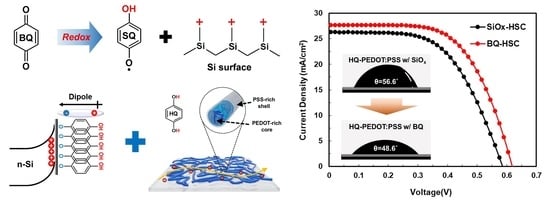
Photovoltaic Device Application of a Hydroquinone-Modified Conductive Polymer and Dual-Functional Molecular Si Surface Passivation Technology
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of PEDOT:PSS Solution
2.2. Device Fabrication
2.3. Characterization
3. Result & Discussion
3.1. Fabrication of Si/PEDOT:PSS Heterojunction Solar Cells (HSCs)
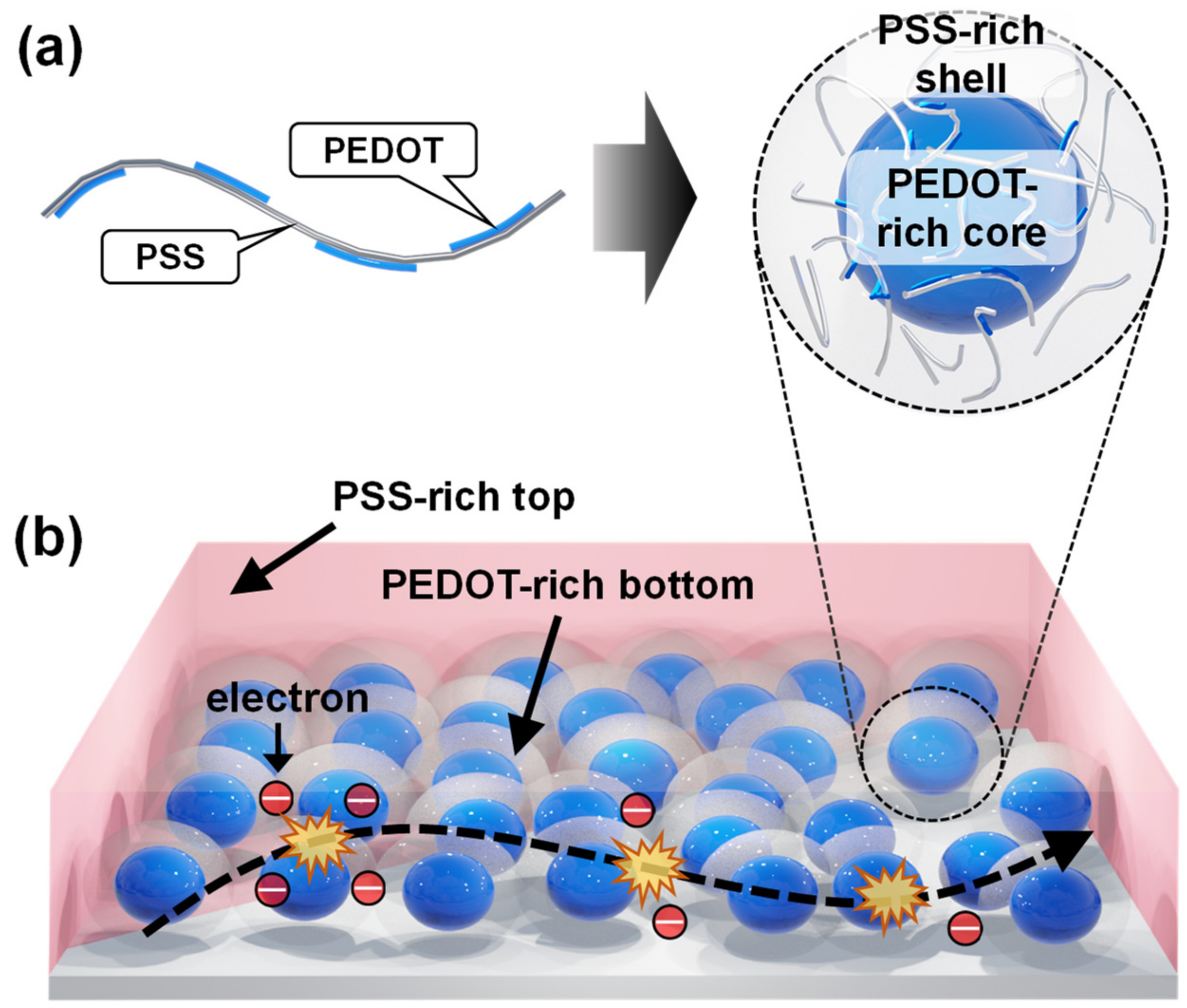
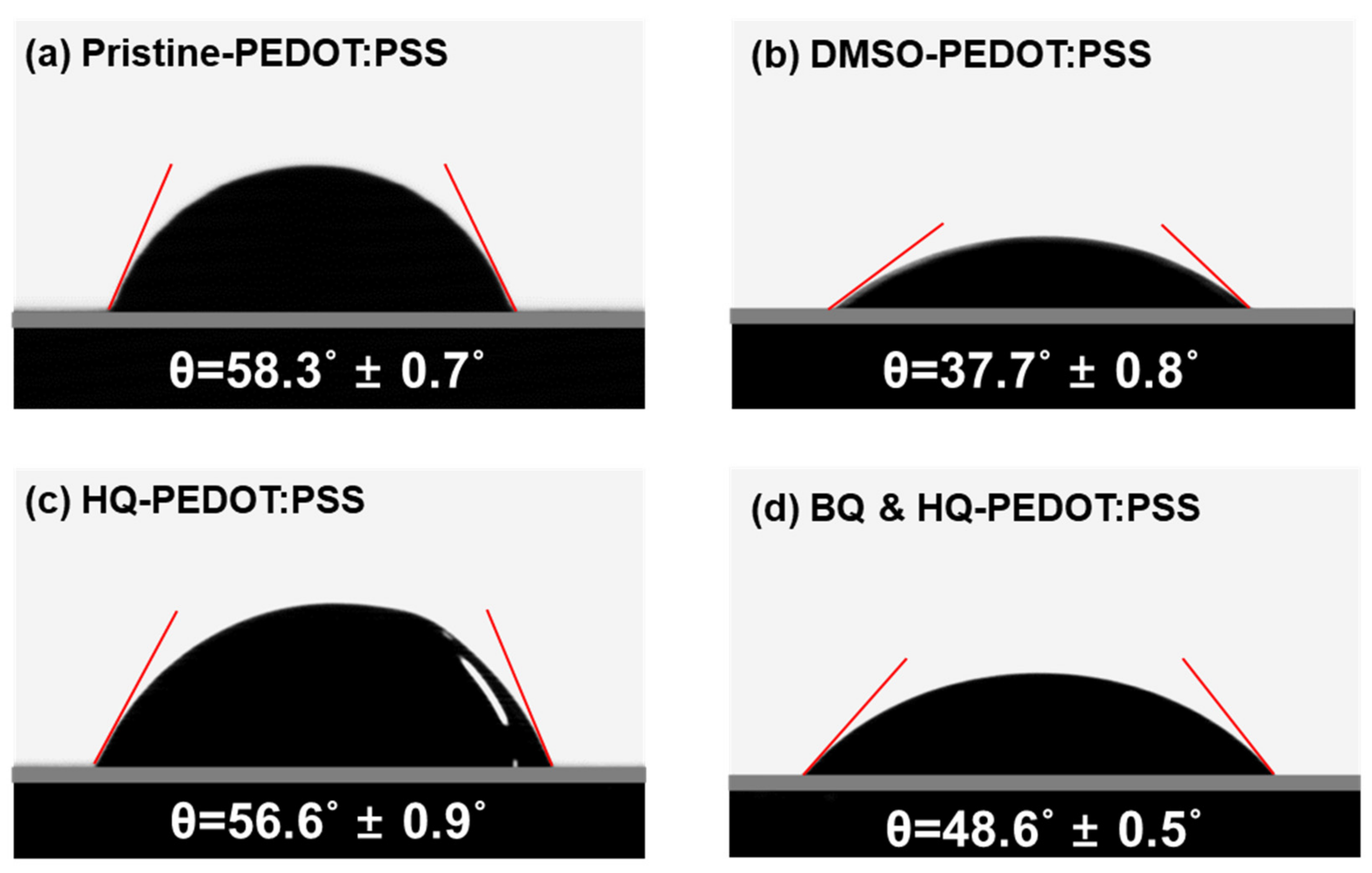
3.2. Comparison between HQ-PEDOT:PSS and Conventional DMSO-PEDOT:PSS
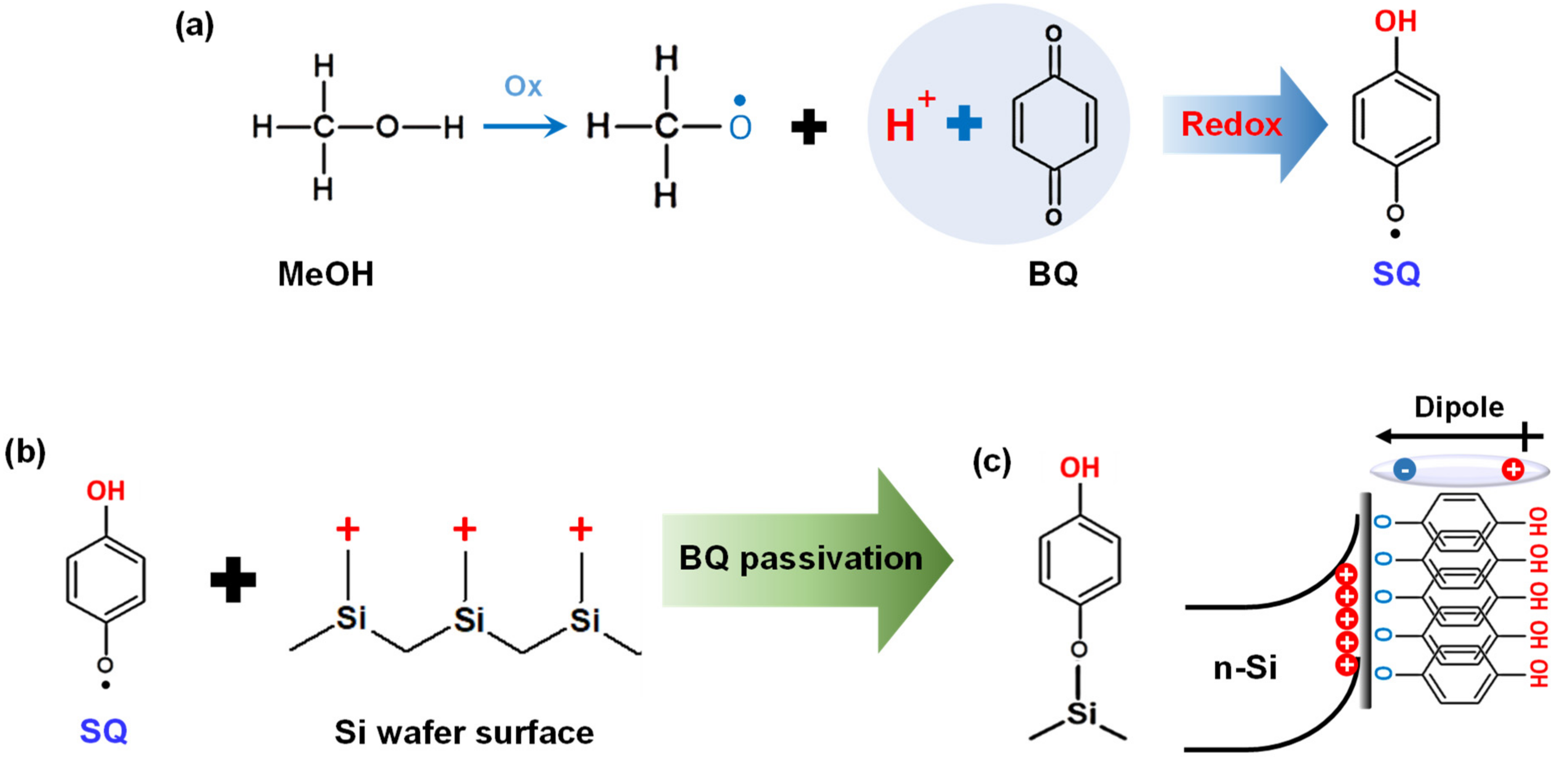
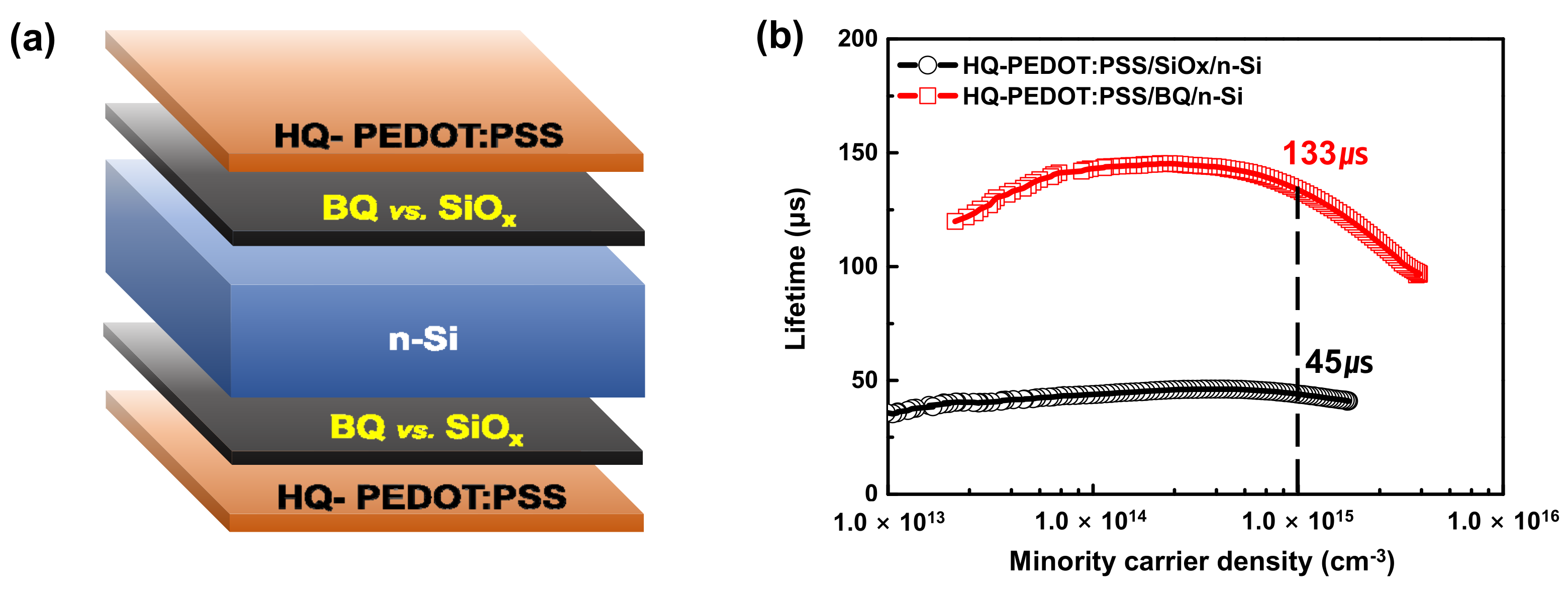
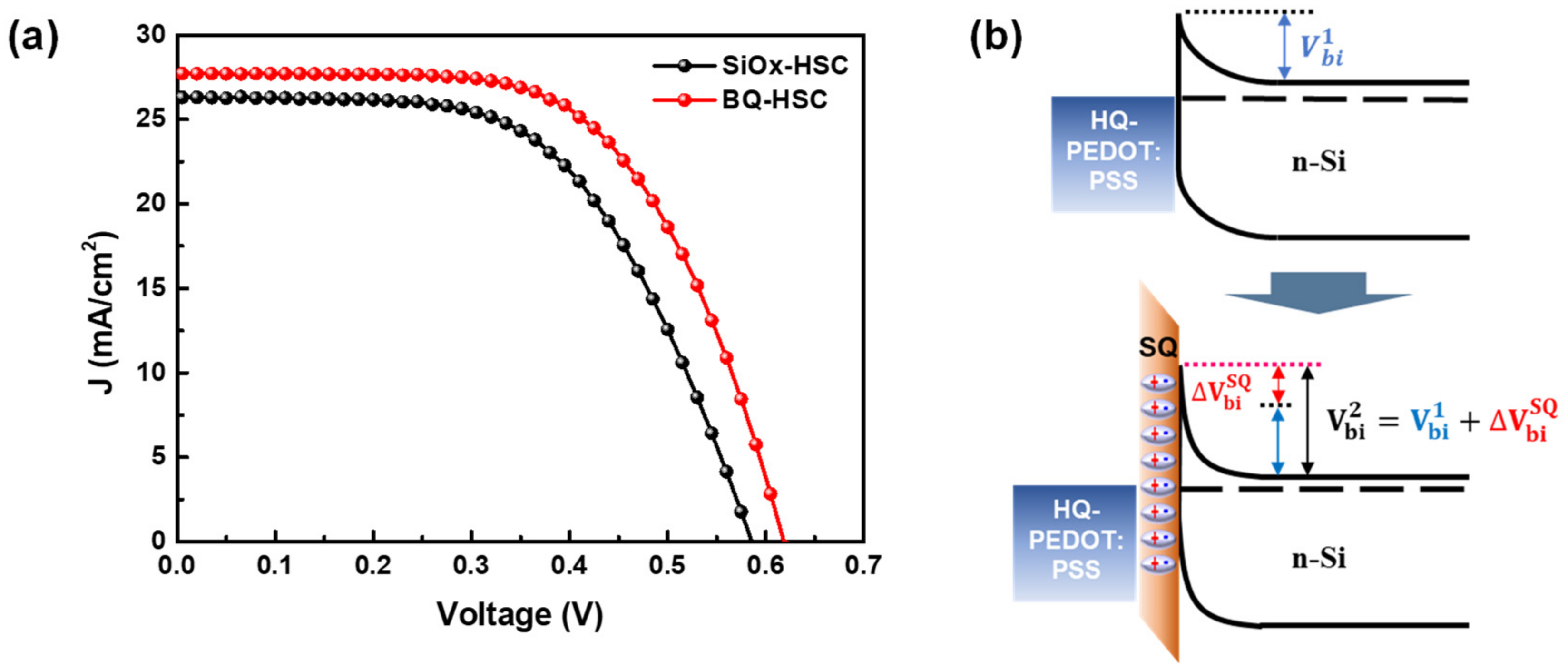
3.3. n-Si/HQ-PEDOT:PSS Interface Engineering with Benzoquinone
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wojciechowski, K.; Forgács, D.; Rivera, T. Industrial opportunities and challenges for perovskite photovoltaic technology. Solar RRL 2019, 3, 1900144. [Google Scholar] [CrossRef]
- Sampaio, P.G.V.; González, M.O.A. Photovoltaic solar energy: Conceptual framework. Renew. Sustain. Energy Rev. 2017, 74, 590–601. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K. Twenty-five years of low-cost solar cells. Nature 2016, 538, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Hermle, M.; Feldmann, F.; Bivour, M.; Goldschmidt, J.C.; Glunz, S.W. Passivating contacts and tandem concepts: Approaches for the highest silicon-based solar cell efficiencies. Appl. Phys. Rev. 2020, 7, 021305. [Google Scholar] [CrossRef]
- Wolden, C.A.; Kurtin, J.; Baxter, J.B.; Repins, I.; Shaheen, S.E.; Torvik, J.T.; Rockett, A.A.; Fthenakis, V.M.; Aydil, E.S. Photovoltaic manufacturing: Present status, future prospects, and research needs. J. Vac. Sci. Technol. A Vac. Surf. Films 2011, 29, 030801. [Google Scholar] [CrossRef] [Green Version]
- Service, R.F. Can the Upstarts Top Silicon? Science 2008, 319, 718–720. [Google Scholar] [CrossRef]
- Chen, D.; Chen, Y.; Wang, Z.; Gong, J.; Liu, C.; Zou, Y.; He, Y.; Wang, Y.; Yuan, L.; Lin, W. 24.58% total area efficiency of screen-printed, large area industrial silicon solar cells with the tunnel oxide passivated contacts (i-TOPCon) design. Sol. Energy Mater. Sol. Cells 2020, 206, 110258. [Google Scholar] [CrossRef]
- Li, M.; Dai, Y.; Ma, W.; Yang, B.; Chu, Q. Review of new technology for preparing crystalline Silicon solar cell materials by metallurgical method. In Earth and Environmental Science, Proceedings of the 3rd International Conference on Energy, Environment and Materials Science (EEMS 2017); Singapore, 28–30 July 2017, IOP Conference Series; IOP Publishing: Bristol, UK, 2017. [Google Scholar]
- Srivastava, S.K.; Kumar, D.; Sharma, M.; Kumar, R.; Singh, P.K. Silver catalyzed nano-texturing of silicon surfaces for solar cell applications. Sol. Energy Mater. Sol. Cells 2012, 100, 33–38. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Kumar, D.; Singh, P.K.; Kumar, V. Silicon nanowire arrays based “black silicon” solar cells. In Proceedings of the 2009 34th IEEE Photovoltaic Specialists Conference (PVSC), Philadelphia, PA, USA, 7–12 June 2009; IEEE: Piscataway, NJ, USA, 2009. [Google Scholar]
- Lee, Y.-T.; Lin, F.-R.; Chen, C.-H.; Pei, Z. A 14.7% Organic/silicon nanoholes hybrid solar cell via interfacial engineering by solution-processed inorganic conformal layer. ACS Appl. Mater. Interfaces 2016, 8, 34537–34545. [Google Scholar] [CrossRef]
- Yu, X.; Shen, X.; Mu, X.; Zhang, J.; Sun, B.; Zeng, L.; Yang, L.; Wu, Y.; He, H.; Yang, D. High efficiency organic/silicon-nanowire hybrid solar cells: Significance of strong inversion layer. Sci. Rep. 2015, 5, 17371. [Google Scholar] [CrossRef] [Green Version]
- Yu, P.; Tsai, C.-Y.; Chang, J.-K.; Lai, C.-C.; Chen, P.-H.; Lai, Y.-C.; Tsai, P.-T.; Li, M.-C.; Pan, H.-T.; Huang, Y.-Y. 13% efficiency hybrid organic/silicon-nanowire heterojunction solar cell via interface engineering. ACS Nano 2013, 7, 10780–10787. [Google Scholar] [CrossRef] [PubMed]
- Tune, D.D.; Flavel, B.S.; Quinton, J.S.; Ellis, A.V.; Shapter, J.G. Single-Walled Carbon Nanotube/Polyaniline/n-Silicon Solar Cells: Fabrication, Characterization, and Performance Measurements. ChemSusChem 2013, 6, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.L.; Jabbour, G.E.; Wang, Q.; Shaheen, S.E.; Ginley, D.S.; Schiff, E.A. Conducting polymer and hydrogenated amorphous silicon hybrid solar cells. Appl. Phys. Lett. 2005, 87, 223504. [Google Scholar] [CrossRef] [Green Version]
- Baek, S.-W.; Jun, S.; Kim, B.; Proppe, A.H.; Ouellette, O.; Voznyy, O.; Kim., C.; Kim., J.; Walters, G.; Song, J.H. Efficient hybrid colloidal quantum dot/organic solar cells mediated by near-infrared sensitizing small molecules. Nat. Energy 2019, 4, 969–976. [Google Scholar] [CrossRef]
- Lim, N.; Cho, N.; Paek, S.; Kim, C.; Lee, J.K.; Ko, J. High-performance organic solar cells with efficient semiconducting small molecules containing an electron-rich benzodithiophene derivative. Chem. Mater. 2014, 26, 2283–2288. [Google Scholar] [CrossRef]
- Troshin, P.A.; Hoppe., H.; Renz, J.; Egginger, M.; Mayorova, J.Y.; Goryachev, A.E.; Peregudov, A.S.; Lyubovskaya, R.N.; Gobsch, G.; Sariciftci, N.S. Material solubility-photovoltaic performance relationship in the design of novel fullerene derivatives for bulk heterojunction solar cells. Adv. Funct. Mater. 2009, 19, 779–788. [Google Scholar] [CrossRef]
- Umeyama, T.; Imahori, H. Isomer effects of fullerene derivatives on organic photovoltaics and perovskite solar cells. Acc. Chem. Res. 2019, 52, 2046–2055. [Google Scholar] [CrossRef]
- Huang, D.; Goh, T.; Kong, J.; Zheng, Y.; Zhao, S.; Xu, Z.; Taylor, A.D. Perovskite solar cells with a DMSO-treated PEDOT: PSS hole transport layer exhibit higher photovoltaic performance and enhanced durability. Nanoscale 2017, 9, 4236–4243. [Google Scholar] [CrossRef]
- Pietsch, M.; Bashouti, M.Y.; Christiansen, S. The role of hole transport in hybrid inorganic/organic silicon/poly (3, 4-ethylenedioxy-thiophene): Poly (styrenesulfonate) heterojunction solar cells. J. Phys. Chem. C 2013, 117, 9049–9055. [Google Scholar] [CrossRef]
- Thomas, J.P.; Leung, K.T. Mixed co-solvent engineering of PEDOT: PSS to enhance its conductivity and hybrid solar cell properties. J. Mater. Chem. A 2016, 4, 17537–17542. [Google Scholar] [CrossRef] [Green Version]
- Reza, K.M.; Gurung, A.; Bahrami, B.; Mabrouk, S.; Elbohy, H.; Pathak, R.; Chen, K.; Chowdhury, A.H.; Rahman, M.T.; Letourneau, S. Tailored PEDOT: PSS hole transport layer for higher performance in perovskite solar cells: Enhancement of electrical and optical properties with improved morphology. J. Energy Chem. 2020, 44, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Sachse, C.; Machala, M.L.; May, C.; Müller-Meskamp, L.; Leo, K. Highly conductive PEDOT: PSS electrode with optimized solvent and thermal post-treatment for ITO-free organic solar cells. Adv. Funct. Mater. 2011, 21, 1076–1081. [Google Scholar] [CrossRef]
- Zhu, Y.; Song, T.; Zhang, F.; Lee, S.-T.; Sun, B. Efficient organic-inorganic hybrid Schottky solar cell: The role of built-in potential. Appl. Phys. Lett. 2013, 102, 113504. [Google Scholar] [CrossRef]
- Snaith, H.J.; Kenrick, H.; Chiesa, M.; Friend, R.H. Morphological and electronic consequences of modifications to the polymer anode ‘PEDOT: PSS’. Polymer 2005, 46, 2573–2578. [Google Scholar] [CrossRef]
- Huang, J.; Miller, P.F.; de Mello, J.C.; de Mello, A.J.; Bradley, D.D.C. Influence of thermal treatment on the conductivity and morphology of PEDOT/PSS films. Synth. Met. 2003, 139, 569–572. [Google Scholar] [CrossRef]
- Friedel, B.; Keivanidis, P.E.; Brenner, T.J.K.; Abrusci, A.; McNeill, C.R.; Friend, R.H.; Greenham, N.C. Effects of layer thickness and annealing of PEDOT: PSS layers in organic photodetectors. Macromolecules 2009, 42, 6741–6747. [Google Scholar] [CrossRef]
- Song, C.; Zhong, Z.; Hu, Z.; Luo, Y.; Wang, L.; Wang, J.; Cao, Y. The effect of solvent treatment on the buried PEDOT: PSS layer. Org. Electron. 2017, 43, 9–14. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.; Wang, J.; Zhang, J.; Yang, Q.; Fu, Y.; Xie, Z. Highly conductive PEDOT: PSS transparent electrode prepared by a post-spin-rinsing method for efficient ITO-free polymer solar cells. Sol. Energy Mater. Sol. Cells 2016, 144, 143–149. [Google Scholar] [CrossRef]
- Lang, U.; Müller, E.; Naujoks, N.; Dual, J. Microscopical investigations of PEDOT: PSS thin films. Adv. Funct. Mater. 2009, 19, 1215–1220. [Google Scholar] [CrossRef]
- Nardes, A.M.; Kemerink, M.; Janssen, R.A.J.; Bastiaansen, J.A.M.; Kiggen, N.M.M.; Langeveld, B.M.W.; Van Breemen, A.J.J.M.; De Kok, M.M. Microscopic understanding of the anisotropic conductivity of PEDOT: PSS thin films. Adv. Mater. 2007, 19, 1196–1200. [Google Scholar] [CrossRef]
- Yan, F.; Parrott, E.P.J.; Ung, B.S.-Y.; Pickwell-MacPherson, E. Solvent doping of PEDOT/PSS: Effect on terahertz optoelectronic properties and utilization in terahertz devices. J. Phys. Chem. C 2015, 119, 6813–6818. [Google Scholar] [CrossRef]
- Nevrela, J.; Micjan, M.; Novota, M.; Kovacova, S.; Pavuk, M.; Juhasz, P.; Kovac, J., Jr.; Jakabovic, J.; Weis, M. Secondary doping in poly (3, 4-ethylenedioxythiophene): Poly (4-styrenesulfonate) thin films. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 1139–1146. [Google Scholar] [CrossRef]
- Chou, T.-R.; Chen, S.-H.; Chiang, Y.-T.; Lin, Y.-T.; Chao, C.-Y. Highly conductive PEDOT: PSS films by post-treatment with dimethyl sulfoxide for ITO-free liquid crystal display. J. Mater. Chem. C 2015, 3, 3760–3766. [Google Scholar] [CrossRef]
- McCarthy, J.E.; Hanley, C.A.; Brennan, L.J.; Lambertini, V.G.; Gun'ko, Y.K. Fabrication of highly transparent and conducting PEDOT: PSS films using a formic acid treatment. J. Mater. Chem. C 2014, 2, 764–770. [Google Scholar] [CrossRef]
- Ouyang, J. “Secondary doping” methods to significantly enhance the conductivity of PEDOT: PSS for its application as transparent electrode of optoelectronic devices. Displays 2013, 34, 423–436. [Google Scholar] [CrossRef]
- Yin, L.; Zhao, Z.; Jiang, F.; Li, Z.; Xiong, S.; Zhou, Y. PEDOT: PSS top electrode prepared by transfer lamination using plastic wrap as the transfer medium for organic solar cells. Org. Electron. 2014, 15, 2593–2598. [Google Scholar] [CrossRef]
- Lee, J.J.; Lee, S.H.; Kim, F.S.; Choi, H.H.; Kim, J.H. Simultaneous enhancement of the efficiency and stability of organic solar cells using PEDOT: PSS grafted with a PEGME buffer layer. Org. Electron. 2015, 26, 191–199. [Google Scholar] [CrossRef]
- Na, S.-I.; Wang, G.; Kim, S.-S.; Kim, T.-W.; Oh, S.-H.; Yu, B.-K.; Lee, T.; Kim, D.-Y. Evolution of nanomorphology and anisotropic conductivity in solvent-modified PEDOT: PSS films for polymeric anodes of polymer solar cells. J. Mater. Chem. 2009, 19, 9045–9053. [Google Scholar] [CrossRef]
- Lee, T.W.; Chung, Y. Control of the surface composition of a conducting-polymer complex film to tune the work function. Adv. Funct. Mater. 2008, 18, 2246–2252. [Google Scholar] [CrossRef]
- Thomas, J.P.; Zhao, L.; Abd-Ellah, M.; Heining, N.F.; Leung, K.T. Interfacial micropore defect formation in PEDOT: PSS-Si hybrid solar cells probed by TOF-SIMS 3D chemical imaging. Anal. Chem. 2013, 85, 6840–6845. [Google Scholar] [CrossRef]
- Sheng, J.; Fam, K.; Wang, D.; Han, C.; Fang, J.; Gao, P.; Ye, J. Improvement of the SiO x passivation layer for high-efficiency Si/PEDOT: PSS heterojunction solar cells. ACS Appl. Mater. Interfaces 2014, 6, 16027–16034. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, A.; Feldmann, F.; Kaufmann, K.; Richter, S.; Werner, M.; Hagendorf, C.; Zimmer, M.; Rentsch, J.; Hermle, M. Tunnel oxide passivated carrier-selective contacts based on ultra-thin SiO2 layers grown by photo-oxidation or wet-chemical oxidation in ozonized water. In Proceedings of the 2015 IEEE 42nd Photovoltaic Specialist Conference (PVSC), New Orleans, LA, USA, 14–19 June 2015; IEEE: Piscataway, NJ, USA, 2015. [Google Scholar]
- Zhang, C.; Zhang, Y.; Guo, H.; Jiang, Q.; Dong, P.; Zhang, C. Efficient planar hybrid n-Si/PEDOT: PSS solar cells with power conversion efficiency up to 13. 31% achieved by controlling the SiOx Interlayer. Energies 2018, 11, 1397. [Google Scholar]
- Deal, B.E.; Grove, A. General relationship for the thermal oxidation of silicon. J. Appl. Phys. 1965, 36, 3770–3778. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.-B.; Asuha, M.T.; Kobayashi, H. Ultrathin SiO2 layer on atomically flat Si (111) surfaces with excellent electrical characteristics formed by nitric acid oxidation method. Appl. Phys. Lett. 2008, 93, 072101. [Google Scholar] [CrossRef]
- Moldovan, A.; Feldmann, F.; Krugel, G.; Zimmer, M.; Rentsch, J.; Hermle, M.; Roth-Fölsch, A.; Kaufmann, K.; Hagendorf, C. Simple cleaning and conditioning of silicon surfaces with UV/ozone sources. Energy Procedia 2014, 55, 834–844. [Google Scholar] [CrossRef]
- He, L.; Jiang, C.; Wang, H.; Lai, D.; Rusli. High efficiency planar Si/organic heterojunction hybrid solar cells. Appl. Phys. Lett. 2012, 100, 073503. [Google Scholar] [CrossRef]
- Chhabra, B.; Bowden, S.; Opila, R.L.; Honsberg, C.B. High effective minority carrier lifetime on silicon substrates using quinhydrone-methanol passivation. Appl. Phys. Lett. 2010, 96, 063502. [Google Scholar] [CrossRef]
- Avasthi, S.; Qi, Y.; Vertelov, G.K.; Schwartz, J.; Kahn, A.; Sturm, J.C. Silicon surface passivation by an organic overlayer of 9, 10-phenanthrenequinone. Appl. Phys. Lett. 2010, 96, 222109. [Google Scholar] [CrossRef] [Green Version]
- Miramond, C.; Vuillaume, D. 1-octadecene monolayers on Si (111) hydrogen-terminated surfaces: Effect of substrate doping. J. Appl. Phys. 2004, 96, 1529–1536. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Zhang, P.; Zhai, X.; Tian, F.; Li, W.; Yang, J.; Liu, Y.; Wang, H.; Wang, W.; Liu, W. Nano-carrier for gene delivery and bioimaging based on carbon dots with PEI-passivation enhanced fluorescence. Biomaterials 2012, 33, 3604–3613. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Y.; Fang, X.; Zhang, F.; Song, T.; Sun, B. An 11%-power-conversion-efficiency organic–inorganic hybrid solar cell achieved by facile organic passivation. IEEE Electron Device Lett. 2013, 34, 345–347. [Google Scholar] [CrossRef]
- Alemu, D.; Wei, H.-Y.; Ho, K.-C.; Chu, C.-W. Highly conductive PEDOT: PSS electrode by simple film treatment with methanol for ITO-free polymer solar cells. Energy Environ. Sci. 2012, 5, 9662–9671. [Google Scholar] [CrossRef]
- Song, I.; Park, N.Y.; Jeong, G.S.; Kang, J.H.; Seo, J.H.; Choi, J.-Y. Conductive channel formation for enhanced electrical conductivity of PEDOT: PSS with high work-function. Appl. Surf. Sci. 2020, 529, 147176. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.-C.; Gao, C.-J. On the mechanism of conductivity enhancement in PEDOT/PSS film doped with multi-walled carbon nanotubes. J. Polym. Res. 2010, 17, 713–718. [Google Scholar] [CrossRef]
- Hosseini, E.; Kollath, V.O.; Karan, K. The key mechanism of conductivity in PEDOT: PSS thin films exposed by anomalous conduction behaviour upon solvent-doping and sulfuric acid post-treatment. J. Mater. Chem. C 2020, 8, 3982–3990. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Woo, S.-H.; Han, T.-H.; Park, M.-H.; Cho, H.; Kim, Y.-H.; Cho, H.; Kim, H.; Yoo, S.; Lee, T.-W. Universal high work function flexible anode for simplified ITO-free organic and perovskite light-emitting diodes with ultra-high efficiency. NPG Asia Mater. 2017, 9, e411. [Google Scholar] [CrossRef]
- Sarkar, B.; Jaiswal, M.; Satapathy, D.K. Swelling kinetics and electrical charge transport in PEDOT: PSS thin films exposed to water vapor. J. Phys. Condens. Matter 2018, 30, 225101. [Google Scholar] [CrossRef]
- Cruz-Cruz, I.; Reyes-Reyes, M.; Aguilar-Frutis, M.A.; Rodriguez, A.G.; López-Sandoval, R. Study of the effect of DMSO concentration on the thickness of the PSS insulating barrier in PEDOT: PSS thin films. Synth. Met. 2010, 160, 1501–1506. [Google Scholar] [CrossRef]
- Mahato, S.; Puigdollers, J.; Voz, C.; Mukhopadhyay, M.; Mukherjee, M.; Hazra, S. Near 5% DMSO is the best: A structural investigation of PEDOT: PSS thin films with strong emphasis on surface and interface for hybrid solar cell. Appl. Surf. Sci. 2020, 499, 143967. [Google Scholar] [CrossRef]
- Thomas, J.P.; Zhao, L.; McGillivray, D.; Leung, K.T. High-efficiency hybrid solar cells by nanostructural modification in PEDOT: PSS with co-solvent addition. J. Mater. Chem. A 2014, 2, 2383–2389. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-J.; Lee, J.-Y.; Chen, S.-M. Changing electrical properties of PEDOT: PSS by incorporating with dimethyl sulfoxide. Chem. Phys. Lett. 2016, 664, 213–218. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, J.; Zhu, Y. Effects of solvent-treated PEDOT: PSS on organic photovoltaic devices. Renew. Energy 2014, 62, 100–105. [Google Scholar] [CrossRef]
- Choi, J.-Y. Understanding of Molecular Contribution of Quinhydrone/Methanol Organic Passivation for Improved Minority Carrier Lifetime on Nanostructured Silicon Surface. Appl. Sci. 2019, 9, 3645. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.; Liu, W.; Wang, D.; Liu, Z.; Jiang, E.; Wu, S.; Zhu, J.; Guo, W.; Sheng, J.; Ye, J. Electron-selective quinhydrone passivated back contact for high-efficiency silicon/organic heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2018, 185, 218–225. [Google Scholar] [CrossRef]
- McIntosh, K.R.; Black, L.E. On effective surface recombination parameters. J. Appl. Phys. 2014, 116, 014503. [Google Scholar] [CrossRef] [Green Version]
- Kato, H.S.; Kawai, M.; Akagi, K.; Tsuneyuki, S. Interaction of condensed water molecules with hydroxyl and hydrogen groups on Si (0 0 1). Surf. Sci. 2005, 587, 34–40. [Google Scholar] [CrossRef]
- Zheng, W.; Sun, C.; Bai, B. Molecular dynamics study on the effect of surface hydroxyl groups on three-phase wettability in oil-water-graphite systems. Polymers 2017, 9, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Wen, X.; Zhou, J.; Zheng, N.; Liu, L.; Xie, Z. Construction of Interface Dipoles by Surface Doping and Their Role in the Open Circuit Voltage in Polymer Solar Cells. Org. Mater. 2020, 2, 71–77. [Google Scholar] [CrossRef] [Green Version]
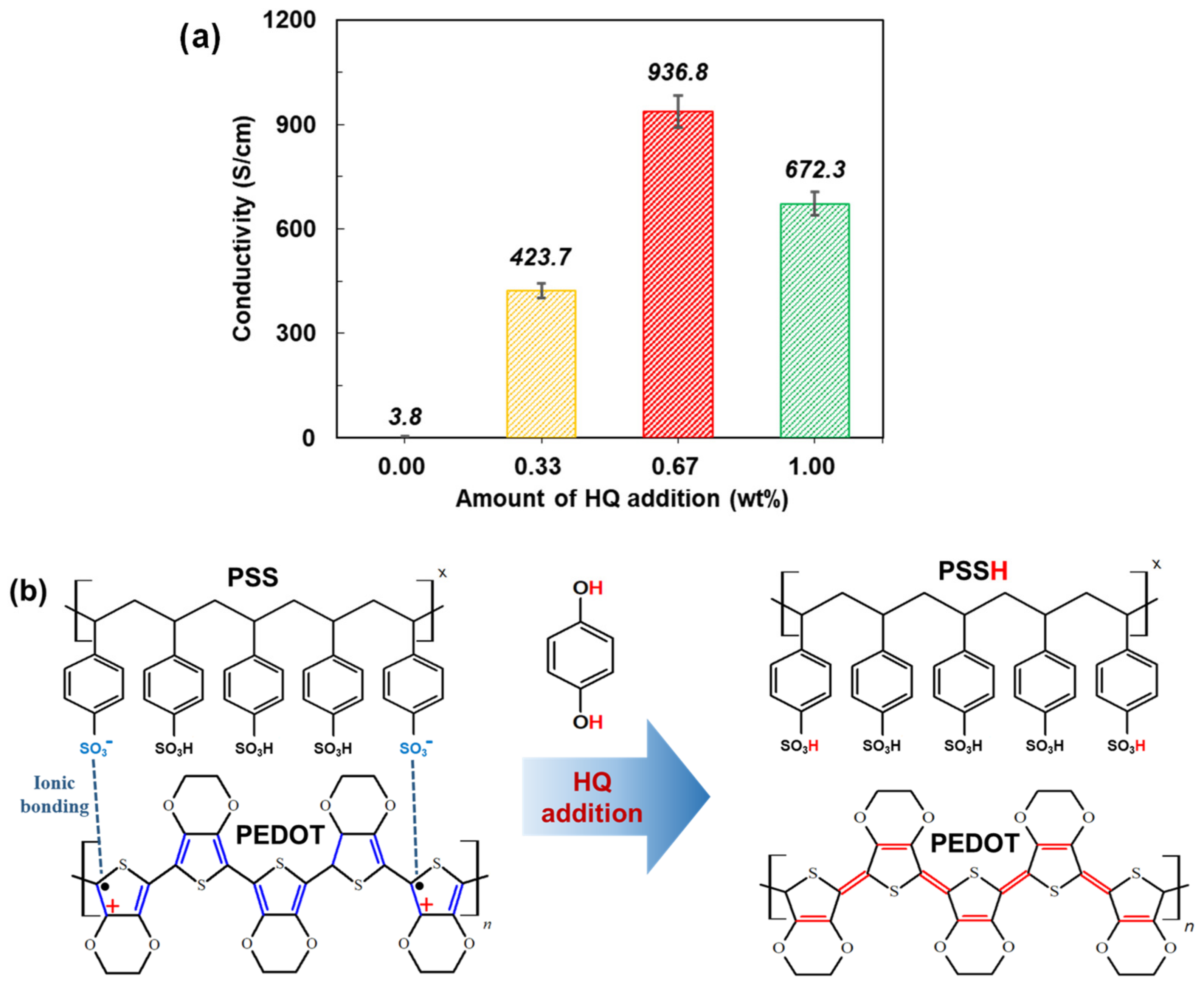
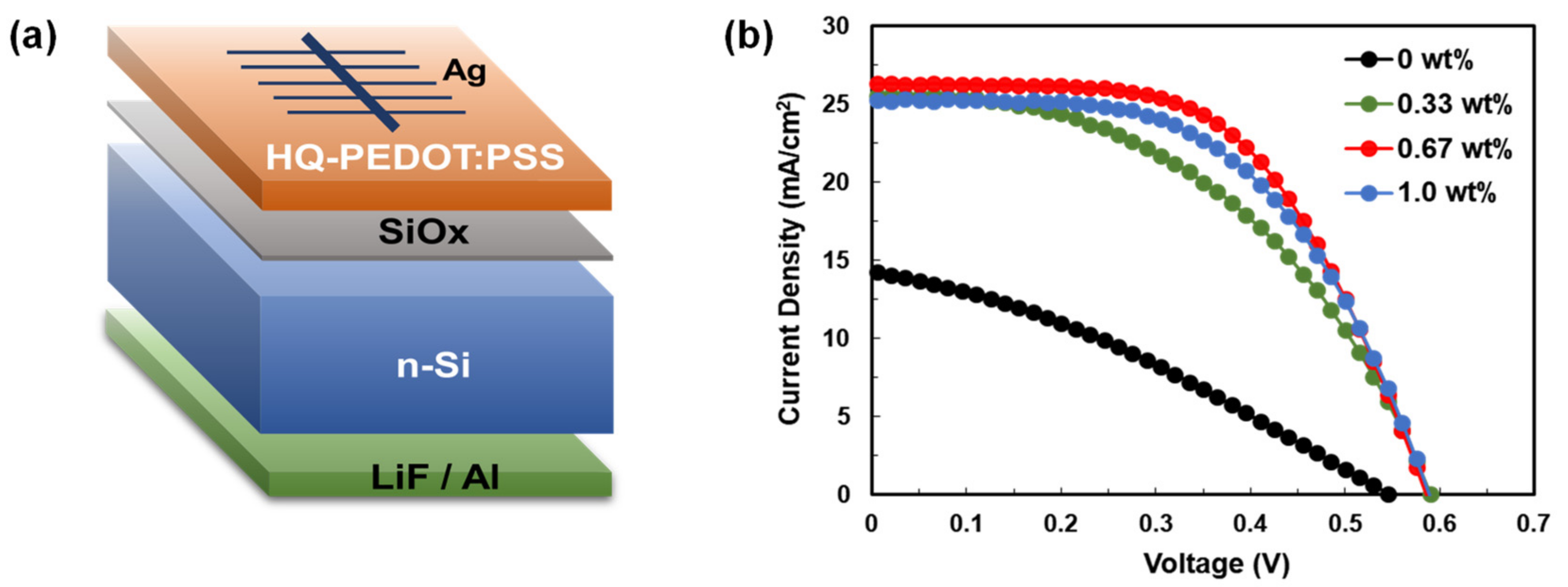
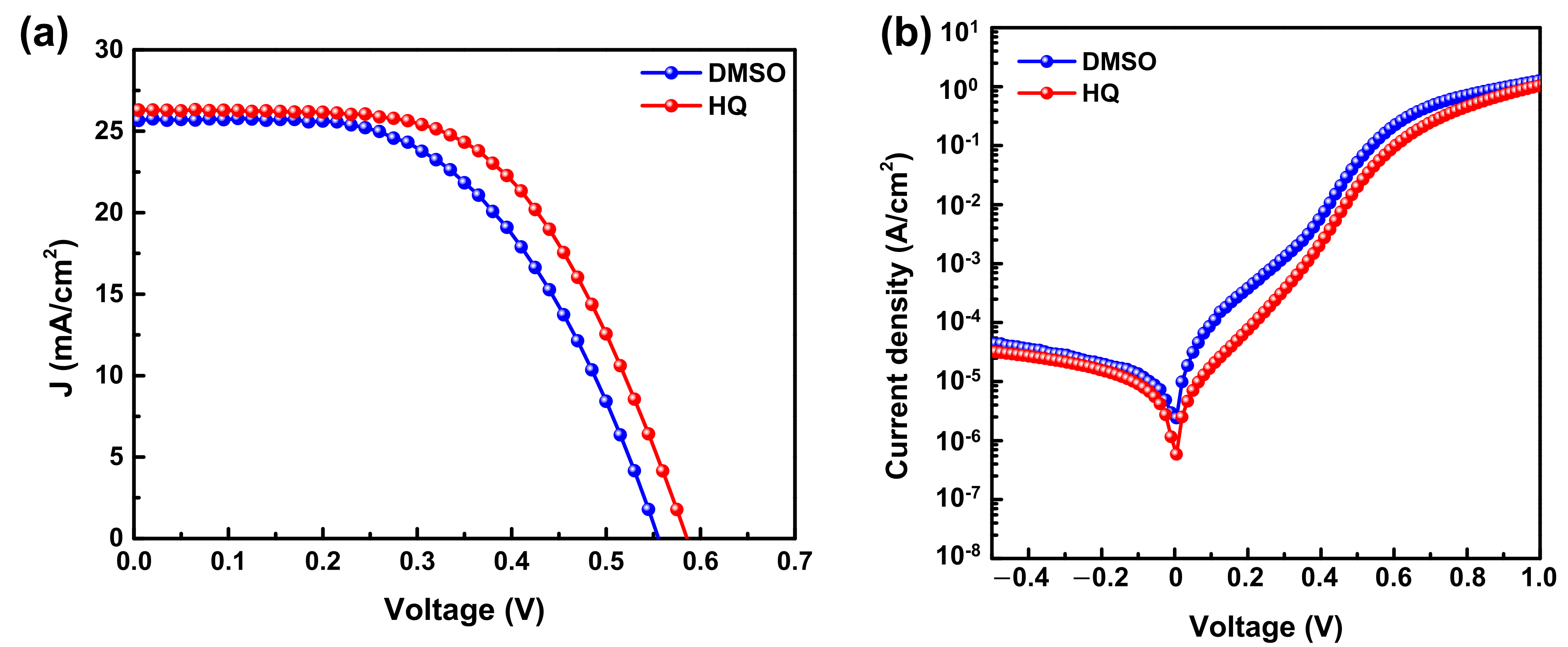
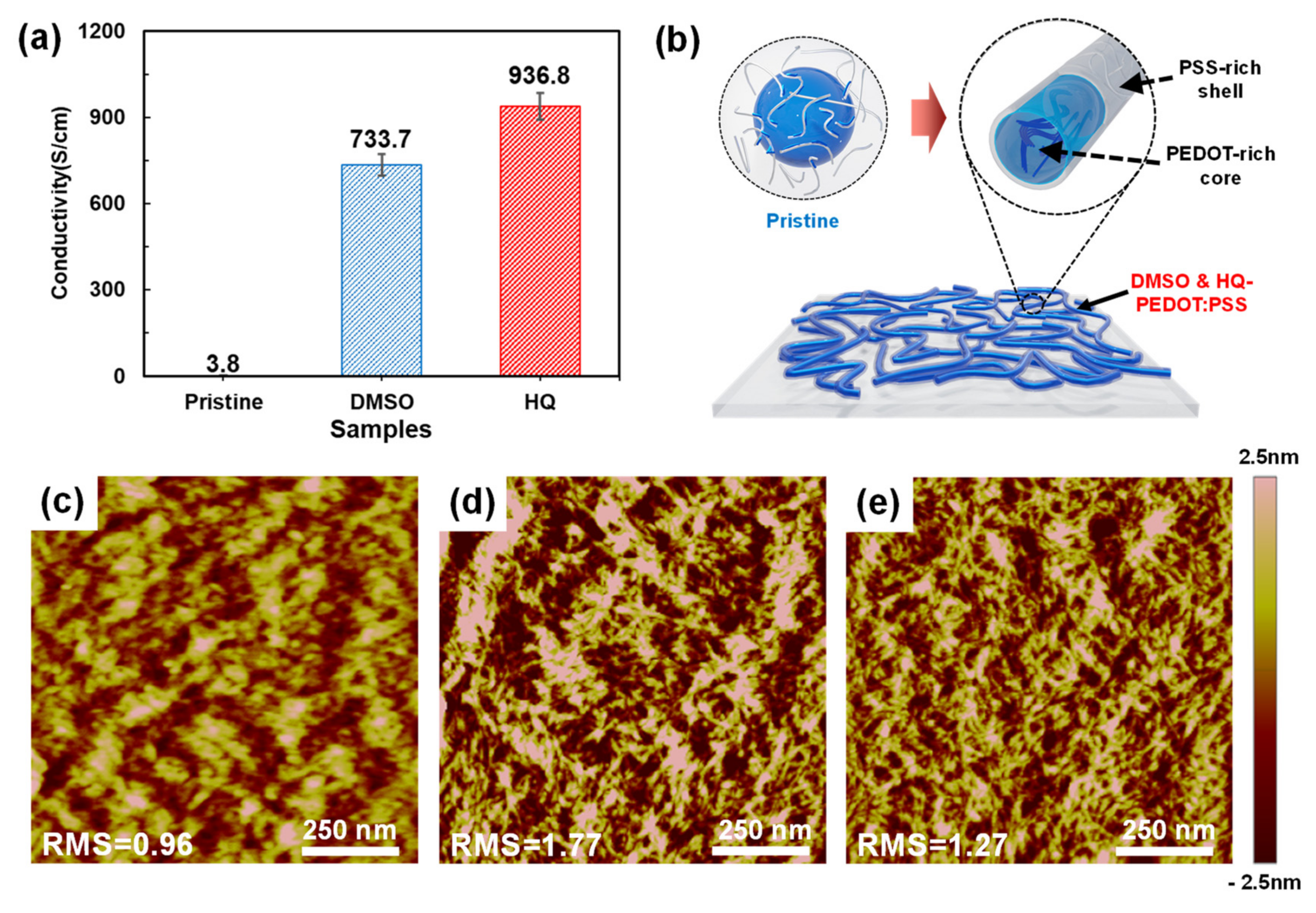
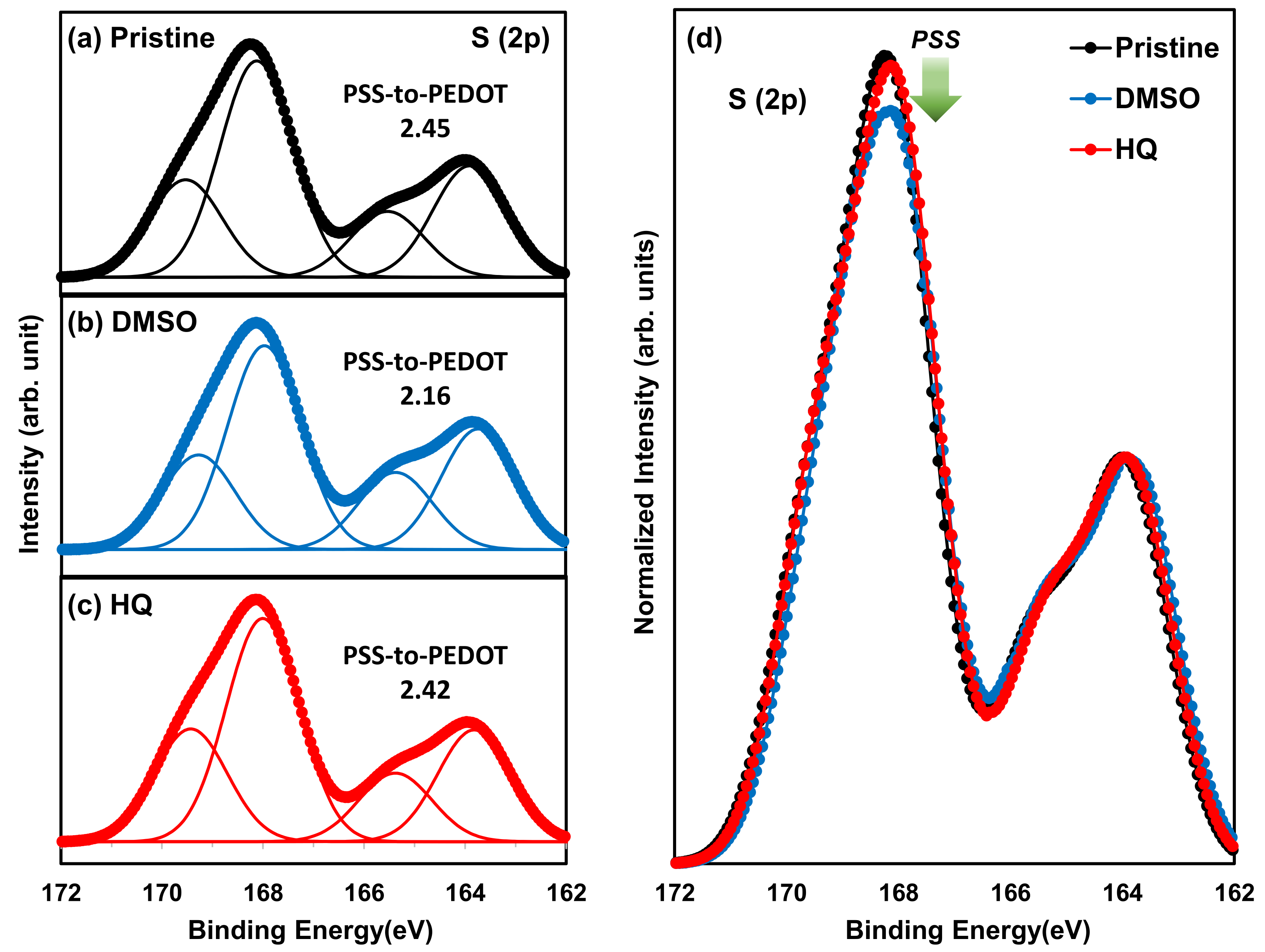
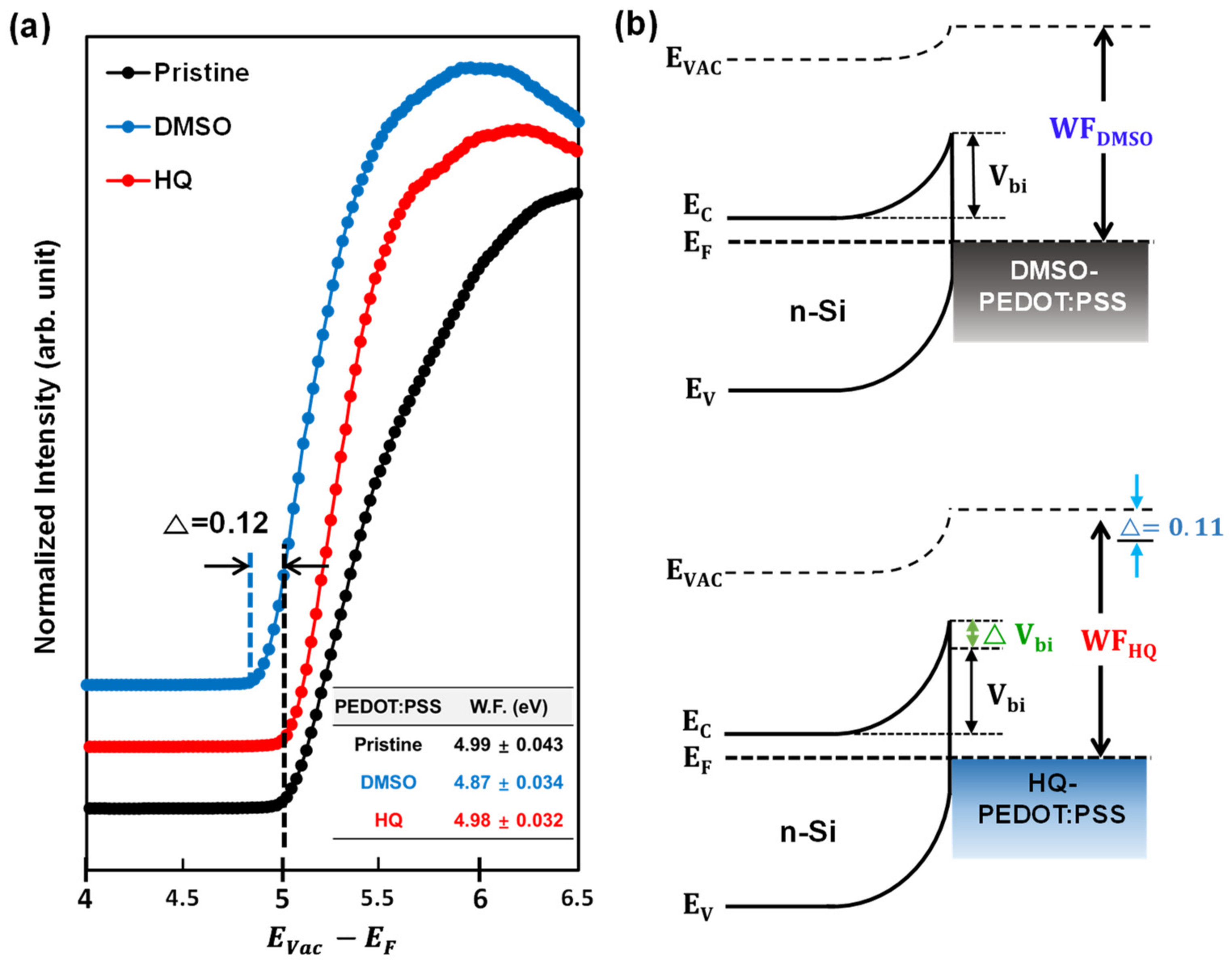
| Amount of HQ Addition | Jsc (mA/cm2) | Voc (mV) | FF (%) | Rsh (Ω·cm2) | Rs (Ω·cm2) | PCE (%) |
|---|---|---|---|---|---|---|
| 0 wt% | 14.3 | 547 | 32.0 | 113.5 | 28.1 | 2.5 ± 0.93 |
| 0.33 wt% | 25.6 | 590 | 47.1 | 504.8 | 6.8 | 7.1 ± 0.60 |
| 0.67 wt% | 26.3 | 586 | 58.6 | 3680.9 | 6.1 | 8.8 ± 0.44 |
| 1.0 wt% | 25.3 | 589 | 55.1 | 1569.1 | 5.8 | 8.2 ± 0.37 |
| PEDOT:PSS | Jsc (mA/cm2) | Voc (mV) | FF (%) | Rsh (Ω·cm2) | Rs (Ω·cm2) | PCE (%) |
|---|---|---|---|---|---|---|
| DMSO | 25.7 | 556 | 53.8 | 1814.2 | 5.9 | 7.7 ± 0.86 |
| HQ | 26.3 | 586 | 58.6 | 3680.2 | 6.1 | 8.8 ± 0.44 |
| Passivation | Jsc (mA/cm2) | Voc (mV) | FF (%) | Rsh (Ω·cm2) | Rs (Ω·cm2) | PCE (%) |
|---|---|---|---|---|---|---|
| SiOx-HSC | 26.3 | 586 | 58.6 | 3680.2 | 6.1 | 8.8 ± 1.36 |
| BQ-HSC | 27.7 | 618 | 61.8 | 4855.3 | 4.6 | 10.6 ± 1.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, N.Y.; Jeong, G.S.; Yu, Y.-J.; Jung, Y.-C.; Lee, J.H.; Seo, J.H.; Choi, J.-Y. Photovoltaic Device Application of a Hydroquinone-Modified Conductive Polymer and Dual-Functional Molecular Si Surface Passivation Technology. Polymers 2022, 14, 478. https://doi.org/10.3390/polym14030478
Park NY, Jeong GS, Yu Y-J, Jung Y-C, Lee JH, Seo JH, Choi J-Y. Photovoltaic Device Application of a Hydroquinone-Modified Conductive Polymer and Dual-Functional Molecular Si Surface Passivation Technology. Polymers. 2022; 14(3):478. https://doi.org/10.3390/polym14030478
Chicago/Turabian StylePark, Na Yeon, Gwan Seung Jeong, Young-Jin Yu, Yoon-Chae Jung, Jin Hee Lee, Jung Hwa Seo, and Jea-Young Choi. 2022. "Photovoltaic Device Application of a Hydroquinone-Modified Conductive Polymer and Dual-Functional Molecular Si Surface Passivation Technology" Polymers 14, no. 3: 478. https://doi.org/10.3390/polym14030478
APA StylePark, N. Y., Jeong, G. S., Yu, Y.-J., Jung, Y.-C., Lee, J. H., Seo, J. H., & Choi, J.-Y. (2022). Photovoltaic Device Application of a Hydroquinone-Modified Conductive Polymer and Dual-Functional Molecular Si Surface Passivation Technology. Polymers, 14(3), 478. https://doi.org/10.3390/polym14030478