Analysis of the Relative Humidity Response of Hydrophilic Polymers for Optical Fiber Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Relative Humidity Responsive Polymers
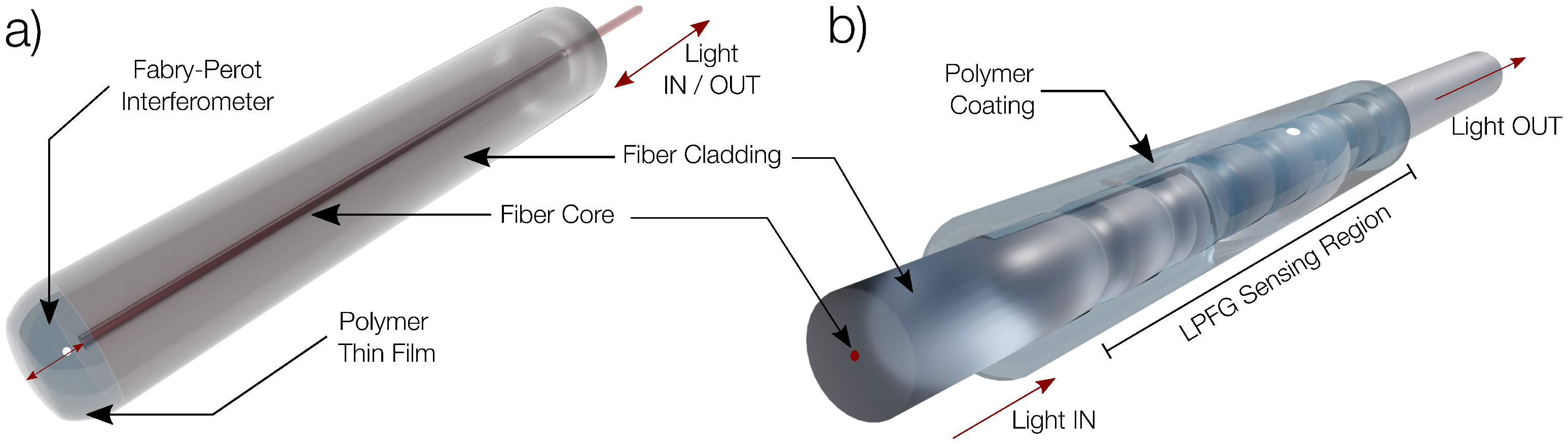
2.2. Fabry–Perot Interferometers and Long-Period Fiber Gratings
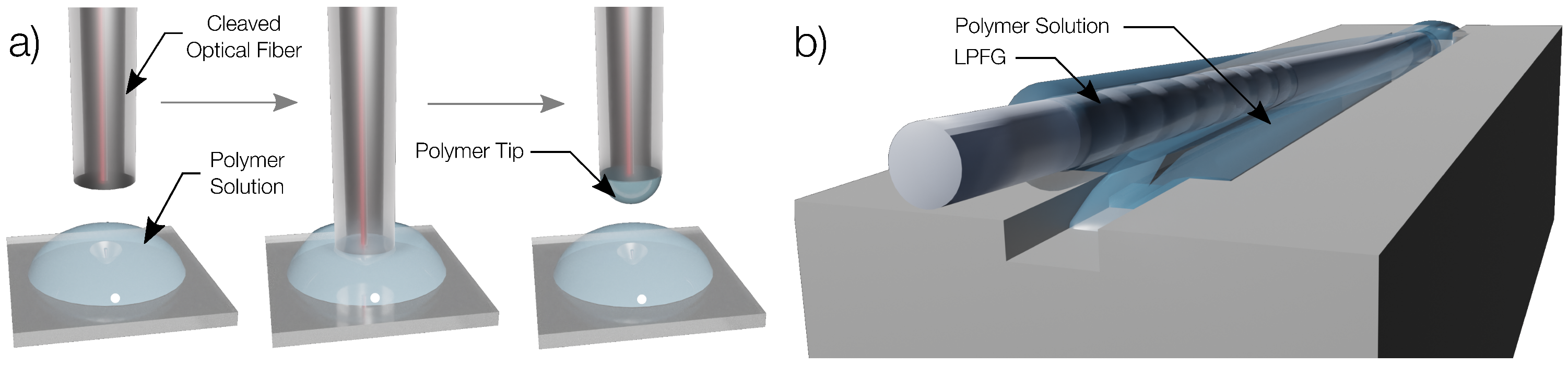
2.3. Polymer Coating of the Optical Structures
2.4. Humidity Measurements
2.5. Simultaneous Measurement of Refractive Index and Thickness of polymers
3. Results
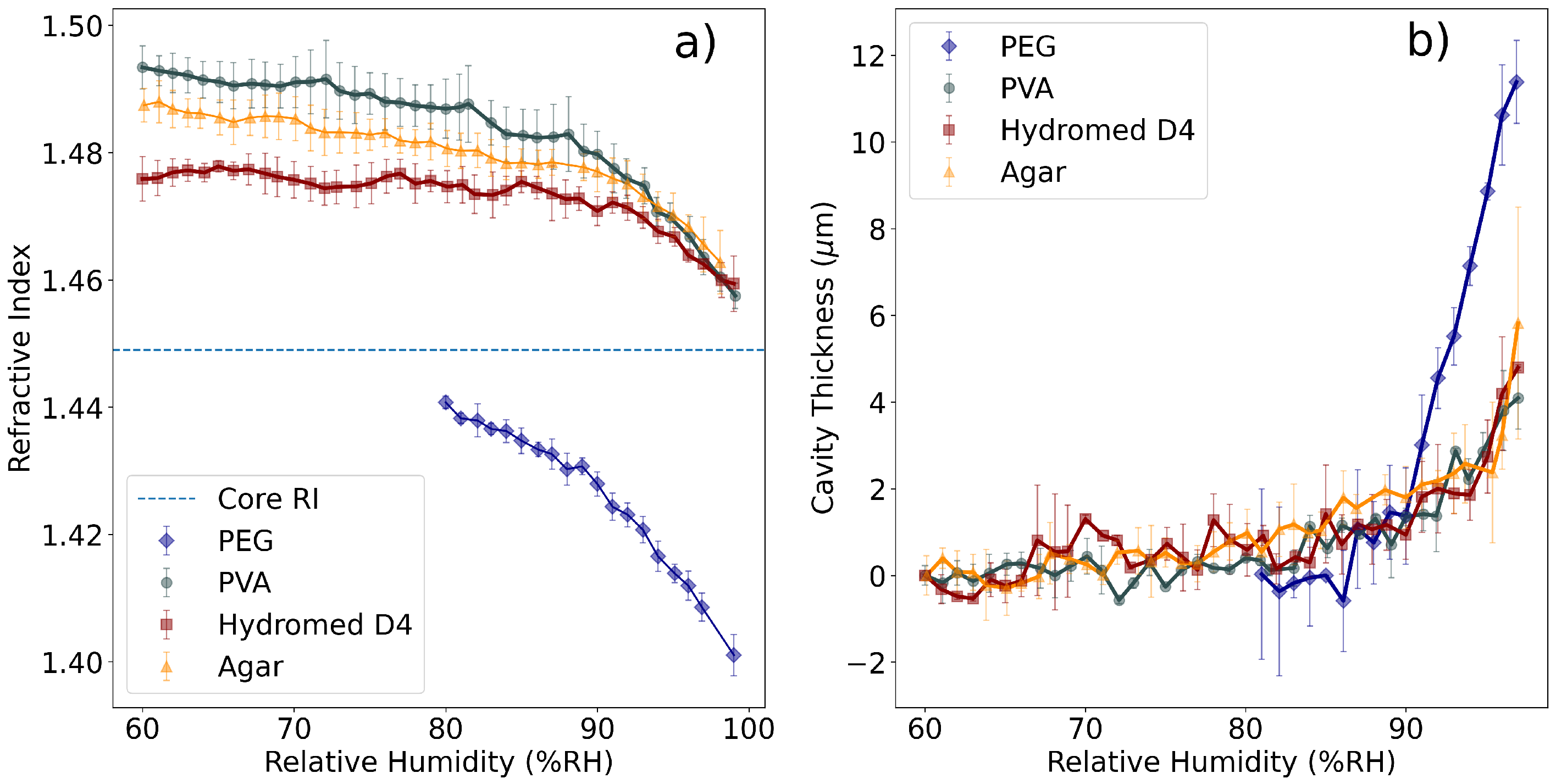
3.1. Measurement of Refractive Index and Cavity Length
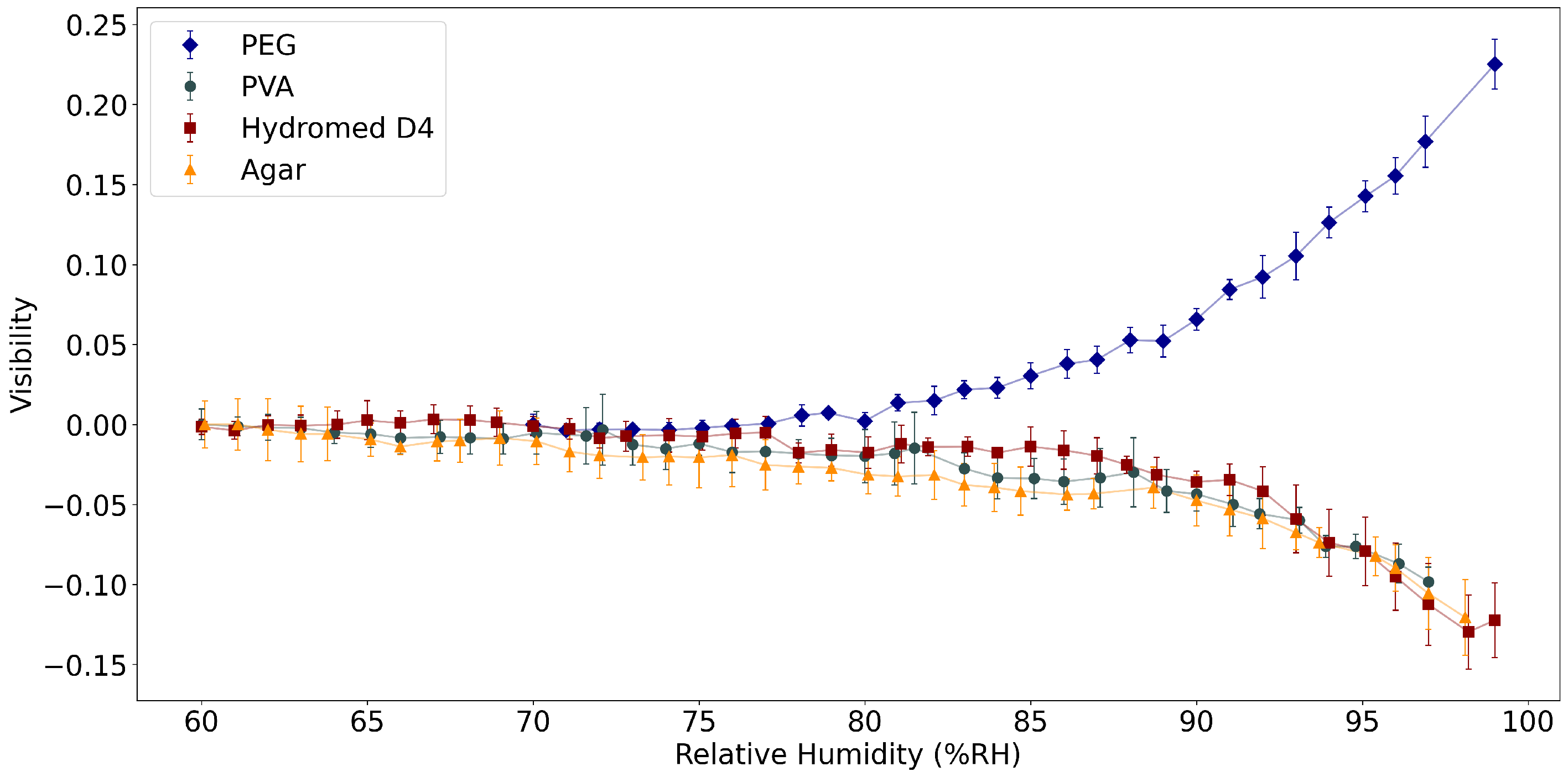
3.2. Fabry–Perot Interferometers as Relative Humidity Sensors
3.3. Long-Period Fiber Gratings as Relative Humidity Sensors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitschke, F. Fiber-optic sensor for humidity. Opt. Lett. 1989, 14, 967–969. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, F.; Pang, F.; Wang, T. An optical fiber humidity sensor based on optical absorption. In Proceedings of the Asia Communications and Photonics Conference and Exhibition. ACP 2011, Shanghai, China, 13–16 November 2011. [Google Scholar] [CrossRef]
- Alwis, L.; Sun, T.; Grattan, K.V. Analysis of polyimide-coated optical fiber long-period grating-based relative humidity sensor. IEEE Sens. J. 2013, 13, 767–771. [Google Scholar] [CrossRef]
- He, C.; Korposh, S.; Correia, R.; Liu, L.; Hayes-Gill, B.R.; Morgan, S.P. Optical fibre sensor for simultaneous temperature and relative humidity measurement: Towards absolute humidity evaluation. Sens. Actuators B Chem. 2021, 344, 130154. [Google Scholar] [CrossRef]
- Hill, K.O.; Fujii, Y.; Johnson, D.C.; Kawasaki, B.S. Photosensitivity in optical fiber waveguides: Application to reflection filter fabrication. Appl. Phys. Lett. 1978, 32, 647. [Google Scholar] [CrossRef]
- Meltz, G.; Morey, W.W.; Glenn, W.H. Formation of Bragg gratings in optical fibers by a transverse holographic method. Opt. Lett. 1989, 14, 823–825. [Google Scholar] [CrossRef]
- Cheng, X.; Bonefacino, J.; Guan, B.O.; Tam, H.Y. All-polymer fiber-optic pH sensor. Opt. Express 2018, 26, 14610–14616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, R.; Pereira, L.; Paixao, T.; Woyessa, G.; Hu, X.; Antunes, P.; Andre, P.; Bang, O.; Pinto, J.; Ortega, B.; et al. Chirped POF Bragg grating production utilizing UV cure adhesive coating for multiparameter sensing. Opt. Fiber Technol. 2021, 65, 102593. [Google Scholar] [CrossRef]
- Li, L.; He, R.; Soares, M.S.; Savović, S.; Hu, X.; Marques, C.; Min, R.; Li, X. Embedded FBG-Based Sensor for Joint Movement Monitoring. IEEE Sens. J. 2021, 21, 26793–26798. [Google Scholar] [CrossRef]
- Pinto, J.L.; Ferreira, M.S.; Novais, S. Optical Fiber Fabry–Perot Tip Sensor for Detection of Water–Glycerin Mixtures. J. Light. Technol. 2018, 36, 1576–1582. [Google Scholar]
- Santos, J.S.; Raimundo, I.M.; Cordeiro, C.M.; Biazoli, C.R.; Gouveia, C.A.; Jorge, P.A. Characterisation of a Nafion film by optical fibre Fabry–Perot interferometry for humidity sensing. Sens. Actuators B Chem. 2014, 196, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Tian, J.; Hu, L.; Yao, Y. High Sensitivity Humidity Fiber-Optic Sensor Based on All-Agar Fabry–Perot Interferometer. IEEE Sens. J. 2018, 18, 4879–4885. [Google Scholar] [CrossRef]
- Vengsarkar, A.M.; Lemaire, P.J.; Judkins, J.B.; Bhatia, V.; Erdogan, T.; Sipe, J.E. Long-period fiber gratings as band-rejection filters. J. Light. Technol. 1996, 14, 58–64. [Google Scholar] [CrossRef]
- Erdogan, T. Cladding-mode resonances in short- and long-period fiber grating filters. JOSA A 1997, 14, 1760–1773. [Google Scholar] [CrossRef]
- Bonal, V.; Quintana, J.A.; Villalvilla, J.M.; Muñoz-Mármol, R.; Mira-Martínez, J.C.; Boj, P.G.; Cruz, M.E.; Castro, Y.; Díaz-García, M.A. Simultaneous Determination of Refractive Index and Thickness of Submicron Optical Polymer Films from Transmission Spectra. Polymer 2021, 13, 2545. [Google Scholar] [CrossRef] [PubMed]
- Flores-Bravo, J.A.; Illarramendi, M.A.; Zubia, J.; Villatoro, J. Optical fiber interferometer for temperature-independent refractive index measuring over a broad range. OptLT 2021, 139, 106977. [Google Scholar] [CrossRef]
- Dias, B.; Mendes, J.P.; de Almeida, J.M.M.M.; Coelho, L.C.C. Characterization and Comparison of the Relative Humidity Response of Hydromorphic Polymers in Long-Period Fiber Grating Structures. Chem. Proc. 2021, 5, 42. [Google Scholar] [CrossRef]
- Acikgoz, S.; Bilen, B.; Demir, M.M.; Menceloglu, Y.Z.; Skarlatos, Y.; Aktas, G.; Inci, M.N. Use of polyethylene glycol coatings for optical fibre humidity sensing. Opt. Rev. 2008, 15, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.; Liu, B.; Zhang, H.; Li, Y.; Zhou, H.; Sun, H.; Zhang, W.; Zhao, Q. Relative humidity sensor based on tilted fiber Bragg grating with polyvinyl alcohol coating. IEEE Photonics Technol. Lett. 2009, 21, 441–443. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Y.; Cao, S.; Huang, Y.; Zhang, L.; Zhang, F.; Liao, C.; Wang, Y. Mechanism and Characteristics of Humidity Sensing with Polyvinyl Alcohol-Coated Fiber Surface Plasmon Resonance Sensor. Sensors 2018, 18, 2029. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Li, T.; Liu, Y.; Li, Y.; Zhao, C.L.; Chan, C.C. Polyvinyl alcohol-coated hybrid fiber grating for relative humidity sensing. J. Biomed. Opt. 2011, 16, 077001. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zou, F.; Jiang, C.; Mou, C.; Wang, T. Humidity Sensor Based on a Long-Period Fiber Grating Coated with Polymer Composite Film. Sensors 2019, 19, 2263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, R.; Jiang, Y.; Ding, W. Agarose gel filled temperature-insensitive photonic crystal fibers humidity sensor based on the tunable coupling ratio. Sens. Actuators B Chem. 2014, 195, 313–319. [Google Scholar] [CrossRef]
- Novais, S.; Ferreira, M.S.; Pinto, J.L. Humidity sensor based on optical fiber coated with agarose gel. Opt. Sens. 2019, 11028, 256–262. [Google Scholar] [CrossRef]
- Fujiwara, E.; Cabral, T.D.; Sato, M.; Oku, H.; Cordeiro, C.M.B. Agarose-based structured optical fibre. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- D’Amato, R.; Caponero, M.A.; Palazzo, B.; Terranova, G.; Polimadei, A. A Novel Fibre Optic Sensor of Relative Humidity for Application in Cultural Heritage. In Proceedings of the IMEKO TC4 International Conference on Metrology for Archaeology and Cultural Heritage, Florence, Italy, 4–6 December 2019; pp. 318–323. [Google Scholar]
- Van Campen, L.; Amidon, G.; Zografi, G. Moisture Sorption Kinetics for Water-Soluble Substances I: Theoretical Considerations of Heat Transport Control. J. Pharm. Sci. 1983, 72, 1381–1388. [Google Scholar] [CrossRef]
- Thijs, H.M.L.; Becer, C.R.; Guerrero-Sanchez, C.; Fournier, D.; Hoogenboom, R.; Schubert, U.S. Water uptake of hydrophilic polymers determined by a thermal gravimetric analyzer with a controlled humidity chamber. J. Mater. Chem. 2007, 17, 4864–4871. [Google Scholar] [CrossRef]
- Baird, J.A.; Olayo-Valles, R.; Rinaldi, C.; Taylor, L.S. Effect of Molecular Weight, Temperature, and Additives on the Moisture Sorption Properties of Polyethylene Glycol. J. Pharm. Sci. 2010, 99, 154–168. [Google Scholar] [CrossRef]
- Bui, D.T.; Nida, A.; Ng, K.C.; Chua, K.J. Water vapor permeation and dehumidification performance of poly(vinyl alcohol)/lithium chloride composite membranes. J. Memb. Sci. 2016, 498, 254–262. [Google Scholar] [CrossRef] [Green Version]
- Bui, T.; Wong, Y.; Thu, K.; Oh, S.; Ja, M.K.; Ng, K.; Raisul, I.; Chua, K. Effect of hygroscopic materials on water vapor permeation and dehumidification performance of poly(vinyl alcohol) membranes. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Vivekh, P.; Bui, D.T.; Wong, Y.; Kumja, M.; Chua, K.J. Performance evaluation of PVA-LiCl coated heat exchangers for next-generation of energy-efficient dehumidification. Appl. Energy 2019, 237, 733–750. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Massarelli, E.; Silva, D.; Pimenta, A.F.; Fernandes, A.I.; Mata, J.L.; Armês, H.; Salema-Oom, M.; Saramago, B.; Serro, A.P. Polyvinyl alcohol/chitosan wound dressings loaded with antiseptics. Int. J. Pharm. 2021, 593, 120110. [Google Scholar] [CrossRef] [PubMed]
- Gastón, A.; Pérez, F.; Sevilla, J. Optical fiber relative-humidity sensor with polyvinyl alcohol film. Appl. Opt. 2004, 43, 4127–4132. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, M.; Tajedin, R.; Farahmandfar, F.; Naeimi, M.; Monajjemi, M. Preparation and characterization of vancomycin-loaded chitosan/PVA/PEG hydrogels for wound dressing. Mater. Res. Express 2020, 7, 095401. [Google Scholar] [CrossRef]
- HydroMed Datasheet; AdvanSource Biomaterials: Wilmington, MA, USA, 2017.
- Hoefer, C.; Santner, J.; Borisov, S.M.; Wenzel, W.W.; Puschenreiter, M. Integrating chemical imaging of cationic trace metal solutes and pH into a single hydrogel layer. Anal. Chim. Acta 2017, 950, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Kenney, R.M.; Boyce, M.W.; Whitman, N.A.; Kromhout, B.P.; Lockett, M.R. A pH-Sensing Optode for Mapping Spatiotemporal Gradients in 3D Paper-Based Cell Cultures. Anal. Chem. 2018, 90, 2376. [Google Scholar] [CrossRef] [PubMed]
- Maierhofer, M.; Rieger, V.; Mayr, T. Optical ammonia sensors based on fluorescent aza-BODIPY dyes—A flexible toolbox. Anal. Bioanal. Chem. 2020, 412, 7559–7567. [Google Scholar] [CrossRef]
- Fuse, T.; Goto, F. Studies on Utilization of AgarPart X. Some Properties of Agarose and Agaropectin Isolated from Various Mucilaginous Substances of Red Seaweeds. Agric. Biol. Chem. 1971, 35, 799–804. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Athukorala, Y.; Lee, J.H. Characterization of Agarose Product from Agar Using DMSO. ALGAE 2005, 20, 61–67. [Google Scholar] [CrossRef]
- Rego, G. Arc-Induced Long Period Fiber Gratings. J. Sens. 2016, 2016, 3598634. [Google Scholar] [CrossRef] [Green Version]
- Rego, G.; Caldas, P.; Ivanov, O.V. Arc-Induced Long-Period Fiber Gratings at INESC TEC. Part I: Fabrication, Characterization and Mechanisms of Formation. Sensors 2021, 21, 4914. [Google Scholar] [CrossRef] [PubMed]
- Liu, T. Digital-Output Relative Humidity & Temperature Sensor/Module DHT22 (DHT22 Also Named as AM2302) Capacitive-Type Humidity and Temperature Module/Sensor; Kiwi Electronics: The Hague, The Netherlands, 2019. [Google Scholar]
- Chiang, K.S.; Liu, W.J.; Liao, X.; Rao, Y.J.; Ran, Z.L. Laser-micromachined Fabry–Perot optical fiber tip sensor for high-resolution temperature-independent measurement of refractive index. Opt. Express 2008, 16, 2252–2263. [Google Scholar] [CrossRef]






| Fabry–Perot Interferometers | Long-Period Fiber Gratings | |
|---|---|---|
| Fabrication | Very simple | Harder |
| Equipment | Reflection Mode interrogation | Transmission Mode interrogation |
| Integration | Easy in capillary tube | Harder in solid environments |
| Sensitivity | Low for polymers near | High (adjusting film thickness) |
| Polymer | Refractive Index Sensitivity (RIU/%RH) | Expansion Coefficient (μm/%RH) | Deliquescence Relative Humidity | Observations |
|---|---|---|---|---|
| PVA | 90%RH | Below DRH | ||
| PEG | 80%RH | Above DRH | ||
| Hydrogel | 90%RH | Below DRH | ||
| Agar | 90%RH | Below DRH |
| Polymer | Working Range (%RH) | Wavelength Sensitivity (nm/%RH) | Optical Power Sensitivity (dB/%RH) |
|---|---|---|---|
| PVA | 65–90 90–100 | ||
| PEG | 65–80 80–100 | - | |
| Hydrogel | 65–95 | ||
| Agar | 65–95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, B.; Carvalho, J.; Mendes, J.P.; Almeida, J.M.M.M.; Coelho, L.C.C. Analysis of the Relative Humidity Response of Hydrophilic Polymers for Optical Fiber Sensing. Polymers 2022, 14, 439. https://doi.org/10.3390/polym14030439
Dias B, Carvalho J, Mendes JP, Almeida JMMM, Coelho LCC. Analysis of the Relative Humidity Response of Hydrophilic Polymers for Optical Fiber Sensing. Polymers. 2022; 14(3):439. https://doi.org/10.3390/polym14030439
Chicago/Turabian StyleDias, Bernardo, João Carvalho, João P. Mendes, José M. M. M. Almeida, and Luís C. C. Coelho. 2022. "Analysis of the Relative Humidity Response of Hydrophilic Polymers for Optical Fiber Sensing" Polymers 14, no. 3: 439. https://doi.org/10.3390/polym14030439
APA StyleDias, B., Carvalho, J., Mendes, J. P., Almeida, J. M. M. M., & Coelho, L. C. C. (2022). Analysis of the Relative Humidity Response of Hydrophilic Polymers for Optical Fiber Sensing. Polymers, 14(3), 439. https://doi.org/10.3390/polym14030439










