Self-Assembled TLR7/8 Agonist-Mannose Conjugate as An Effective Vaccine Adjuvant for SARS-CoV-2 RBD Trimer
Abstract
1. Introduction
2. Materials and Methods
2.1. TLR Reporter Assay
2.2. In Vitro BMDCs Assay and Cytokine Assay
2.3. Formulations of SARS-CoV-2 RBD Trimer Vaccines and Animals
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Statistical Analysis
3. Results
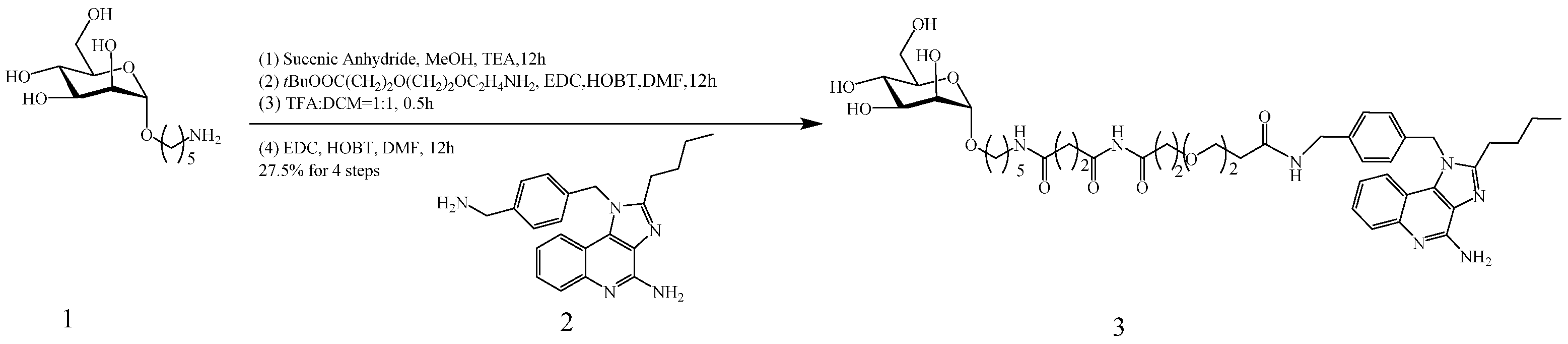
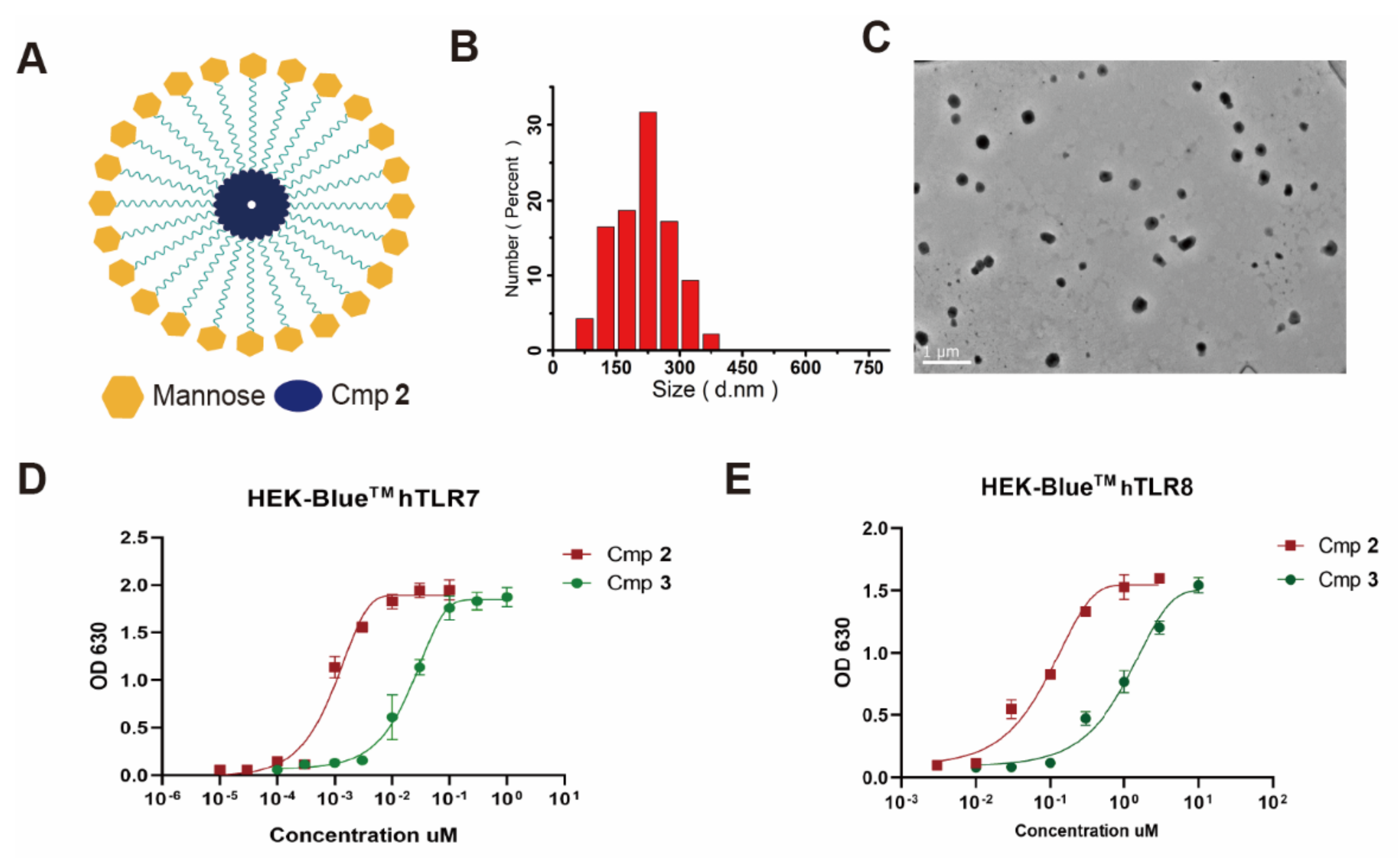
3.1. Synthesis and Characterization of Compound 3
3.2. Compound 3 Induced BMDC Maturation and Stimulate Cytokines Secretion
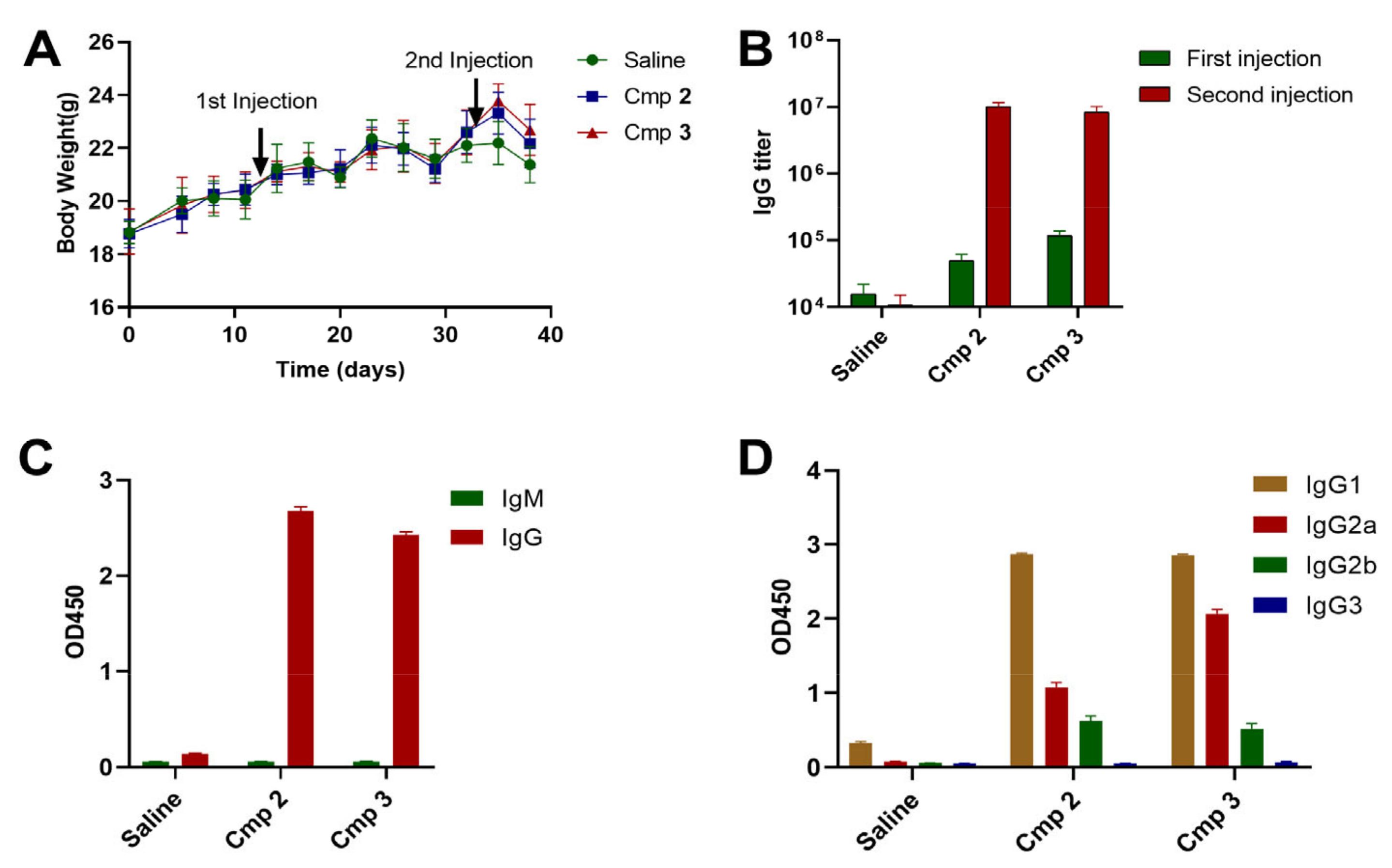
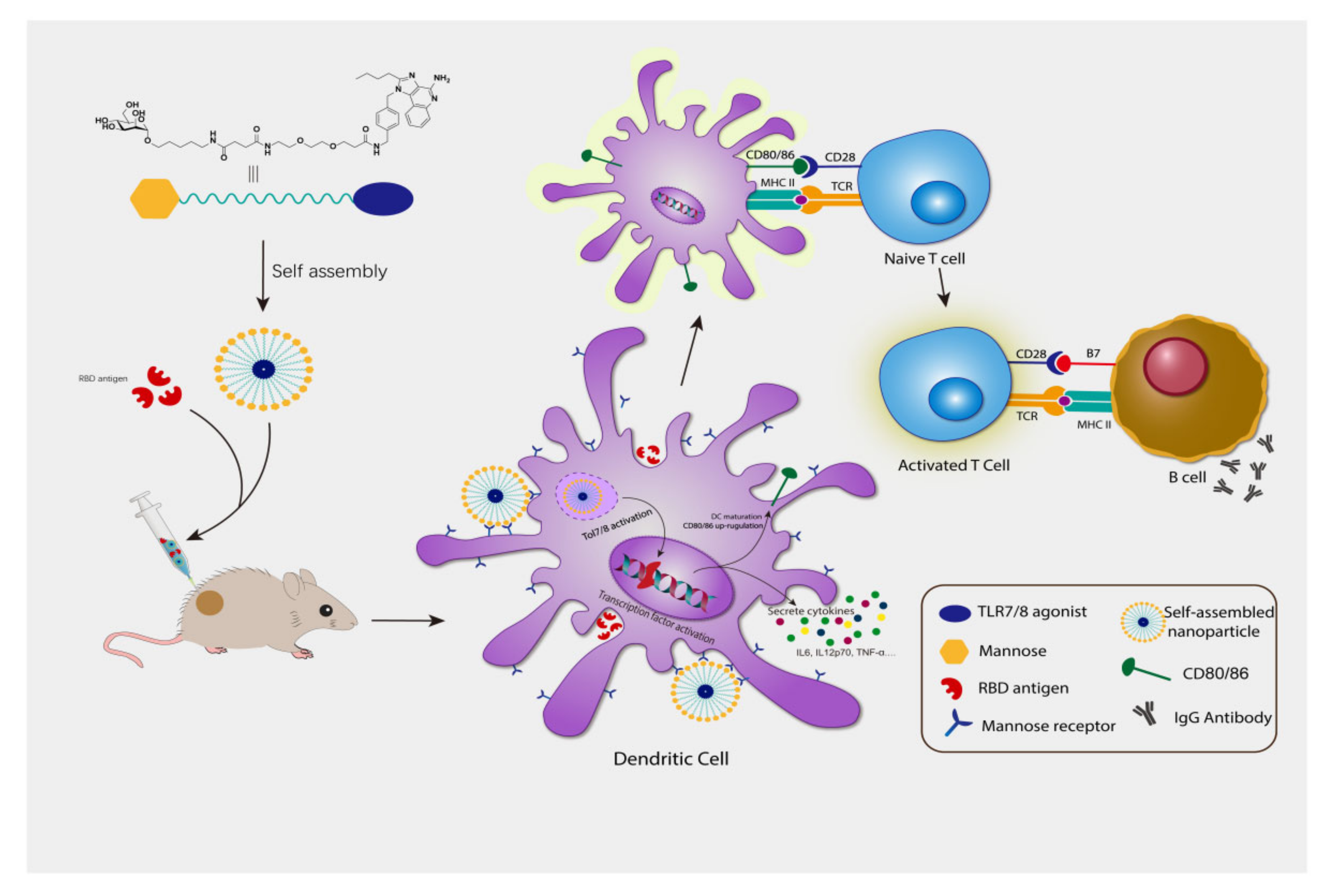
3.3. Compound 3 as an Effective Vaccine Adjuvant for SARS-CoV2 RBD Trimer Antigen
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chow, J.C.; Young, D.W.; Golenbock, D.T.; Christ, W.J.; Gusovsky, F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 1999, 274, 10689–10692. [Google Scholar] [CrossRef]
- Miller, S.M.; Cybulski, V.; Whitacre, M.; Bess, L.S.; Livesay, M.T.; Walsh, L.; Burkhart, D.; Bazin, H.G.; Evans, J.T. Novel Lipidated Imidazoquinoline TLR7/8 Adjuvants Elicit Influenza-Specific Th1 Immune Responses and Protect Against Heterologous H3N2 Influenza Challenge in Mice. Front. Immunol. 2020, 11, 406. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; McKenzie, I.F. Role of the mannose receptor in the immune response. Curr. Mol. Med. 2001, 1, 469–474. [Google Scholar] [CrossRef]
- Gao, C.; Stavenhagen, K.; Eckmair, B.; McKitrick, T.R.; Mehta, A.Y.; Matsumoto, Y.; McQuillan, A.M.; Hanes, M.S.; Eris, D.; Baker, K.J.; et al. Differential recognition of oligomannose isomers by glycan-binding proteins involved in innate and adaptive immunity. Sci. Adv. 2021, 7. [Google Scholar] [CrossRef]
- Sallusto, F.; Cella, M.; Danieli, C.; Lanzavecchia, A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: Downregulation by cytokines and bacterial products. J. Exp. Med. 1995, 182, 389–400. [Google Scholar] [CrossRef]
- Le Moignic, A.; Malard, V.; Benvegnu, T.; Lemiegre, L.; Berchel, M.; Jaffres, P.A.; Baillou, C.; Delost, M.; Macedo, R.; Rochefort, J.; et al. Preclinical evaluation of mRNA trimannosylated lipopolyplexes as therapeutic cancer vaccines targeting dendritic cells. J. Control. Release 2018, 278, 110–121. [Google Scholar] [CrossRef]
- Wilson, D.S.; Hirosue, S.; Raczy, M.M.; Bonilla-Ramirez, L.; Jeanbart, L.; Wang, R.; Kwissa, M.; Franetich, J.F.; Broggi, M.A.S.; Diaceri, G.; et al. Antigens reversibly conjugated to a polymeric glyco-adjuvant induce protective humoral and cellular immunity. Nat. Mater. 2019, 18, 175–185. [Google Scholar] [CrossRef]
- Ingale, S.; Wolfert, M.A.; Gaekwad, J.; Buskas, T.; Boons, G.J. Robust immune responses elicited by a fully synthetic three-component vaccine. Nat. Chem. Biol. 2007, 3, 663–667. [Google Scholar] [CrossRef]
- Sun, X.; Stefanetti, G.; Berti, F.; Kasper, D.L. Polysaccharide structure dictates mechanism of adaptive immune response to glycoconjugate vaccines. Proc. Natl. Acad. Sci. USA 2019, 116, 193–198. [Google Scholar] [CrossRef]
- Liang, Z.; Zhu, H.; Wang, X.; Jing, B.; Li, Z.; Xia, X.; Sun, H.; Yang, Y.; Zhang, W.; Shi, L.; et al. Adjuvants for Coronavirus Vaccines. Front. Immunol. 2020, 11, 589833. [Google Scholar] [CrossRef]
- Karuturi, B.V.K.; Tallapaka, S.B.; Yeapuri, P.; Curran, S.M.; Sanderson, S.D.; Vetro, J.A. Encapsulation of an EP67-Conjugated CTL Peptide Vaccine in Nanoscale Biodegradable Particles Increases the Efficacy of Respiratory Immunization and Affects the Magnitude and Memory Subsets of Vaccine-Generated Mucosal and Systemic CD8(+) T Cells in a Diameter-Dependent Manner. Mol. Pharm. 2017, 14, 1469–1481. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.; Wu, G.; Chen, L.; Hu, P.; Ren, H.; Hu, H. Coffee Consumption Decreases Risks for Hepatic Fibrosis and Cirrhosis: A Meta-Analysis. PLoS ONE 2015, 10, e0142457. [Google Scholar] [CrossRef]
- Chen, C.; Aldarouish, M.; Li, Q.; Liu, X.; Han, F.; Liu, H.; Qian, Q. Triggered Immune Response Induced by Antigenic Epitopes Covalently Linked with Immunoadjuvant-Pulsed Dendritic Cells as a Promising Cancer Vaccine. J. Immunol. Res. 2020, 2020, 3965061. [Google Scholar] [CrossRef]
- LeBlanc, J.J.; Li, Y.; Bastien, N.; Forward, K.R.; Davidson, R.J.; Hatchette, T.F. Switching gears for an influenza pandemic: Validation of a duplex reverse transcriptase PCR assay for simultaneous detection and confirmatory identification of pandemic (H1N1) 2009 influenza virus. J. Clin. Microbiol. 2009, 47, 3805–3813. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Cao, H.; Liu, C. SARS-CoV-2 S1 is superior to the RBD as a COVID-19 subunit vaccine antigen. J. Med. Virol. 2021, 93, 892–898. [Google Scholar] [CrossRef]
- Shi, R.; Zeng, J.; Xu, L.; Wang, F.; Duan, X.; Wang, Y.; Wu, Z.; Yu, D.; Huang, Q.; Yao, Y.G.; et al. A combination vaccine against SARS-CoV-2 and H1N1 influenza based on receptor binding domain trimerized by six-helix bundle fusion core. EBioMedicine 2022, 85, 104297. [Google Scholar] [CrossRef]
- Patel, M.K.; Vijayakrishnan, B.; Koeppe, J.R.; Chalker, J.M.; Doores, K.J.; Davis, B.G. Analysis of the dispersity in carbohydrate loading of synthetic glycoproteins using MALDI-TOF mass spectrometry. Chem. Commun. 2010, 46, 9119–9121. [Google Scholar] [CrossRef]
- Kimber, I.; Pichowski, J.S.; Betts, C.J.; Cumberbatch, M.; Basketter, D.A.; Dearman, R.J. Alternative approaches to the identification and characterization of chemical allergens. Toxicol. Vitr. 2001, 15, 307–312. [Google Scholar] [CrossRef]
- Shevlin, E.; Miggin, S.M. The TIR-domain containing adaptor TRAM is required for TLR7 mediated RANTES production. PLoS ONE 2014, 9, e107141. [Google Scholar] [CrossRef][Green Version]
- Lim, T.S.; Goh, J.K.; Mortellaro, A.; Lim, C.T.; Hammerling, G.J.; Ricciardi-Castagnoli, P. CD80 and CD86 differentially regulate mechanical interactions of T-cells with antigen-presenting dendritic cells and B-cells. PLoS ONE 2012, 7, e45185. [Google Scholar] [CrossRef]
- Rodell, C.B.; Arlauckas, S.P.; Cuccarese, M.F.; Garris, C.S.; Li, R.; Ahmed, M.S.; Kohler, R.H.; Pittet, M.J.; Weissleder, R. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2018, 2, 578–588. [Google Scholar] [CrossRef]
- Dearman, R.J.; Cumberbatch, M.; Maxwell, G.; Basketter, D.A.; Kimber, I. Toll-like receptor ligand activation of murine bone marrow-derived dendritic cells. Immunology 2009, 126, 475–484. [Google Scholar] [CrossRef]
- Mullen, A.C.; High, F.A.; Hutchins, A.S.; Lee, H.W.; Villarino, A.V.; Livingston, D.M.; Kung, A.L.; Cereb, N.; Yao, T.P.; Yang, S.Y.; et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science 2001, 292, 1907–1910. [Google Scholar] [CrossRef]
- Kishimoto, T. IL-6: From its discovery to clinical applications. Int. Immunol. 2010, 22, 347–352. [Google Scholar] [CrossRef]
- Bleier, J.I.; Pillarisetty, V.G.; Shah, A.B.; DeMatteo, R.P. Increased and long-term generation of dendritic cells with reduced function from IL-6-deficient bone marrow. J. Immunol. 2004, 172, 7408–7416. [Google Scholar] [CrossRef]
- Daudelin, J.F.; Mathieu, M.; Boulet, S.; Labrecque, N. IL-6 production by dendritic cells is dispensable for CD8+ memory T-cell generation. BioMed Res. Int. 2013, 2013, 126189. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Ma, C.S.; Deenick, E.K.; Batten, M.; Tangye, S.G. The origins, function, and regulation of T follicular helper cells. J. Exp. Med. 2012, 209, 1241–1253. [Google Scholar] [CrossRef]
- Huber, V.C.; McKeon, R.M.; Brackin, M.N.; Miller, L.A.; Keating, R.; Brown, S.A.; Makarova, N.; Perez, D.R.; Macdonald, G.H.; McCullers, J.A. Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin. Vaccine Immunol. 2006, 13, 981–990. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Ravetch, J.V. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science 2005, 310, 1510–1512. [Google Scholar] [CrossRef]
- Mozdzanowska, K.; Furchner, M.; Washko, G.; Mozdzanowski, J.; Gerhard, W. A pulmonary influenza virus infection in SCID mice can be cured by treatment with hemagglutinin-specific antibodies that display very low virus-neutralizing activity in vitro. J. Virol. 1997, 71, 4347–4355. [Google Scholar] [CrossRef]
- Gerhard, W.; Mozdzanowska, K.; Furchner, M.; Washko, G.; Maiese, K. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol. Rev. 1997, 159, 95–103. [Google Scholar] [CrossRef]
- Wilson, J.A.; Hevey, M.; Bakken, R.; Guest, S.; Bray, M.; Schmaljohn, A.L.; Hart, M.K. Epitopes involved in antibody-mediated protection from Ebola virus. Science 2000, 287, 1664–1666. [Google Scholar] [CrossRef]
- Schlesinger, J.J.; Chapman, S. Neutralizing F(ab’)2 fragments of protective monoclonal antibodies to yellow fever virus (YF) envelope protein fail to protect mice against lethal YF encephalitis. J. Gen. Virol. 1995, 76 Pt 1, 217–220. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, C.; Meng, X.; Hu, Y.; Mao, H.; Li, H.; Yang, J.; Sun, T.; Meng, S.; Zong, C. Self-Assembled TLR7/8 Agonist-Mannose Conjugate as An Effective Vaccine Adjuvant for SARS-CoV-2 RBD Trimer. Polymers 2022, 14, 5466. https://doi.org/10.3390/polym14245466
Teng C, Meng X, Hu Y, Mao H, Li H, Yang J, Sun T, Meng S, Zong C. Self-Assembled TLR7/8 Agonist-Mannose Conjugate as An Effective Vaccine Adjuvant for SARS-CoV-2 RBD Trimer. Polymers. 2022; 14(24):5466. https://doi.org/10.3390/polym14245466
Chicago/Turabian StyleTeng, Changcai, Xiongyan Meng, Yeqin Hu, Hongzhao Mao, Huiting Li, Jing Yang, Tiantian Sun, Shuai Meng, and Chengli Zong. 2022. "Self-Assembled TLR7/8 Agonist-Mannose Conjugate as An Effective Vaccine Adjuvant for SARS-CoV-2 RBD Trimer" Polymers 14, no. 24: 5466. https://doi.org/10.3390/polym14245466
APA StyleTeng, C., Meng, X., Hu, Y., Mao, H., Li, H., Yang, J., Sun, T., Meng, S., & Zong, C. (2022). Self-Assembled TLR7/8 Agonist-Mannose Conjugate as An Effective Vaccine Adjuvant for SARS-CoV-2 RBD Trimer. Polymers, 14(24), 5466. https://doi.org/10.3390/polym14245466






