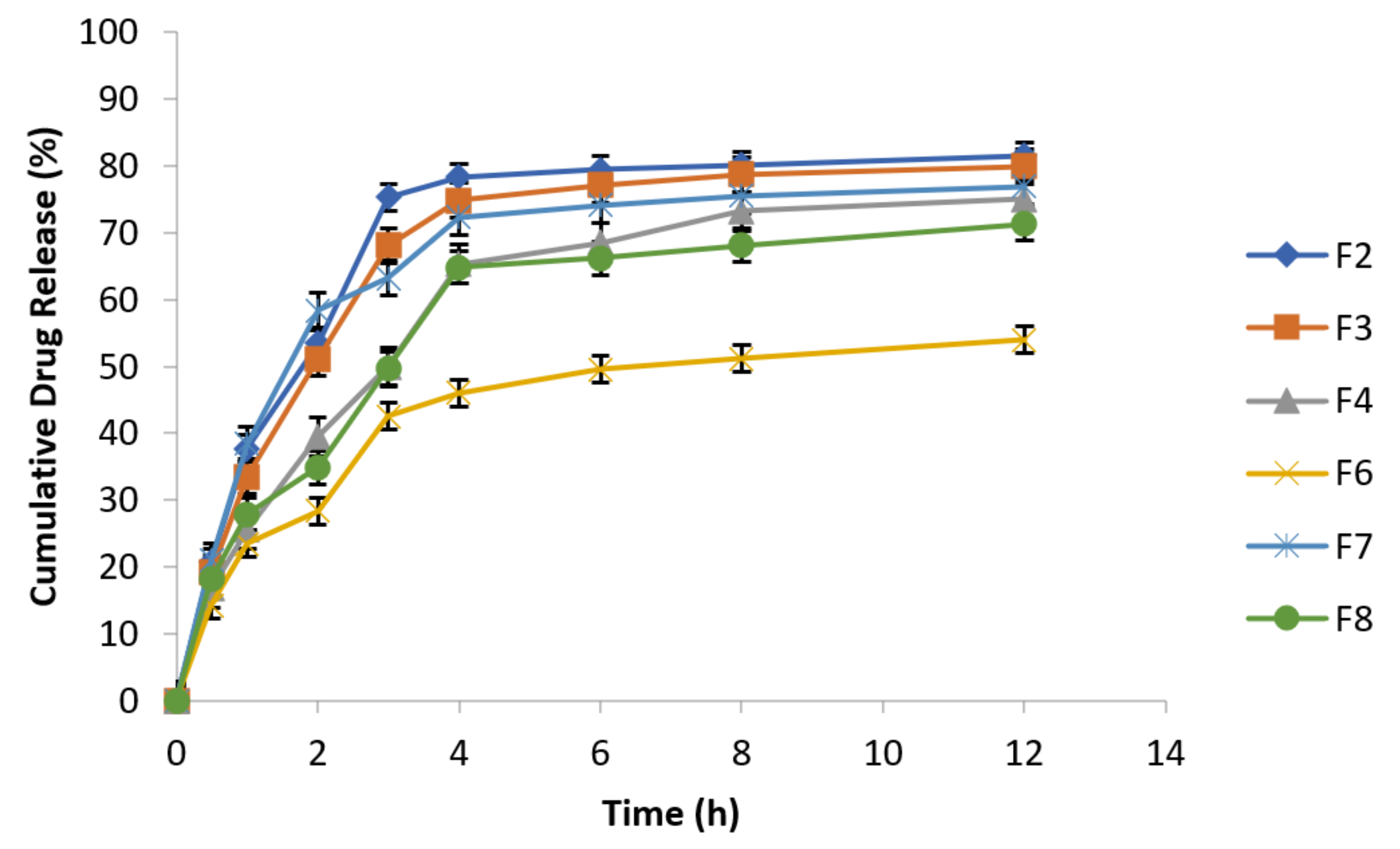
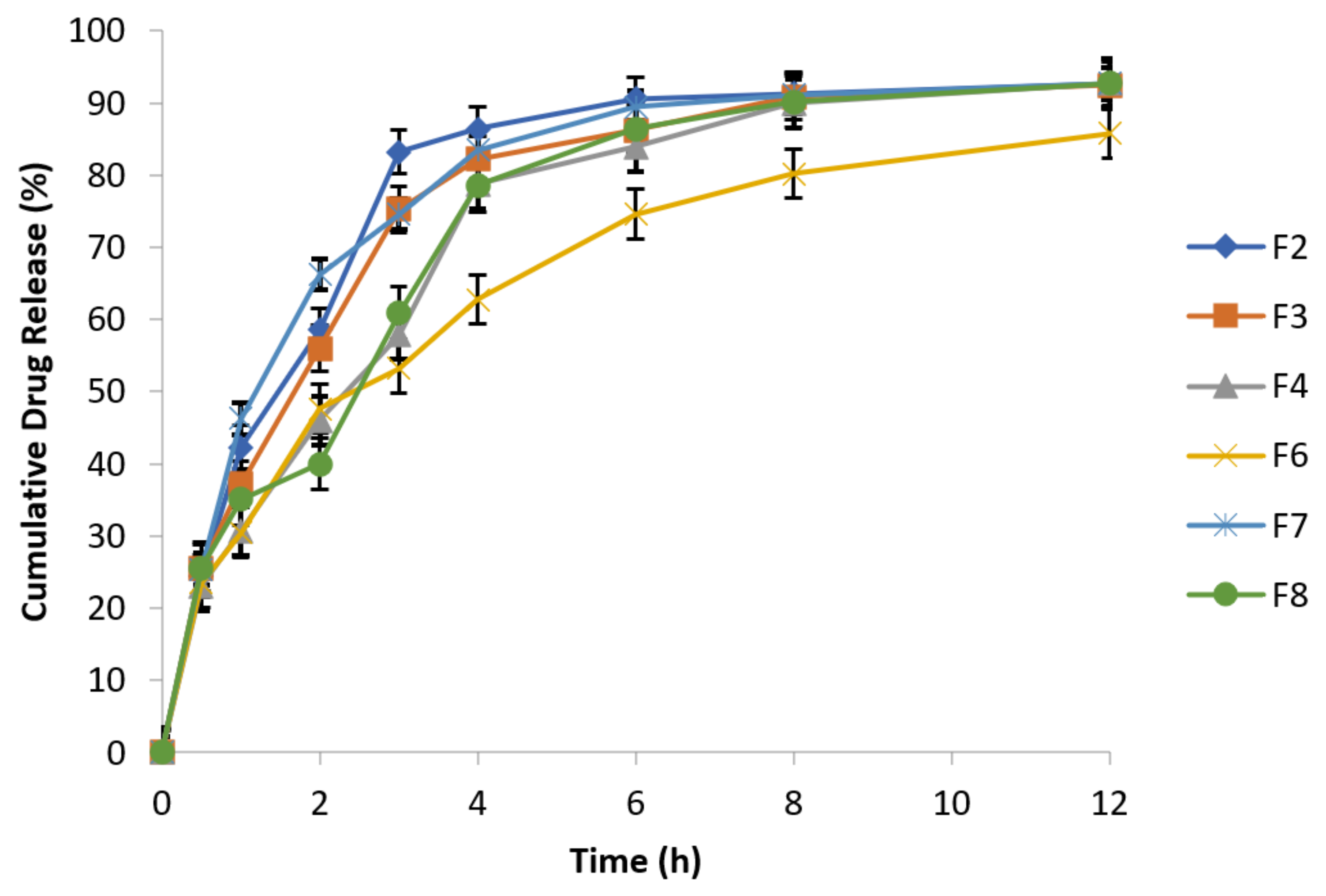
3.6.10. Effect of Agitational Intensity on Drug Release Data
3.6.11. Effect of pH on In Vitro Drug Release
3.6.12. Effect of Osmotic Pressure on Drug Release
3.6.13. In Vitro Drug Release Kinetic Profiling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gambhire, M.N.; Ambade, K.W.; Kurmi, S.D.; Kadam, V.J.; Jadhav, K.R. Development and in vitro evaluation of an oral floating matrix tablet formulation of diltiazem hydrochloride. AAPS PharmSciTech 2007, 8, 313. [Google Scholar] [CrossRef] [PubMed]
- Uǧurlu, T.; Karaçi̧cek, U.; Rayaman, E. Optimization and evaluation of clarithromycin floating tablets using experimental mixture design. Acta Pol. Pharm. Drug Res. 2014, 71, 311–321. [Google Scholar]
- Emara, L.H.; Abdou, A.R.; El-Ashmawy, A.A.; Mursi, N.M. Preparation and evaluation of metronidazole sustained release floating tablets. Int. J. Pharm. Pharm. Sci. 2014, 6, 198–204. [Google Scholar]
- Prasanthi, C.H.; Prasanthi, N.L.; Manikiran, S.S.; Rao, N.R. Focus on current trends in the treatment of helicobacter pylori infection: An update. Int. J. Pharm. Sci. Rev. Res. 2011, 9, 9. [Google Scholar]
- Youssef, N.A.H.A.; Kassem, A.A.; El-Massik, M.A.E.; Boraie, N.A. Development of gastroretentive metronidazole floating raft system for targeting Helicobacter pylori. Int. J. Pharm. 2015, 486, 297–305. [Google Scholar] [CrossRef]
- Krishnaiah, Y.S.R.; Raju, P.V.; Kumar, B.D.; Jayaram, B.; Rama, B.; Raju, V.; Bhaskar, P. Pharmacokinetic evaluation of guar gum-based colon-targeted oral drug delivery systems of metronidazole in healthy volunteers. Eur. J. Drug Metab. Pharmacokinet. 2003, 28, 287–294. [Google Scholar] [CrossRef]
- Arora, S.; Budhiraja, R. Effect of polymers and excipients on the release kinetics, bioadhesion, and floatability of metronidazole tablet. Asian J. Pharm. 2011, 5, 215. [Google Scholar] [CrossRef]
- Dudhipala, N.; Narala, A. Amoxycillin Trihydrate Floating-Bioadhesive Drug Delivery System for Eradication of Helicobacter pylori: Preparation, In Vitro and Ex Vivo Evaluation. J. Bioequiv. Bioavailab. 2013, 8, 118–124. [Google Scholar] [CrossRef]
- Guimarães, J. Helicobacter pylori: Fatores relacionados à sua patogênese. Rev. Para. Med. 2008, 22, 33–38. [Google Scholar]
- Malfertheiner, P.; Megraud, F.; O’Morain, A.C.; Atherton, J.; Axon, A.T.R.; Bazzoli, F.; Gensini, G.F.; Gisbert, J.P.; Graham, D.Y.; Rokkas, T.; et al. Management ofHelicobacter pyloriinfection—the Maastricht IV/Florence Consensus Report. Gut 2012, 61, 646–664. [Google Scholar] [CrossRef]
- García, A.; Salas-Jara, M.J.; Herrera, C.; González, C. Biofilm andHelicobacter pylori: From environment to human host. World J. Gastroenterol. 2014, 20, 5632–5638. [Google Scholar] [CrossRef] [PubMed]
- Payão, S.L.M. Helicobacter pyloriand its reservoirs: A correlation with the gastric infection. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Bardonnet, P.; Faivre, V.; Pugh, W.; Piffaretti, J.; Falson, F. Gastroretentive dosage forms: Overview and special case of Helicobacter pylori. J. Control. Release 2006, 111, 1–18. [Google Scholar] [CrossRef]
- Barrocas, P.M.D.C.; Santos, D.F.G.; Ferreira, D.C.; Coelho, P.M.B.D.S.; Oliveira, R.C.S.; Veiga, F.J.D.B. Sistemas farmacêuticos gastrorretentivos flutuantes. Rev. Bras. Ciências Farm. 2007, 43, 325–334. [Google Scholar] [CrossRef][Green Version]
- Ferrari, P.C.; Grossklauss, D.B.B.D.S.; Alvarez, M.; Paixão, F.C.; Andreis, U.; Crispim, A.G.; De Castro, A.D.; Evangelista, R.C.; Miranda, J.R.D.A. A novel automated alternating current biosusceptometry method to characterization of controlled-release magnetic floating tablets of metronidazole. Drug Dev. Ind. Pharm. 2014, 40, 1123–1131. [Google Scholar] [CrossRef]
- Umamaheshwari, R.B.; Jain, S.; Bhadra, D.; Jain, N.K. Floating microspheres bearing acetohydroxamic acid for the treatment of Helicobacter pylori. J. Pharm. Pharmacol. 2003, 55, 1607–1613. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Chaurasia, H.; Chaurasia, D.; Prajapati, S.K.; Singh, S. Formulation and in-vitro evaluation of floating microballoons of indomethacin. Acta Pol. Pharm. Drug Res. 2010, 67, 291–298. [Google Scholar]
- Jain, S.K.; Awasthi, A.M.; Jain, N.K.; Agrawal, G.P. Calcium silicate based microspheres of repaglinide for gastroretentive floating drug delivery: Preparation and in vitro characterization. J. Control. Release 2005, 107, 300–309. [Google Scholar] [CrossRef]
- Ovenseri, A.C.; Uhumwangho, U.M.; Obarisiagbon, A.J.; Umechukwu, C.P. Formulation of non-effervescent floating matrix tablets of metronidazole using Abelmoschus esculentus gum as binder and 2-camphanone as sublimating agent. J. Phytomed. Ther. 2020, 19, 387–397. [Google Scholar] [CrossRef]
- Misra, R.; Bhardwaj, P. Development and Characterization of Novel Floating-Mucoadhesive Tablets Bearing Venlafaxine Hydrochloride. Scientifica 2016, 2016, 4282986. [Google Scholar] [CrossRef]
- Kumar, S.A.; Debnath, M.; Anusha, K.; Manna, K.; Rao, J.S. Studies on Formulation Development and Evaluations on Losartan Floating and Bioadhesive Drug Delivery System. PJPR 2017, 3, 84–98. [Google Scholar] [CrossRef]
- Kailash, V.; Gali, V.; Prathiba, C. Preformulation Studies of Pharmaceutical New Drug Molecule & Products: An Preformulation Studies of Pharmaceutical New Drug Molecule & Products: An Overview. Am. J. Pharm. Health Res. 2013, 1, 1–20. [Google Scholar]
- Aoki, H.; Iwao, Y.; Mizoguchi, M.; Noguchi, S.; Itai, S. Clarithromycin highly-loaded gastro-floating fine granules prepared by high-shear melt granulation can enhance the efficacy of Helicobacter pylori eradication. Eur. J. Pharm. Biopharm. 2015, 92, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Geldart, D.; Abdullah, E.; Hassanpour, A.; Nwoke, L.; Wouters, I. Characterization of powder flowability using measurement of angle of repose. China Particuology 2006, 4, 104–107. [Google Scholar] [CrossRef]
- Singh, I.; Saini, V. Formulation and optimization of floating matrix tablets of clarithromycin using simplex lattice design. Pak. J. Pharm. Sci. 2016, 29, 511–519. [Google Scholar] [PubMed]
- Suriyaprakash, T.N.K.; Prabu, S.L.; Arumugarajan, A.; Sumathi, A. Formulation and Evaluation of Oral Controlled Release Clarithromycin Matrix Tablets using Hydrophilic Polymer. Int. J. Pharm. Sci. Nanotechnol. 2013, 6, 2255–2259. [Google Scholar] [CrossRef]
- Kumar, K.K.; Swathi, M.; Srinivas, L.; Basha, S.N. Formulation and evaluation of floating in situ gelling system of losartan potassium. Der. Pharm. Lett. 2015, 7, 98–112. [Google Scholar]
- Mohapatra, P.K.; Tomer, V.; Gupta, M.K. Design and development of losartan potassium floating drug delivery systems. Int. J. Appl. Pharm. 2018, 10, 168–173. [Google Scholar] [CrossRef]
- Eswaraiah, M.; Jaya, S. Design and in Vitro Characterization of Floating Tablets of Metronidazole. Asian J. Pharm. Clin. Res. 2019, 12, 539–544. [Google Scholar] [CrossRef]
- Aguilar-Díaz, J.E.; García-Montoya, E.; Suñe-Negre, J.M.; Pérez-Lozano, P.; Miñarro, M.; Ticó, J.R. Predicting orally disintegrating tablets formulations of ibuprophen tablets: An application of the new SeDeM-ODT expert system. Eur. J. Pharm. Biopharm. 2012, 80, 638–648. [Google Scholar] [CrossRef]
- Kumar, G.; Jain, V.; Shende, S.; Jain, P.; Kumar, C.G. Formulation and evaluation of bilayer tablets of clarithromycin and omeprazole against Helicobacter pylori infection. Pharma Innov. J. 2019, 8, 407–412. [Google Scholar]
- Chavanpatil, M.D.; Jain, P.; Chaudhari, S.; Shear, R.; Vavia, P. Novel sustained release, swellable and bioadhesive gastroretentive drug delivery system for ofloxacin. Int. J. Pharm. 2006, 316, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Ho, H.-O.; Liu, D.-Z.; Siow, W.-S.; Sheu, M.-T. Swelling/Floating Capability and Drug Release Characterizations of Gastroretentive Drug Delivery System Based on a Combination of Hydroxyethyl Cellulose and Sodium Carboxymethyl Cellulose. PLoS ONE 2015, 10, e0116914. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Shukla, V.K.; Easwari, T.S.; Kumar, S.A.N.J.O.O.; Chaudhary, R.A.M.K.U.M.A.R.; Saraswat, S. Formulation development and evaluation of mucoadhesive oral dosage form containing clarithromycin using different mucoadhesive polymers. Int. J. Pharm. Sci. Health Care 2012, 2, 166. [Google Scholar]
- Adibkia, K.; Hamedeyazdan, S.; Javadzadeh, Y. Drug release kinetics and physicochemical characteristics of floating drug delivery systems. Expert Opin. Drug Deliv. 2011, 8, 891–903. [Google Scholar] [CrossRef]
- Heng, W.; Wei, Y.; Zhou, S.; Ma, D.; Gao, Y.; Zhang, J.; Qian, S. Effects of Temperature and Ionic Strength of Dissolution Medium on the Gelation of Amorphous Lurasidone Hydrochloride. Pharm. Res. 2019, 36, 72. [Google Scholar] [CrossRef]
- Nayak, A.K.; Hasnain, M.S. Sterculia gum based multiple units for oral drug delivery. In Plant Polysaccharides-Based Multiple-Unit Systems for Oral Drug Delivery; Springer: Singapore, 2019; pp. 67–82. [Google Scholar]
- Yin, C.; Li, X. Anomalous diffusion of drug release from a slab matrix: Fractional diffusion models. Int. J. Pharm. 2011, 418, 78–87. [Google Scholar] [CrossRef]
- Panda, S.; Sailada, N.S.; Devi, B.; Pattnaik, S.; Maharana, L. Design of floating drug delivery systems: An update on polymeric advancements with special reference from natural origin. Int. J. Pharm. Sci. Rev. Res. 2016, 39, 125–132. [Google Scholar]
- Choudhury, P.; Murthy, P.; Tripathy, N.; Panigrahi, R.; Behera, S. Investigation of Drug Polymer Compatibility: Formulation and Characterization of Metronidazole Microspheres for Colonic Delivery. Webmed. Cent. Pharm Sci. 2012, 3, 1–19. [Google Scholar]
- Patel, S.S.; Ray, S.; Thakur, R.S. Formulation and evaluation of floating drug delivery system containing clarithromycin for helicobacter pylori. Acta Pol. Pharm. 2006, 63, 53–61. [Google Scholar]
- Chandira, M.; Chandramohan, D.; Chiranjib, B.J.; Kumar, K.S. Design and characterization of sustained release gastro retentive floating tablets of Diltiazem hydrochloride. Sch. Res. Libr. Der. Pharm. Lett. 2009, 1, 25–38. [Google Scholar]
- Labot, J.M.; Manzo, R.H.; Allemandi, D.A. Double-layered mucoadhesive tablets containing nystatin. AAPS PharmSciTech 2002, 3, E22. [Google Scholar] [CrossRef]
- Thahera, P.D.; Latha, K.; Shailaja, T.; Nyamathulla, S.; Uhumwangho, M.U. Formulation and evaluation of Norfloxacin gastro retentive drug delivery systems using natural polymers. Int. Curr. Pharm. J. 2012, 1, 155–164. [Google Scholar] [CrossRef]
- Manoj, K.K.; Harikumar, S.L. Formulation, optimization and in-vitro evaluation of metronidazole 500mg floating tablets for the treatment of helicobacter pylori induced gastric inflammatory disorder. World J. Pharm. Res. 2017, 6, 809–835. [Google Scholar] [CrossRef]
- Hashem, F.M.; Shaker, D.S.; Nasr, M.; Saad, I.E.; Ragaey, R. Guar gum and hydroxy propyl methylcellulose compressed coated tablets for colonic drug delivery: In vitro and in vivo evaluation in healthy human volunteers. Drug Discov. Ther. 2011, 5, 90–95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- da Ebithal, A.; Nidhal, K.M. Formulation and evaluation of trifluoperazine hcl as orodispersible tablets. Int. J. Pharm. 2014, 6, 294–300. [Google Scholar]
- Kumar, P.; Singh, S.; Mishra, B. Gastroretentive drug delivery system of Ranitidine hydrochloride based on osmotic technology: Development and evaluation. Curr. Drug Deliv. 2008, 5, 332–342. [Google Scholar] [CrossRef]
- Bhattacharyya, L.; Cecil, T.; Dabbah, R.; Roll, D.; Schuber, S.; Sheinin, E.B.; Williams, R.L. The Value of USP Public Standards for Therapeutic Products and the USP Council of Experts Executive Committee 2. 2004. Available online: www.usp.org (accessed on 6 April 2021).
- Purushothaman, M.; Ratna, V. Formulation Optimization and Release Kinetics of Metronidazole Matrix, Compression and Spray Coated Tablets: Effect of Organic Acid on Colon Targeted Drug Delivery System. Int. J. Res. Pharm. Sci. 2010, 1, 551–562. [Google Scholar]
- Ca, A.; Mu, U.; Ma, I. Evaluation of Ciprofloxacin Floating-Bioadhesive Tablet Formulated with Okra Gum as Multifunctional Polymer. Pharm. Biosci. J. 2018, 6, 1–11. [Google Scholar] [CrossRef]
- Pare, A.; Yadav, S.K.; Patil, U.K. Formulation and Evaluation of Effervescent Floating Tablet of Amlodipine besylate. Res. J. Pharm. Technol. 2008, 1, 526–530. [Google Scholar]
- Shin, S.; Kim, T.H.; Jeong, S.W.; Chung, S.E.; Lee, D.Y.; Kim, D.-H.; Shin, B.S. Development of a gastroretentive delivery system for acyclovir by 3D printing technology and its in vivo pharmacokinetic evaluation in Beagle dogs. PLoS ONE 2019, 14, e0216875. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, F.A.; Hussain, M.A.; Ashraf, M.U.; Haseeb, M.T.; Farid-Ul-Haq, M. Linseed hydrogel based floating drug delivery system for fluoroquinolone antibiotics: Design, in vitro drug release and in vivo real-time floating detection. Saudi Pharm. J. 2020, 28, 538–549. [Google Scholar] [CrossRef] [PubMed]





| Tablets Weight | Allowed Variation (%) |
|---|---|
| Average weight 130 mg or less | ±10 |
| More than 130 mg up to 324 mg | ±7.5 |
| Above 324 mg | ±5 |
| Prescribed Solvents | Solubility (mg/mL) at 25 °C | Solubility (mg/mL) at 37 °C |
|---|---|---|
| Water | 9.235 ± 1.135 | 10.568 ± 1.637 |
| 0.1 N HCl | 3.315 ± 1.983 | 3.973 ± 1.996 |
| Phosphate Buffer pH 4.5 | 0.0231 ± 0.0012 | 0.0276 ± 1.0023 |
| Phosphate Buffer pH 6.8 | 0.0356 ± 0.0017 | 0.0365 ± 0.0019 |
| Phosphate Buffer pH 7.4 | 0.0388 ± 0.0014 | 0.0425 ± 0.0021 |
| Batch Code | Angle of Repose | Bulk Density (gm/mL) | Tapped Density (gm/mL) | Compressibility Index (%) | Hausner’s Ratio |
|---|---|---|---|---|---|
| F1 | 27.6 ± 0.04 | 0.438 ± 0.05 | 0.497 ± 0.01 | 11.45 ± 0.06 | 1.12 ± 0.04 |
| F2 | 28.11 ± 0.01 | 0.433 ± 0.11 | 0.4871 ± 0.05 | 10.71 ± 0.03 | 1.12 ± 0.02 |
| F3 | 25.6 ± 0.02 | 0.435 ± 0.06 | 0.4861 ± 0.02 | 10.57 ± 0.05 | 1.13 ± 0.02 |
| F4 | 25.3 ± 0.05 | 0.438 ± 0.01 | 0.4971 ± 0.08 | 9.18 ± 0.03 | 1.13 ± 0.07 |
| F5 | 28.1 ± 0.08 | 0.439 ± 0.05 | 0.5091 ± 0.04 | 12.13 ± 0.16 | 1.15 ± 0.05 |
| F6 | 25.8 ± 0.02 | 0.432 ± 0.10 | 0.4751 ± 0.02 | 11.52 ± 0.03 | 1.10 ± 0.02 |
| F7 | 31.6 ± 0.08 | 0.443 ± 0.03 | 0.5160 ± 0.05 | 13.48 ± 0.01 | 1.15 ± 0.07 |
| F8 | 26.5 ± 0.03 | 0.438 ± 0.04 | 0.5041 ± 0.05 | 13.91 ± 0.03 | 1.12 ± 0.04 |
| Ingredients | F1 (mg) | F2 (mg) | F3 (mg) | F4 (mg) | F5 (mg) | F6 (mg) | F7 (mg) | F8 (mg) |
|---|---|---|---|---|---|---|---|---|
| Metronidazole | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 |
| Carbopol 934P | 150 | 150 | 150 | 150 | 150 | 150 | 150 | 150 |
| Chitosan | 150 | - | - | - | 75 | - | - | - |
| Guar Gum | - | 150 | - | - | 75 | - | - | 75 |
| Sodium alginate | - | - | 150 | - | - | 75 | - | - |
| HPMC | - | - | - | 150 | - | 75 | - | 75 |
| MCC | 75 | 75 | 75 | 75 | 75 | 75 | 125 | 75 |
| Lactose | - | - | - | - | - | - | 100 | - |
| NaHCO3 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 |
| Talc | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Magnesium stearate | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Batch Codes | Hardness (kg/cm2) | Friability | Thickness (mm) | Diameter (mm) | Weigh Variation (mg) | Drug Content (%) |
|---|---|---|---|---|---|---|
| F1 | 5.4 ± 0.25 | 0.41 ± 0.02 | 3.56 ± 0.015 | 15.11 ± 0.006 | 648 ± 4.12 | 95.21 |
| F2 | 5.8 ± 0.29 | 0.78 ± 0.21 | 3.68 ± 0.032 | 15.11 ± 0.063 | 647 ± 4.23 | 98.09 |
| F3 | 6.2 ± 0.10 | 0.83 ± 0.11 | 3.42 ± 0.010 | 15.13 ± 0.023 | 651 ± 3.87 | 98.63 |
| F4 | 6.3 ± 0.45 | 0.73 ± 0.33 | 3.45 ± 0.033 | 15.23 ± 0.002 | 652 ± 2.25 | 96.13 |
| F5 | 5.2 ± 0.33 | 0.95 ± 0.09 | 3.67 ± 0.043 | 15.10 ± 0.34 | 645 ± 3.30 | 96.71 |
| F6 | 6.8 ± 0.42 | 0.67 ± 0.54 | 3.70 ± 0.022 | 15.28 ± 0.054 | 649 ± 4.25 | 99.03 |
| F7 | 5.8 ± 0.21 | 0.54 ± 0.43 | 3.54 ± 0.011 | 15.19 ± 0.051 | 643 ± 4.32 | 98.13 |
| F8 | 6.1 ± 0.22 | 0.62 ± 0.01 | 3.63 ± 0.012 | 15.22 ± 0.032 | 648 ± 4.24 | 99.01 |
| Formulations | Tablet Density (g/cm3) | Floating Lag Time (sec) | Total Floating Time (h) |
|---|---|---|---|
| F1 | 0.984 | NA * | NA * |
| F2 | 0.957 | 18 | 6 |
| F3 | 0.975 | 12 | >12 |
| F4 | 0.987 | 4 | >12 |
| F5 | 0.993 | NA * | NA * |
| F6 | 0.979 | 5 | >12 |
| F7 | 0.982 | 35 | >12 |
| F8 | 0.989 | 20 | 6 |
| Apparatus | USP Type Ⅰ (Basket) |
|---|---|
| Agitation speed | 100 rpm |
| Medium | 0.1 N HCl, pH 1.2 |
| Volume | 900 mL |
| Temperature | 37 ± 1 °C |
| Time | 0.5, 1, 2, 3, 4, 6, 8 and 12 h |
| Wavelength | 277 nm |
| Power Law | ||||
|---|---|---|---|---|
| Formulation Codes | K ± SD | R2 | n | Release Mechanism |
| F2 | 0.001 ± 0.0088 | 0.9945 | 0.333 | Does not follow power law kinetics |
| F3 | 0.001 ± 0.00142 | 0.9956 | 0.432 | Quasi Fickian diffusion |
| F4 | 0.001 ± 0.00273 | 0.9978 | 0.52 | Anomalous non-Fickian diffusion |
| F6 | 0.003 ± 0.007 | 0.9988 | 0.51 | Anomalous non-Fickian diffusion |
| F7 | 0.001 ± 0.0078 | 0.9939 | 0.52 | Anomalous non-Fickian diffusion |
| F8 | 0.001 ± 0.0014 | 0.9978 | 0.54 | Anomalous non-Fickian diffusion |
| Power Law | ||||
|---|---|---|---|---|
| Formulation Codes | K ± SD | R2 | n | Release Mechanism |
| F2 | 0.001 ± 0.0013 | 0.9896 | 0.342 | Does not follow power law kinetics (Fickian) |
| F3 | 0.001 ± 0.0016 | 0.9925 | 0.406 | Quasi Fickian diffusion |
| F4 | 0.001 ± 0.0029 | 0.9965 | 0.50 | Anomalous non-Fickian diffusion |
| F6 | 0.003 ± 0.007 | 0.9981 | 0.51 | Anomalous non-Fickian diffusion |
| F7 | 0.001 ± 0.0008 | 0.9945 | 0.54 | Anomalous non-Fickian diffusion |
| F8 | 0.001 ± 0.0017 | 0.9967 | 0.57 | Anomalous non-Fickian diffusion |
| Power Law | ||||
|---|---|---|---|---|
| Formulation Codes | K ± SD | R2 | n | Release Mechanism |
| F2 | 0.001 ± 0.067 | 0.9268 | 0.318 | Does not follow power law kinetics |
| F3 | 0.001 ± 0.0008 | 0.9438 | 0.42 | Quasi Fickian diffusion |
| F4 | 0.001 ± 0.0012 | 0.9937 | 0.42 | Quasi Fickian diffusion |
| F6 | 0.001 ± 0.00146 | 0.9437 | 0.41 | Quasi Fickian diffusion |
| F7 | 0.001 ± 0.0007 | 0.881 | 0.321 | Does not follow power law kinetics |
| F8 | 0.001 ± 0.0006 | 0.9822 | 0.40 | Quasi Fickian diffusion |
| Power Law | ||||
|---|---|---|---|---|
| Formulation Codes | K ± SD | R2 | n | Release Mechanism |
| F2 | 0.001 ± 0.00028 | 0.9306 | 0.300 | Does not follow power law kinetics |
| F3 | 0.0001 ± 0.00017 | 0.9591 | 0.44 | Quasi Fickian diffusion |
| F4 | 0.001 ± 0.0003 | 0.9941 | 0.43 | Quasi Fickian diffusion |
| F6 | 0.001 ± 0.0003 | 0.9499 | 0.44 | Quasi Fickian diffusion |
| F7 | 0.0001 ± 0.00022 | 0.8792 | 0.278 | Does not follow power law kinetics |
| F8 | 0.0001 ± 0.00014 | 0.9981 | 0.45 | Quasi Fickian diffusion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naseem, F.; Shah, S.U.; Rashid, S.A.; Farid, A.; Almehmadi, M.; Alghamdi, S. Metronidazole Based Floating Bioadhesive Drug Delivery System for Potential Eradication of H. pylori: Preparation and In Vitro Characterization. Polymers 2022, 14, 519. https://doi.org/10.3390/polym14030519
Naseem F, Shah SU, Rashid SA, Farid A, Almehmadi M, Alghamdi S. Metronidazole Based Floating Bioadhesive Drug Delivery System for Potential Eradication of H. pylori: Preparation and In Vitro Characterization. Polymers. 2022; 14(3):519. https://doi.org/10.3390/polym14030519
Chicago/Turabian StyleNaseem, Faiza, Shefaat Ullah Shah, Sheikh Abdur Rashid, Arshad Farid, Mazen Almehmadi, and Saad Alghamdi. 2022. "Metronidazole Based Floating Bioadhesive Drug Delivery System for Potential Eradication of H. pylori: Preparation and In Vitro Characterization" Polymers 14, no. 3: 519. https://doi.org/10.3390/polym14030519
APA StyleNaseem, F., Shah, S. U., Rashid, S. A., Farid, A., Almehmadi, M., & Alghamdi, S. (2022). Metronidazole Based Floating Bioadhesive Drug Delivery System for Potential Eradication of H. pylori: Preparation and In Vitro Characterization. Polymers, 14(3), 519. https://doi.org/10.3390/polym14030519







