Highly Transparent Aromatic Polyamides from Unsymmetrical Diamine with Trifluoromethyl Groups
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
2.3. Model Reaction
2.4. Polymerization
2.5. Preparation of Poly(Amide-Imide) Films
3. Results and Discussion
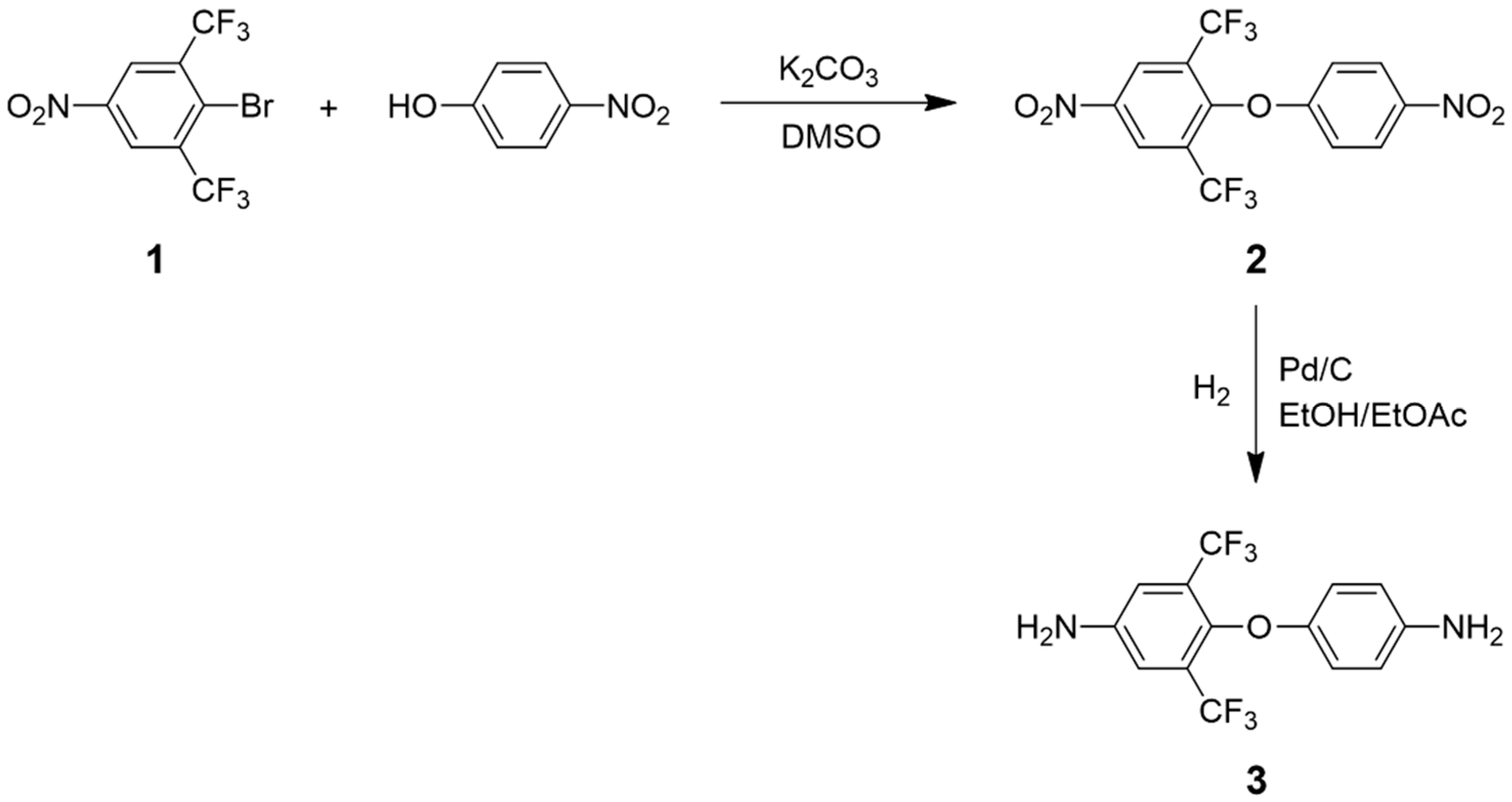
3.1. Monomer Synthesis
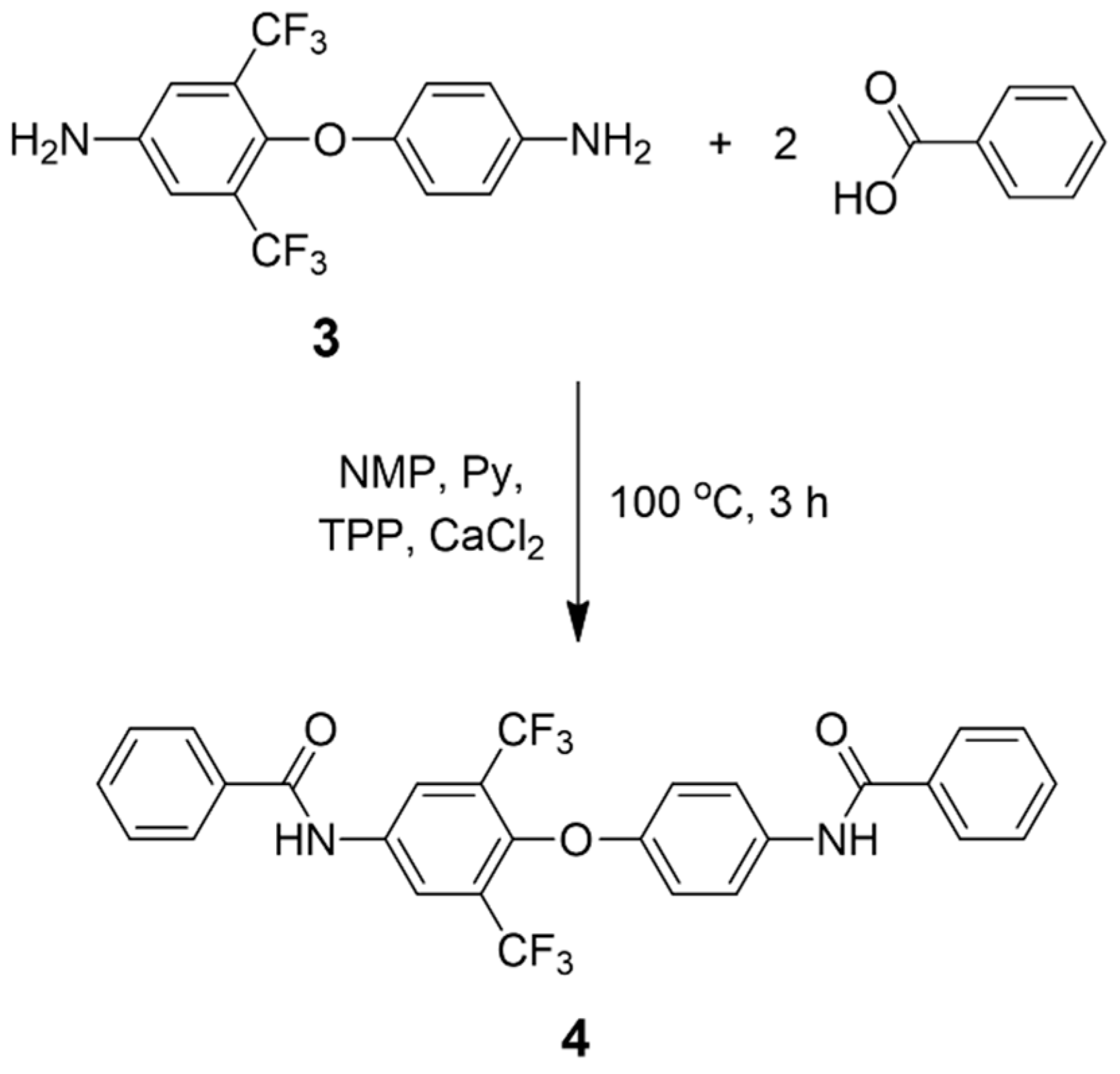
3.2. Model Reaction
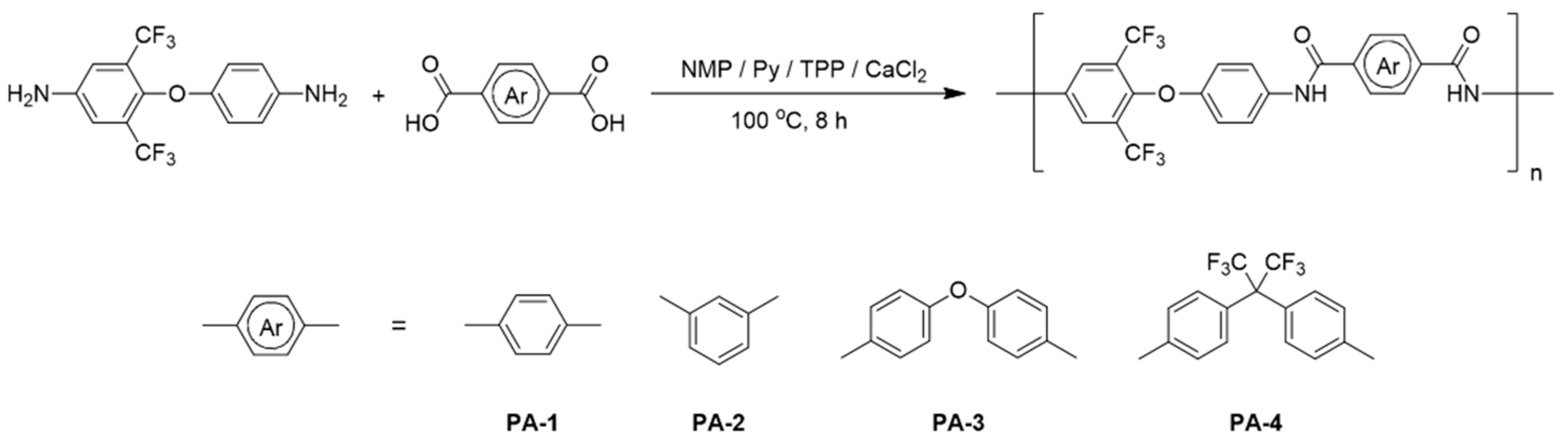
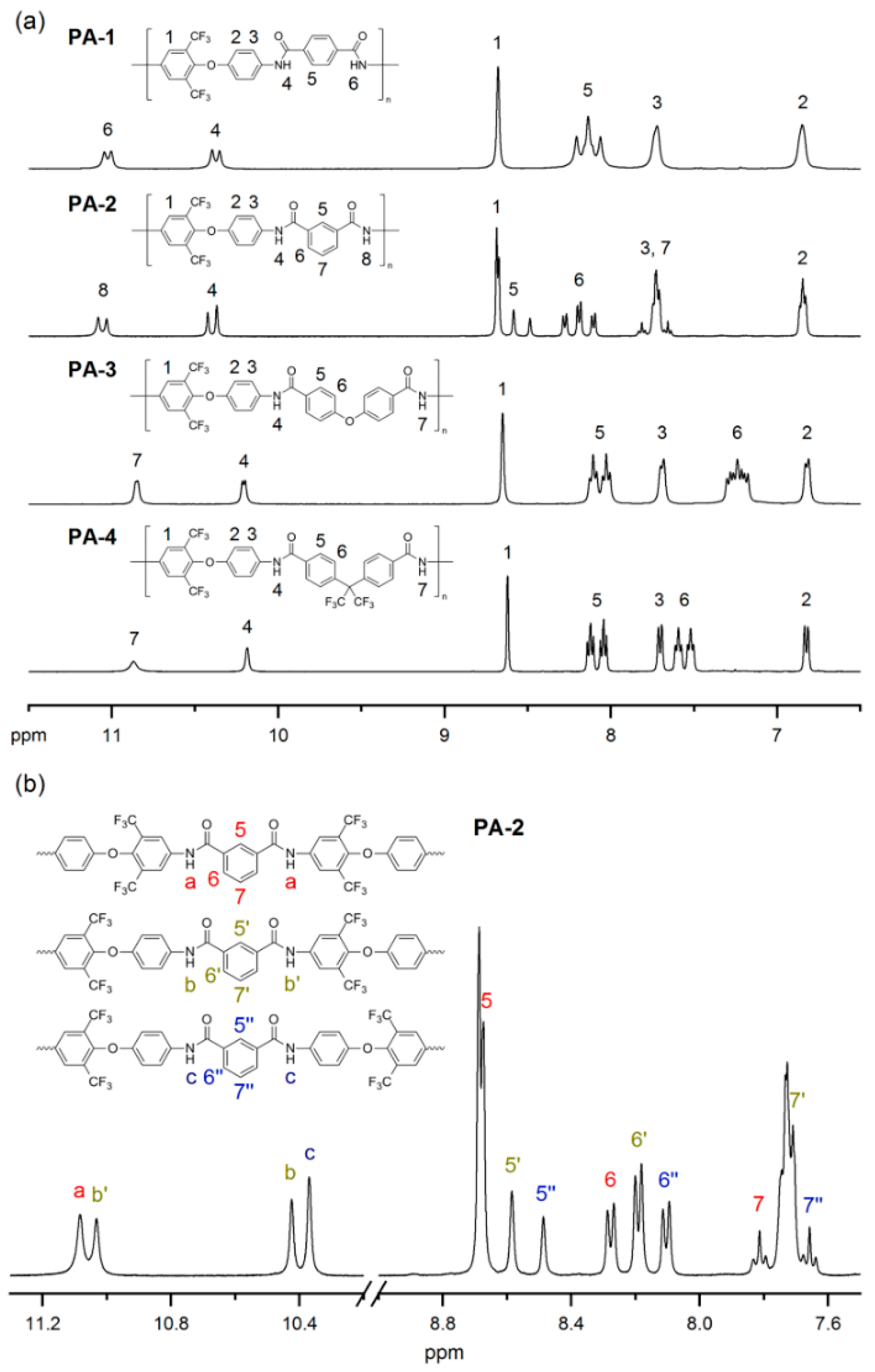
3.3. Polymerization
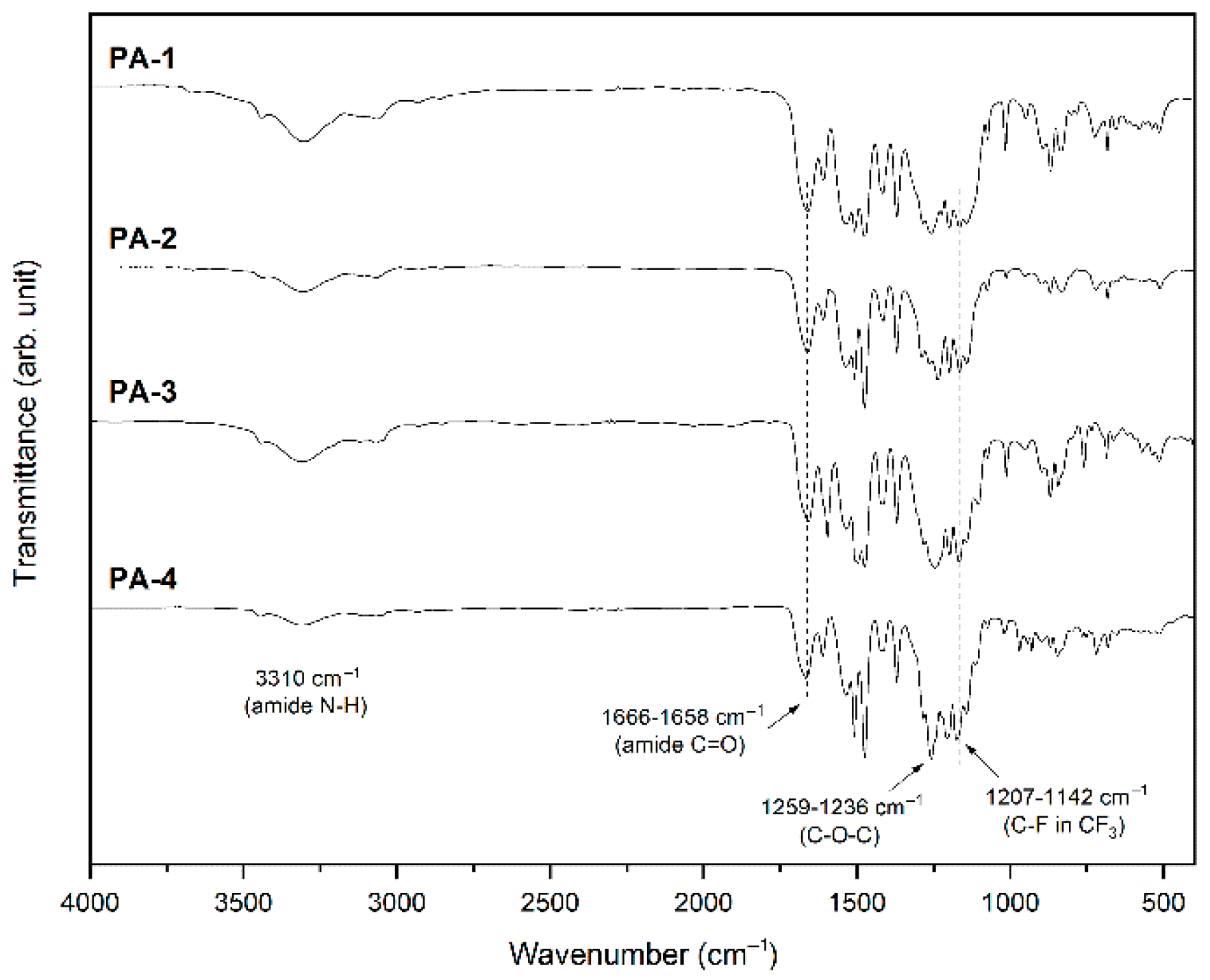
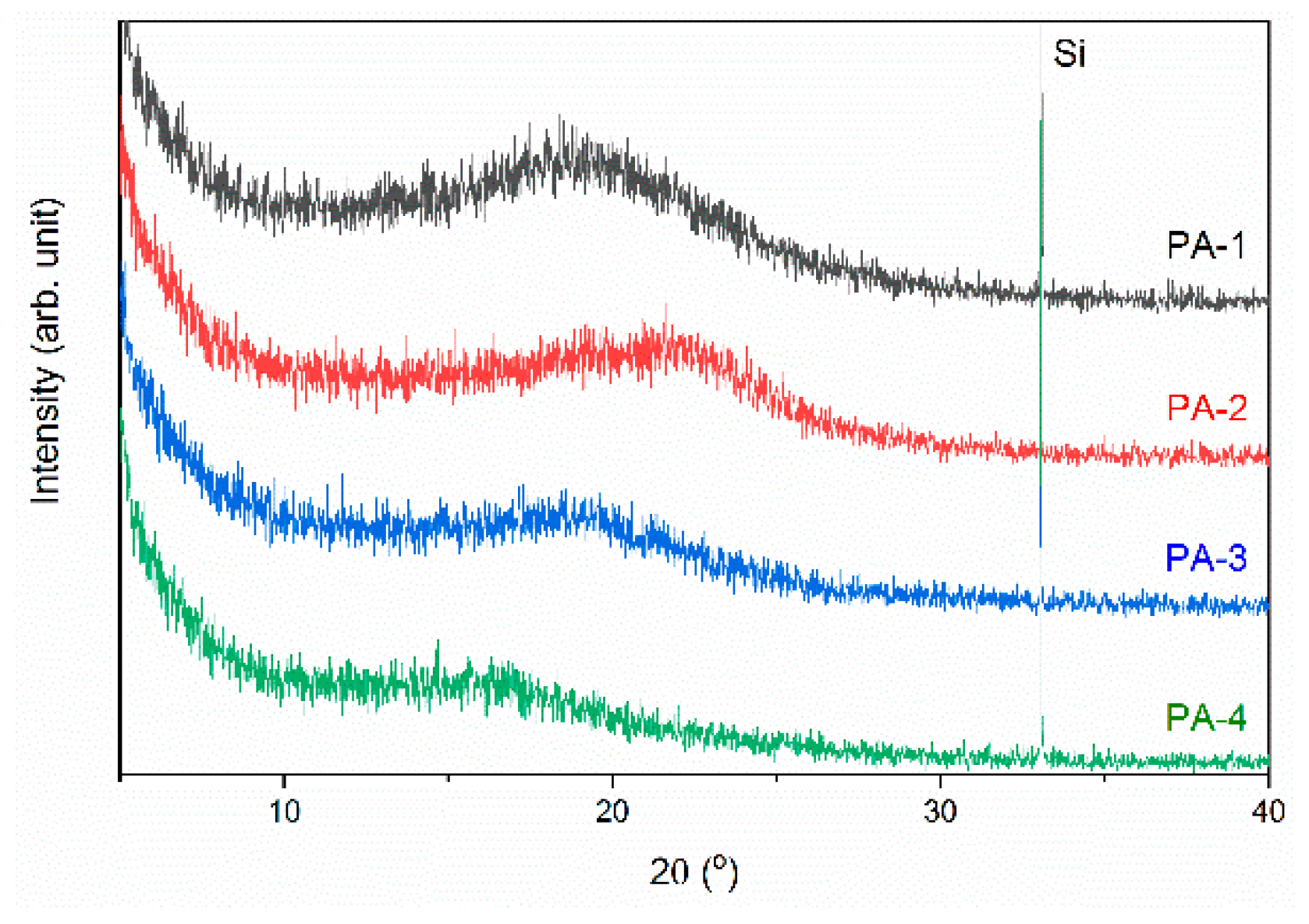
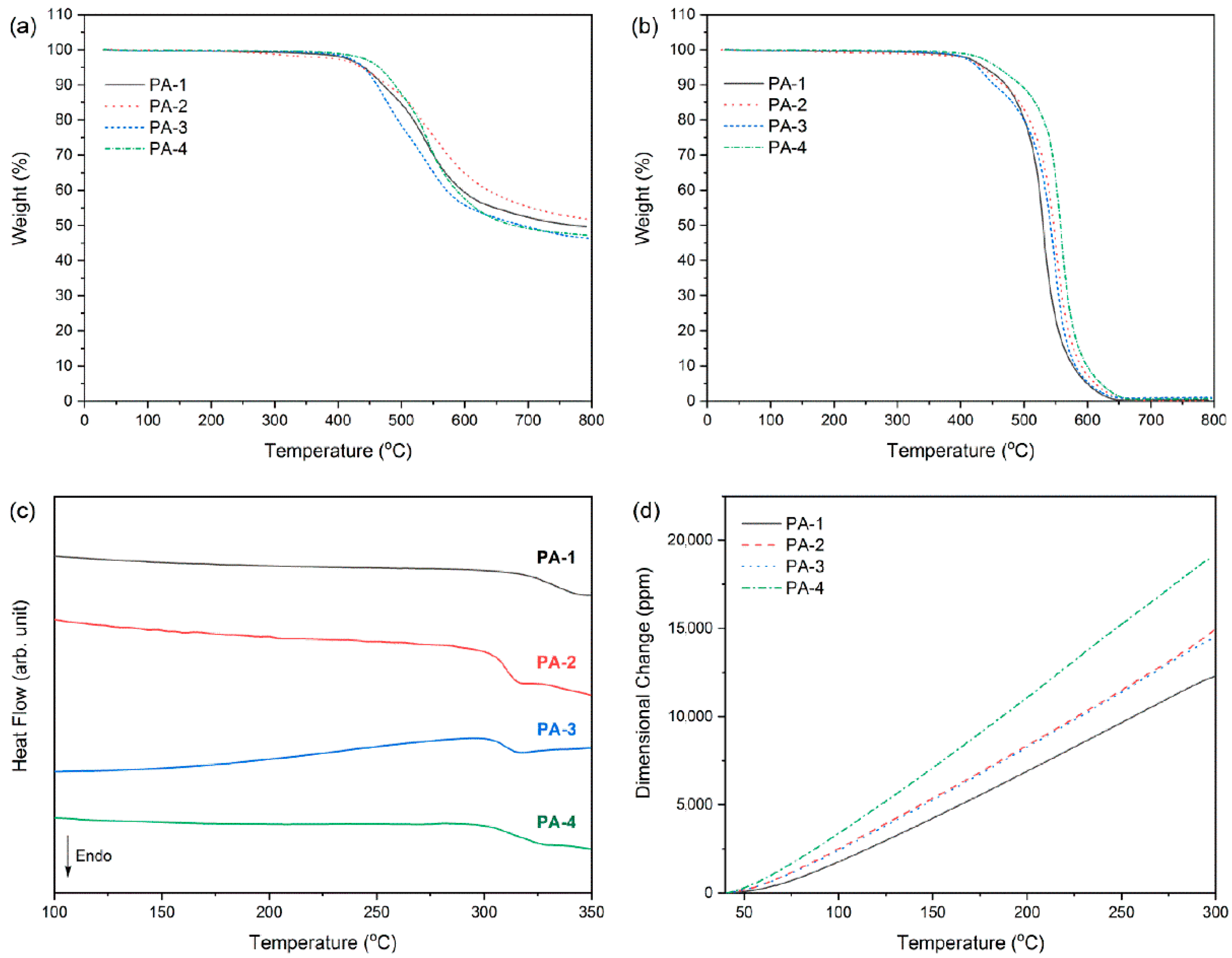
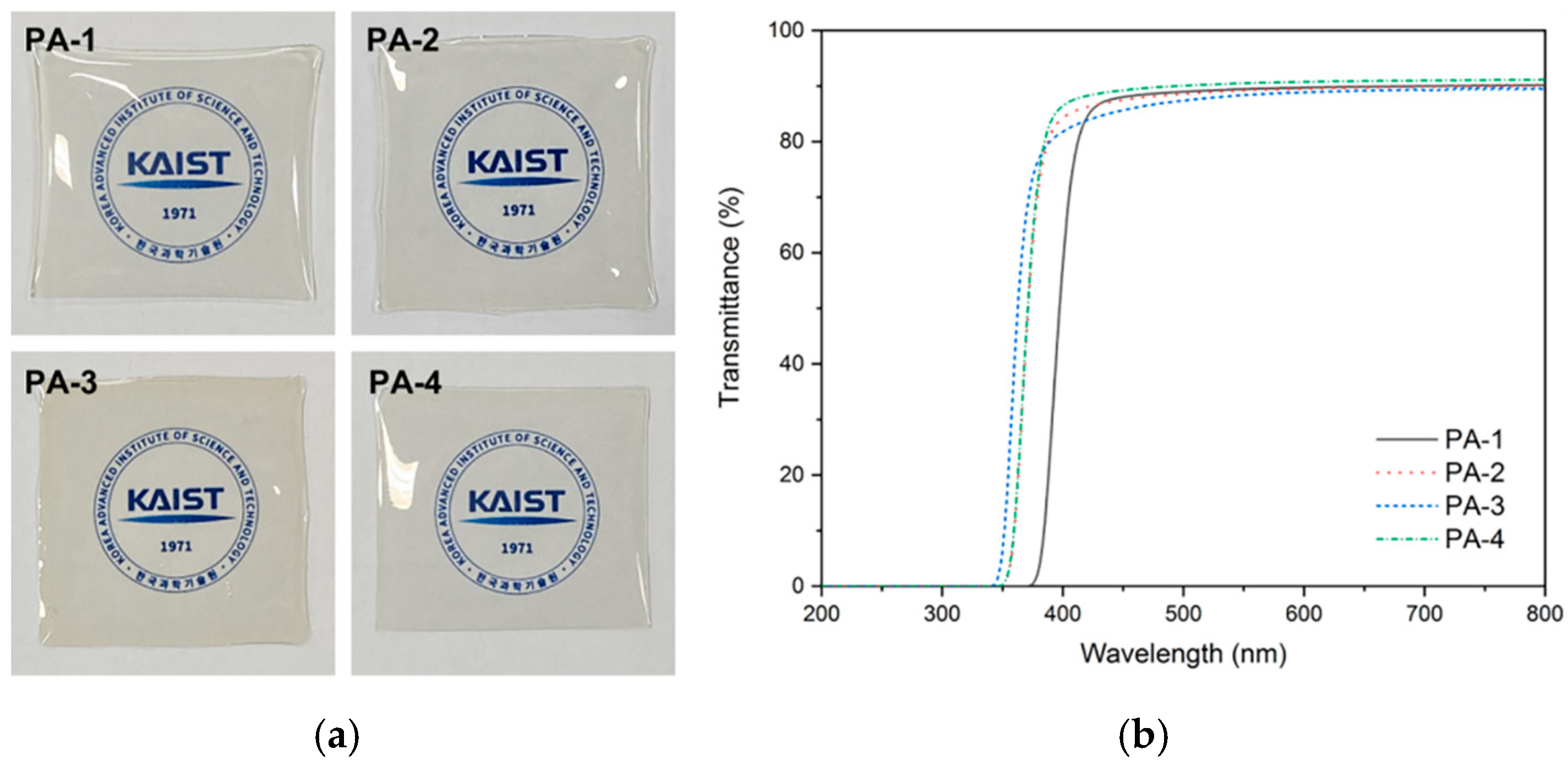
3.4. Polymer Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, M.-C.; Kim, Y.; Ha, C.-S. Polymers for flexible displays: From material selection to device applications. Prog. Polym. Sci. 2008, 33, 581–630. [Google Scholar] [CrossRef]
- Logothetidis, S. Flexible organic electronic devices: Materials, process and applications. Mater. Sci. Eng. B 2008, 152, 96–104. [Google Scholar] [CrossRef]
- Lim, H.; Cho, W.J.; Ha, C.S.; Ando, S.; Kim, Y.K.; Park, C.H.; Lee, K. Flexible organic electroluminescent devices based on fluorine-containing colorless polyimide substrates. Adv. Mater. 2002, 14, 1275–1279. [Google Scholar] [CrossRef]
- Yan, M.; Kim, T.W.; Erlat, A.G.; Pellow, M.; Foust, D.F.; Liu, J.; Schaepkens, M.; Heller, C.M.; Mcconnelee, P.A.; Feist, T.P.; et al. A transparent, high barrier, and high heat substrate for organic electronics. Proc. IEEE 2005, 93, 1468–1477. [Google Scholar] [CrossRef]
- Nakano, S.; Saito, N.; Miura, K.; Sakano, T.; Ueda, T.; Sugi, K.; Yamaguchi, H.; Amemiya, I.; Hiramatsu, M.; Ishida, A. Highly reliable a-IGZO TFTs on a plastic substrate for flexible AMOLED displays. J. Soc. Inf. Disp. 2012, 20, 493–498. [Google Scholar] [CrossRef]
- Zhu, H.; Shin, E.S.; Liu, A.; Ji, D.; Xu, Y.; Noh, Y.Y. Printable Semiconductors for Backplane TFTs of Flexible OLED Displays. Adv. Funct. Mater. 2020, 30, 1904588. [Google Scholar] [CrossRef]
- Yu, M.C.; Ruan, D.B.; Liu, P.T.; Chien, T.C.; Chiu, Y.C.; Gan, K.J.; Sze, S.M. High Performance Transparent a-IGZO Thin Film Transistors with ALD-HfO2Gate Insulator on Colorless Polyimide Substrate. IEEE Trans. Nanotechnol. 2020, 19, 481–485. [Google Scholar] [CrossRef]
- MacDonald, W.A. Engineered films for display technologies. J. Mater. Chem. 2004, 14, 4–10. [Google Scholar] [CrossRef]
- Yi, L.; Huang, W.; Yan, D. Polyimides with side groups: Synthesis and effects of side groups on their properties. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 533–559. [Google Scholar] [CrossRef]
- Tapaswi, P.K.; Ha, C.-S. Recent Trends on Transparent Colorless Polyimides with Balanced Thermal and Optical Properties: Design and Synthesis. Macromol. Chem. Phys. 2019, 220, 1800313. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horie, K. Photophysics, photochemistry, and optical properties of polyimides. Prog. Polym. Sci. 2001, 26, 259–335. [Google Scholar] [CrossRef]
- Chern, Y.T.; Tsai, J.Y. Low dielectric constant and high organosolubility of novel polyimide derived from unsymmetric l,4-Bis(4-aminophenoxy)-2,6-di-tert-butylbenzene. Macromolecules 2008, 41, 9556–9564. [Google Scholar] [CrossRef]
- Bong, S.; Yeo, H.; Goh, M.; Ku, B.C.; Kim, Y.Y.; Bong, P.H.; Park, B.; You, N.H. Synthesis and characterization of colorless polyimides derived from 4-(4-aminophenoxy)-2,6-dimethylaniline. Macromol. Res. 2016, 24, 1091–1097. [Google Scholar] [CrossRef]
- Yang, C.-P.; Hsiao, S.-H.; Yang, H.-W. Organosoluble optically transparent poly(ether imide)s based on atert-butylhydroquinone bis(ether anhydride). Macromol. Chem. Phys. 2000, 201, 409–418. [Google Scholar] [CrossRef]
- Dhara, M.G.; Banerjee, S. Fluorinated high-performance polymers: Poly(arylene ether)s and aromatic polyimides containing trifluoromethyl groups. Prog. Polym. Sci. 2010, 35, 1022–1077. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ishigami, T.; Ishii, J.; Sugiura, K.; Fujii, M. Solution-processable transparent polyimides with low coefficients of thermal expansion and self-orientation behavior induced by solution casting. Eur. Polym. J. 2013, 49, 3657–3672. [Google Scholar] [CrossRef]
- Hasegawa, M.; Watanabe, Y.; Tsukuda, S.; Ishii, J. Solution-processable colorless polyimides with ultralow coefficients of thermal expansion for optoelectronic applications. Polym. Int. 2016, 65, 1063–1073. [Google Scholar] [CrossRef]
- Reglero Ruiz, J.A.; Trigo-López, M.; García, F.C.; García, J.M. Functional aromatic polyamides. Polymers 2017, 9, 414. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Mao, D.; Fan, B. Copolyamide-Imide Membrane with Low CTE and CME for Potential Space Optical Applications. Polymers 2021, 13, 1001. [Google Scholar] [CrossRef]
- In, I.; Kim, S.Y. Soluble wholly aromatic polyamides containing unsymmetrical pyridyl ether linkages. Polymer 2006, 47, 547–552. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Lin, K.H. Soluble aromatic polyamides bearing asymmetrical diaryl ether groups. Polymer 2004, 45, 7877–7885. [Google Scholar] [CrossRef]
- Lee, B.; Byun, T.; Kim, S.D.; Kang, H.A.; Kim, S.Y.; Chung, I.S. Soluble para-linked aromatic polyamides with pendent groups. Macromol. Res. 2015, 23, 838–843. [Google Scholar] [CrossRef]
- Sheng, S.R.; Pei, X.L.; Huang, Z.Z.; Liu, X.L.; Song, C.S. Novel soluble fluorinated aromatic polyamides derived from 2-(4-trifluoromethylphenoxy)terephthaloyl chloride with various aromatic diamines. Eur. Polym. J. 2009, 45, 230–236. [Google Scholar] [CrossRef]
- Behniafar, H.; Khosravi-borna, S. Synthesis and characterization of novel aromatic polyamides derived from 2, 2′-bis(p-phenoxyphenyl)-4, 4′-diaminodiphenyl ether. Polym. Int. 2009, 58, 1299–1307. [Google Scholar] [CrossRef]
- Sheng, S.R.; Ma, C.X.; Jiang, J.W.; Huang, Z.Z.; Song, C.S. Synthesis and properties of novel aromatic polyamides with xanthene cardo groups. J. Appl. Polym. Sci. 2010, 116, 1650–1659. [Google Scholar] [CrossRef]
- Liou, G.-S.; Maruyama, M.; Kakimoto, M.-A.; Imai, Y. Preparation and properties of aromatic polyamides from 2,2′-bis(p-aminophenoxy) biphenyl or 2,2′-bis(p-aminophenoxy)-1,1′-binaphthyl and aromatic dicarboxylic acids. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 2499–2506. [Google Scholar] [CrossRef]
- Banerjee, S. Handbook of Specialty Fluorinated Polymers, 1st ed.; William, A., Ed.; Kidlington: Oxford, UK, 2015; ISBN 9780323357920. [Google Scholar]
- Rogers, H.G.; Gaudiana, R.A.; Hollinsed, W.C.; Kalyanaraman, P.S.; Manello, J.S.; Mcgowan, C.; Minns, R.A.; Sahatjian, R. Highly Amorphous, Birefringent, Para-Linked Aromatic Polyamides. Macromolecules 1985, 18, 1058–1068. [Google Scholar] [CrossRef]
- Gaudiana, R.A.; Minns, R.A.; Rogers, H.G.; Sinta, R.; Taylor, L.D.; Kalyanaraman, P.; McGowan, C. Molecular factors affecting solubility in rigid-rod polyamides. J. Polym. Sci. Part A Polym. Chem. 1987, 25, 1249–1271. [Google Scholar] [CrossRef]
- Takada, K.; Mae, Y.; Kaneko, T. Fluorinated and bio-based polyamides with high transparencies and low yellowness index. Polymers 2018, 10, 1311. [Google Scholar] [CrossRef] [Green Version]
- Murad, A.R.; Iraqi, A.; Aziz, S.B.; Hi, H.; Abdullah, S.N.; Brza, M.A.; Abdulwahid, R.T. Influence of Fluorine Substitution on the Optical, Thermal, Electrochemical and Structural Properties of Carbazole-Benzothiadiazole Dicarboxylic Imide Alternate Copolymers. Polymers 2020, 12, 2910. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.D.; Lee, B.; Byun, T.; Chung, I.S.; Park, J.; Shin, I.; Ahn, N.Y.; Seo, M.; Lee, Y.; Kim, Y.; et al. Poly(amide-imide) materials for transparent and flexible displays. Sci. Adv. 2018, 4, eaau1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuura, T.; Hasuda, Y.; Nishi, S.; Yamada, N. Polyimide derived from 2,2′-bis(trifluoromethyl)-4,4′-diaminobiphenyl. 1. Synthesis and characterization of polyimides prepared with 2,2′-bis(3,4-dicarboxyphenyl)hexafluoropropane dianhydride or pyromellitic dianhydride. Macromolecules 1991, 24, 5001–5005. [Google Scholar] [CrossRef]
- Ma, C.X.; Sheng, S.R.; Wei, M.H.; He, W.; Song, C.S. High-optical transparency and low-dielectric constant of new organosoluble polyamides containing trifluoromethyl and xanthene groups. J. Appl. Polym. Sci. 2010, 118, 2959–2968. [Google Scholar] [CrossRef]
- Wang, C.Y.; Li, P.H.; Li, G.; Jiang, J.M. High optical transparency and low dielectric constant of novel organosoluble poly(ether ketone amide)s derived from an unsymmetrical diamine containing trifluoromethyl and methyl pendant groups. Colloid Polym. Sci. 2009, 287, 495–500. [Google Scholar] [CrossRef]
- Liaw, D.J. Optically high transparency and light color of organosoluble polyamides containing trifluoromethyl and kink diphenylmethylene linkage. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 4559–4569. [Google Scholar] [CrossRef]
- Liaw, D.J.; Huang, C.C.; Chen, W.H. Color lightness and highly organosoluble fluorinated polyamides, polyimides and poly(amide-imide)s based on noncoplanar 2,2′-dimethyl-4,4′- biphenylene units. Polymer 2006, 47, 2337–2348. [Google Scholar] [CrossRef]
- Li, P.; Wang, C.; Li, G.; Jiang, J. High optical transparency and low dielectric constant of organosoluble poly(aryl ether amides) based on 1, 2-bis (4-amino-2-trifluoromethylphenoxy) benzene and aromatic dicarboxylic acids. J. Macromol. Sci. Part A Pure Appl. Chem. 2010, 47, 55–60. [Google Scholar] [CrossRef]
- Kim, S.D.; Kim, S.Y.; Chung, I.S. Soluble and transparent polyimides from unsymmetrical diamine containing two trifluoromethyl groups. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4413–4422. [Google Scholar] [CrossRef]
- Yamazaki, N.; Higashi, F.; Kawabata, J. Studies on reactions of the N-phosphonium salts of pyridines. XI. Preparation of polypeptides and polyamides by means of triaryl phosphites in pyridine. J. Polym. Sci. Polym. Chem. Ed. 1974, 12, 2149–2154. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Wang, X.L.; Liu, G.C.; Chen, L.; Wang, Y.Z. Thermal transition behaviors, solubility, and mechanical properties of wholly aromatic para-, meta -poly(ether-amide)s: Effect on numbers of para -aryl ether linkages. RSC Adv. 2016, 6, 84284–84293. [Google Scholar] [CrossRef]
- Yang, C.P.; Chen, R.S.; Chen, K.H.; Chen, Y.P. Synthesis and properties of novel fluorinated polyamides based on 2-trifluoromethyl-4,4′-diaminodiphenylether. J. Chinese Chem. Soc. 2002, 49, 927–934. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, R.; Seo, M.; Lee, H.-S. Double-activated nucleophilic aromatic substitution polymerization by bis-ortho-trifluoromethyl groups to soluble para-poly(biphenylene oxide). Polymer 2020, 188, 122124. [Google Scholar] [CrossRef]
- Ando, S.; Sekiguchi, K.; Mizoroki, M.; Okada, T.; Ishige, R. Anisotropic Linear and Volumetric Thermal-Expansion Behaviors of Self-Standing Polyimide Films Analyzed by Thermomechanical Analysis (TMA) and Optical Interferometry. Macromol. Chem. Phys. 2018, 219, 1700354. [Google Scholar] [CrossRef]
- Hasegawa, M. Development of Solution-Processable, Optically Transparent Polyimides with Ultra-Low Linear Coefficients of Thermal Expansion. Polymers 2017, 9, 520. [Google Scholar] [CrossRef]
- Wooten, F. Optical Properties of Solids; Elsevier: Amsterdam, The Netherlands, 1972; ISBN 9780127634500. [Google Scholar]


| Polymer Code | ηinh (dL/g) a | Mn (kDa) b | Mw (kDa) b | Mw/Mn b | Formula of PA (Formula Weight) | C | H | N | |
|---|---|---|---|---|---|---|---|---|---|
| PA-1 | 1.28 | 61.8 | 128.4 | 2.07 | (C22H12F6N2O3)n (466.33)n | Calcd Found | 56.66 54.78 | 2.59 2.77 | 6.01 5.78 |
| PA-2 | 0.80 | 55.0 | 133.0 | 2.42 | (C22H12F6N2O3)n (466.33)n | Calcd Found | 56.66 55.43 | 2.59 2.77 | 6.01 5.83 |
| PA-3 | 1.06 | 45.4 | 119.0 | 2.62 | (C28H16F6N2O4)n (558.43)n | Calcd Found | 60.22 58.50 | 2.89 3.00 | 5.02 4.73 |
| PA-4 | 0.81 | 51.8 | 119.7 | 2.31 | (C31H16F12N2O3)n (692.45)n | Calcd Found | 53.77 52.77 | 2.33 2.46 | 4.05 3.84 |
| Solvents b | PA-1 | PA-2 | PA-3 | PA-4 |
|---|---|---|---|---|
| NMP | ++ | ++ | ++ | ++ |
| DMAc | ++ | ++ | ++ | ++ |
| DMF | ++ | ++ | ++ | ++ |
| DMSO | ++ | ++ | ++ | + |
| m-cresol | ++ | ++ | ++ | ++ |
| THF | ++ | ++ | ++ | ++ |
| Acetone | -s | -s | -s | ++ |
| Ethyl acetate | - | - | - | -s |
| Anisole | - | - | - | - |
| Chloroform | - | - | - | - |
| Acetonitrile | - | - | - | - |
| Polymer Code | Td5 (°C) a | Tg (°C) b | CTE (ppm/°C) c | Char Yield (%) d | ||
|---|---|---|---|---|---|---|
| In N2 | In Air | 2nd Run | 3rd Run | |||
| PA-1 | 441 | 437 | 334 | 49.8 | 49.7 | 50 |
| PA-2 | 437 | 434 | 310 | 58.1 | 58.0 | 52 |
| PA-3 | 440 | 427 | 308 | 57.9 | 57.9 | 46 |
| PA-4 | 465 | 458 | 311 | 76.1 | 76.7 | 47 |
| Polymer Code | Cutoff Wavelength (nm) | Transmittance | YI a | Film Thickness (μm) | |
|---|---|---|---|---|---|
| At 400 nm | At 550 nm | ||||
| PA-1 | 368 | 58.4 | 89.4 | 2.5 | ~30 |
| PA-2 | 344 | 84.3 | 89.2 | 2.3 | ~30 |
| PA-3 | 337 | 81.8 | 88.4 | 3.6 | ~30 |
| PA-4 | 343 | 86.5 | 90.5 | 1.8 | ~30 |
| λ (nm) | Polymer Code | nTEa | nTMb | navc | Δn d | ε e | d (μm) f |
|---|---|---|---|---|---|---|---|
| 633 | PA-1 | 1.6042 | 1.5414 | 1.5833 | 0.0628 | 2.76 | 6.8 |
| PA-2 | 1.5901 | 1.5534 | 1.5779 | 0.0367 | 2.74 | 8.8 | |
| PA-3 | 1.6122 | 1.5628 | 1.5957 | 0.0494 | 2.80 | 4.4 | |
| PA-4 | 1.5444 | 1.5112 | 1.5333 | 0.0332 | 2.59 | 8.4 | |
| 1310 | PA-1 | 1.5743 | 1.5190 | 1.5559 | 0.0553 | 2.66 | 6.7 |
| PA-2 | 1.5625 | 1.5298 | 1.5516 | 0.0327 | 2.65 | 8.8 | |
| PA-3 | 1.5830 | 1.5387 | 1.5682 | 0.0443 | 2.71 | 4.3 | |
| PA-4 | 1.5213 | 1.4918 | 1.5115 | 0.0295 | 2.51 | 8.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.J.; Kang, I.; Byun, T.; So, J.; Kim, S.Y. Highly Transparent Aromatic Polyamides from Unsymmetrical Diamine with Trifluoromethyl Groups. Polymers 2022, 14, 501. https://doi.org/10.3390/polym14030501
Kim SJ, Kang I, Byun T, So J, Kim SY. Highly Transparent Aromatic Polyamides from Unsymmetrical Diamine with Trifluoromethyl Groups. Polymers. 2022; 14(3):501. https://doi.org/10.3390/polym14030501
Chicago/Turabian StyleKim, Seong Jong, Inah Kang, Taejoon Byun, Jongho So, and Sang Youl Kim. 2022. "Highly Transparent Aromatic Polyamides from Unsymmetrical Diamine with Trifluoromethyl Groups" Polymers 14, no. 3: 501. https://doi.org/10.3390/polym14030501
APA StyleKim, S. J., Kang, I., Byun, T., So, J., & Kim, S. Y. (2022). Highly Transparent Aromatic Polyamides from Unsymmetrical Diamine with Trifluoromethyl Groups. Polymers, 14(3), 501. https://doi.org/10.3390/polym14030501






