Characterization of High Molecular Weight Pneumococcal Conjugate by SEC-MALS and AF4-MALS
Abstract
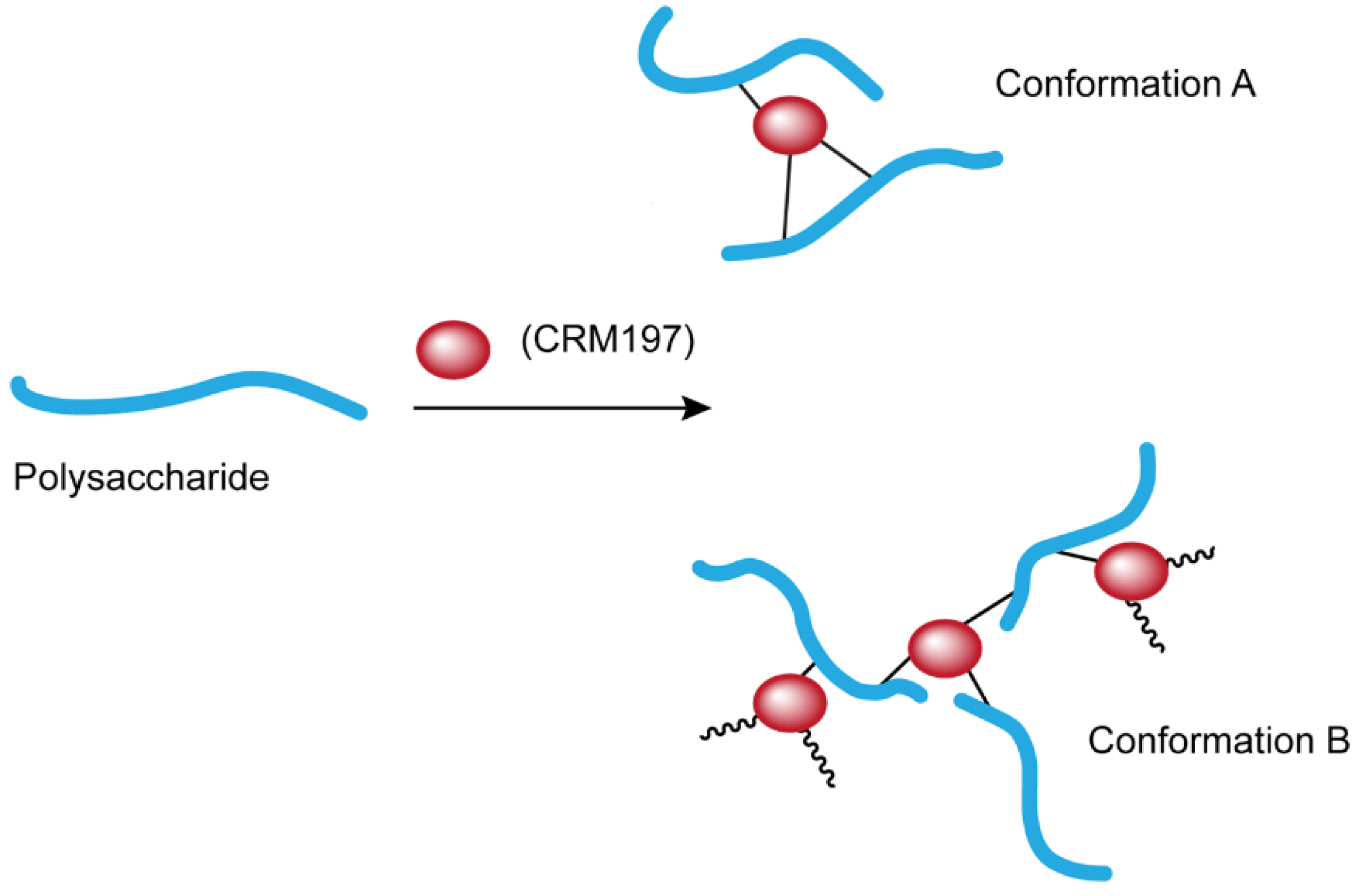
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. SEC-MALS Method
2.3. AF4-MALS Method
2.4. MALS Measurement
2.5. Monovalent Conjugate (MVC) Samples
3. Results and Discussion
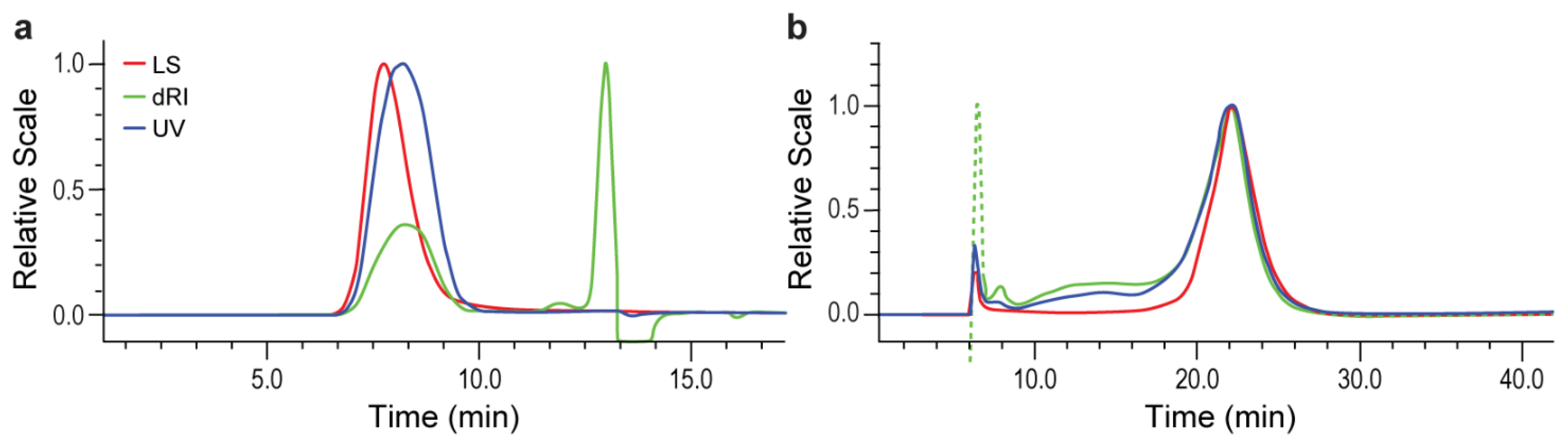
3.1. SEC Measurement of High Mw MVCs
3.2. AF4 Measurement of High Mw MVCs
3.3. SEC-MALS and AF4-MALS Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daniels, C.C.; Rogers, P.D.; Shelton, C.M. A Review of Pneumococcal Vaccines: Current Polysaccharide Vaccine Recommendations and Future Protein Antigens. J. Pediatr. Pharmacol. Ther. 2016, 21, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Geno, K.A.; Gilbert, G.L.; Song, J.Y.; Skovsted, I.C.; Klugman, K.P.; Jones, C.; Konradsen, H.B.; Nahm, M.H. Pneumococcal capsules and their types: Past, present, and future. Clin. Microbiol. Rev. 2015, 28, 871–899. [Google Scholar] [CrossRef] [PubMed]
- Niedermana, M.S.; Folaranmib, T.; Buchwald, U.K.; Musey, L.; Cripps, A.W.; Johnson, K.D. Efficacy and effectiveness of a 23-valent polysaccharide vaccine against invasive and noninvasive pneumococcal disease and related outcomes: A review of available evidence. Expert Rev. Vaccines 2021, 20, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Koff, W.C.; Burton, D.R.; Johnson, P.R.; Walker, B.D.; King, C.R.; Nabel, G.J.; Ahmed, R.; Bhan, M.K.; Plotkin, S.A. Accelerating Next-Generation Vaccine Development for Global Disease Prevention. Science 2013, 340, 1064. [Google Scholar] [CrossRef] [PubMed]
- Musher, D.M.; Chapman, A.J.; Goree, A.; Jonsson, S.; Briles, D.; Baughn, R.E. Natural and vaccine-related immunity to Streptococcus pneumoniae. J. Infect. Dis. 1986, 154, 245–256. [Google Scholar] [CrossRef]
- Weller, S.; Reynaud, C.A.; Weill, J.C. Vaccination against encapsulated bacteria in humans: Paradoxes. Trends Immunol. 2005, 26, 85–89. [Google Scholar] [CrossRef]
- Masomian, M.; Ahmad, Z.; Gew, L.T.; Poh, C.L. Development of next generation Streptococcus pneumonia vaccines conferring broad protection. Vaccines 2020, 8, 132. [Google Scholar] [CrossRef]
- Gruber, W.C.; Scott, D.A.; Emini, E. Development and clinical evaluation of Prevnar 13, a 13-valent pneumococcal CRM197 conjugate vaccine. Ann. N. Y. Acad. Sci. 2012, 1263, 15–26. [Google Scholar] [CrossRef]
- Cannon, K.; Elder, C.; Young, M.; Scott, D.A.; Scully, I.L.; Baugher, G.; Peng, Y.; Jansen, K.U.; Gruber, W.C.; Watson, W. A trial to evaluate the safety and immunogenicity of a 20-valentpneumococcal conjugate vaccine in populations of adults ≥65 years of age with different prior pneumococcal vaccination. Vaccine 2021, 39, 7494–7502. [Google Scholar] [CrossRef]
- Prymula, R.; Schuerman, L. 10-valent pneumococcal nontypeable Haemophilus influenzae PD conjugate vaccine: Synflorix. Expert Rev. Vaccines 2009, 8, 1479–1500. [Google Scholar] [CrossRef]
- Platt, H.L.; Cardona, J.F.; Haranaka, M.; Schwartz, H.I.; Perez, S.N.; Dowell, A.; Chang, C.; Dagan, R.; Tamms, G.M.; Sterling, T.; et al. A phase 3 trial of safety, tolerability, and immunogenicity of V114, 15-valent pneumococcal conjugate vaccine, compared with 13-valent pneumococcal conjugate vaccine in adults 50 years of age and older (PNEU-AGE). Vaccine 2022, 40, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Caro-Aguilar, I.; Indrawati, L.; Kaufhold, R.M.; Gaunt, C.; Zhang, Y.; Nawrocki, D.K.; Giovarelli, C.; Winters, M.A.; Smith, W.J.; Heinrichs, J.; et al. Immunogenicity differences of a 15-valent pneumococcal polysaccharide conjugate vaccine (PCV15) based on vaccine dose, route of immunization and mouse strain. Vaccine 2017, 35, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Kalin, M. Pneumococcal serotypes and their clinical relevance. Thorax 1998, 53, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Jacques, L.C.; Panagiotou, S.; Baltazar, M.; Senghore, M.; Khandaker, S.; Xu, R.; Bricio-Moreno, L.; Yang, M.; Dowson, C.G.; Everett, D.B.; et al. Increased pathogenicity of pneumococcal serotype 1 is driven by rapid autolysis and release of pneumolysin. Nat. Commun. 2020, 11, 1892. [Google Scholar] [CrossRef] [PubMed]
- Beall, B.; Walker, H.; Tran, T.; Li, Z.; Varghese, J.; McGee, L.; Li, Y.; Metcalf, B.J.; Gierke, R.; Mosites, E.; et al. Upsurge of Conjugate Vaccine Serotype 4 Invasive Pneumococcal Disease Clusters Among Adults Experiencing Homelessness in California, Colorado, and New Mexico. J. Infect. Dis. 2021, 223, 1241. [Google Scholar] [CrossRef]
- Ndlangis, K.; Plessis, M.; Allam, M.; Wolter, N.; De Gouveia, L.; Klugman, K.P.; Cohen, C.; Gladstone, R.A.; Gottberg, A.V. Invasive Disease Caused Simultaneously by Dual Serotypes of Streptococcus pneumoniae. J. Clin. Microbiol. 2018, 56, e01149-17. [Google Scholar]
- Lindberg, B.; Lindqvist, B.; Lönngren, J.; Powell, D.A. Structural studies of the capsular polysaccharide from Streptococcus pneumoniae type 1. Carbohydr. Res. 1980, 78, 111–117. [Google Scholar] [CrossRef]
- Stroop, C.J.M.; Xu, Q.; Retzlaff, M.; Abeygunawardana, C.; Bush, A. Structural analysis and chemical depolymerization of the capsular polysaccharide of Streptococcus pneumoniae type 1. Carbohydr. Res. 2002, 337, 335–344. [Google Scholar] [CrossRef]
- Jones, C.; Currie, F. The Pneumococcal Polysaccharide S4: A Structural Reassessment. Carbohydr. Res. 1988, 184, 279–284. [Google Scholar] [CrossRef]
- Frasch, C.E. Preparation of bacterial polysaccharide–protein conjugates: Analytical and manufacturing challenges. Vaccine 2009, 27, 6468–6470. [Google Scholar] [CrossRef]
- Biemans, R.; Micoli, F.; Romano, M.R. Glycoconjugate vaccines, production and characterization. Recent Trends Carbohydr. Chem. 2020, 2, 285–313. [Google Scholar]
- Hadidi, M.; Buckley, J.J.; Zydney, A.L. Effects of solution conditions on characteristics and size exclusion chromatography of pneumococcal polysaccharides and conjugate vaccines. Carbohydr. Polym. 2016, 152, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.Z.; Rustandi, R.R.; Barbacci, D.; Swartz, A.; Gulasarian, A.; Loughney, J.W. RP-UPLC method for Oncolytic Coxsackievirus Viral Protein Separation and Empty to Full Capsid Quantification. Hum. Gene Ther. 2022, 33, 765–775. [Google Scholar] [CrossRef]
- Bednar, B.; Hennessey, J.P., Jr. Molecular size analysis of capsular polysaccharide preparations from Streptococcus pneumoniae. Carbohydr. Res. 1993, 243, 115–130. [Google Scholar] [CrossRef]
- Deng, J.Z.; Rustandi, R.R.; Swartz, A.; Shieh, Y.; Baker, J.B.; Vlasak, J.; Wang, S.; Loughney, J.W. SEC Coupled with In-line Multiple Detectors for the Characterization of an Oncolytic Coxsackievirus. Mol. Ther. Oncolytic 2022, 24, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Thiebaud, J.; Fangeta, I.; Jaudinaud, I.; Fourrichon, L.; Sabouraud, A.; Talaga, P.; Uhlrich, S. Development and validation of high-performance size exclusion chromatography methods to determine molecular size parameters of Haemophilus influenzae type b polysaccharides and conjugates. Anal. Biochem. 2014, 453, 22–28. [Google Scholar] [CrossRef]
- Lockyera, K.; Gao, F.; Francis, R.J.; Eastwood, D.; Khatri, B.; Stebbings, R.; Derrick, J.P.; Bolgiano, B. Higher mass meningococcal group C-tetanus toxoid vaccines conjugated with carbodiimide correlate with greater immunogenicity. Vaccine 2020, 38, 2859–2869. [Google Scholar] [CrossRef]
- Deng, J.Z.; Lancaster, C.; Winters, M.A.; Phillips, K.M.; Zhuang, P.; Ha, S. Multi-attribute Characterization of Pneumococcal Conjugate Vaccine by Size-exclusion Chromatography Coupled with UV-MALS-RI Detections. Vaccine 2022, 40, 1464–1471. [Google Scholar] [CrossRef]
- Harding, S.E.; Abdelhameed, A.S.; Morris, G.A.; Adams, G.; Laloux, O.; Cerny, L.; Bonnier, B.; Duvivier, P.; Conrath, K.; Lenfant, C. Solution properties of capsular polysaccharides from Streptococcus pneumoniae. Carbohydr. Polym. 2012, 90, 237–242. [Google Scholar] [CrossRef]
- Podzimek, S. Light Scattering, Size Exclusion Chromatography and Asymmetric Flow Field Flow Fractionation: Powerful Tools for the Characterization of Polymers, Proteins and Nanoparticles; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Muza, U.L.; Boye, S.; Lederer, A. Dealing with the complexity of conjugated and self-assembled polymer-nano structures using field-flow fractionation. Anal. Sci. Adv. 2021, 2, 95–108. [Google Scholar] [CrossRef]
- Pitkänen, L.; Striegel, A.M. Polysaccharide characterization by hollow-fiber flow field-flow fractionation with on-line multi-angle static light scattering and differential refractometry. J. Chromatogr. A 2015, 1380, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Ventouri, I.K.; Loeber, S.; Somsen, G.W.; Schoenmakers, P.J.; Astefanei, A. Field-flow fractionation for molecular-interaction studies of labile and complex systems: A critical review. Anal. Chim. Acta 2022, 1193, 339396. [Google Scholar] [CrossRef] [PubMed]
- Plavchak, C.L.; Smith, W.C.; Bria, C.R.M.; Williams, S.K.R. New Advances and Applications in Field-Flow Fractionation. Annu. Rev. Anal. Chem. 2021, 14, 257–279. [Google Scholar] [CrossRef]
- Huglin, M.B. Light Scattering from Polymer Solutions; Book Chapter 5; Academic Press: Cambridge, MA, USA, 1972. [Google Scholar]
- Wyatt, P.J. Light Scattering and the Absolute Characterization of Macromolecules. Anal. Chim. Acta 1993, 272, 1–40. [Google Scholar] [CrossRef]
- Zimm, B.H.J. The scattering of light and the radial distribution function of high polymer solutions. J. Chem. Phys. 1948, 16, 1093–1099. [Google Scholar] [CrossRef]
- Andersson, M.; Wittgren, B.; Wahlund, K. Accuracy in Multiangle Light Scattering Measurements for Molar Mass and Radius Estimations. Model Calculations and Experiments. Anal. Chem. 2003, 75, 4279–4291. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, B.S.; Kerwin, B.A.; Chang, B.S.; Philo, J.S. Online Size-Exclusion High-Performance Liquid Chromatography Light Scattering and Differential Refractometry Methods to Determine Degree of Polymer Conjugation to Proteins and Protein-Protein or Protein-Ligand Association States. Anal. Biochem. 2001, 299, 136–146. [Google Scholar] [CrossRef]
- Theisen, A.; Johann, C.; Deacon, M.P.; Harding, S.E. Refractive Increment Data-Book; Nottingham University Press: Nottingham, UK, 2000. [Google Scholar]
- Netopilík, M.; Podzimek, S. Retention Mechanism of Branched Macromolecules in Size Exclusion Chromatography. ACS Omega 2020, 5, 14254–14260. [Google Scholar] [CrossRef]
- Podzimek, S.; Lebeda, P.; Johann, C. Asymmetric flow field flow fractionation: A powerful method for polymer characterization. LC-GC N. Am. 2009, 27, 62–69. [Google Scholar]
- Broyles, B.S.; Shalliker, R.A.; Cherrak, D.E.; Guiochon, G. Visualization of viscous fingering in chromatographic columns. J. Chromatogr. A 1998, 822, 173–187. [Google Scholar] [CrossRef]
- Chremos, A.; Horkay, F.; Douglas, J.F. Structure and Conformational Properties of Ideal Nanogel Particles in Athermal Solutions. J. Chem. Phys. 2021, 155, 134905. [Google Scholar] [CrossRef] [PubMed]
- Jiwani, S.I.; Gillis, R.B.; Besong, D.; Almutairi, F.; Erten, T.; Kök, S.M.; Harding, S.E.; Paulsen, B.S.; Adams, G.G. Isolation and Biophysical Characterisation of Bioactive Polysaccharides from Cucurbita Moschata. Polymers 2020, 12, 1650. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, A.S.; Morris, G.A.; Almutairi, F.; Adams, G.G.; Duvivier, P.; Conrath, K.; Harding, S.E. Solution conformation and flexibility of capsular polysaccharides from Neisseria meningitidis and glycoconjugates with the tetanus toxoid protein. Nature 2016, 6, 35588. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Podzimek, S. Truths and Myths about the Determination of Molar Mass Distribution of Synthetic and Natural Polymers by Size Exclusion Chromatography. J. Appl. Polym. Sci. 2014, 131, 40111. [Google Scholar] [CrossRef]




| Injection Volume (µL) | MVC Standard | ST-1 MVC | ST-4 MVC | |||
|---|---|---|---|---|---|---|
| Mw (kDa) | Rz (nm) | Mw (kDa) | Rz (nm) | Mw (kDa) | Rz (nm) | |
| 50 | 4167 | 50 | 5059 | 140 | 6986 | 272 |
| 100 | 4147 | 51 | 6345 | 201 | 9713 | 363 |
| 150 | 4085 | 51 | 7863 | 287 | 12,066 | 419 |
| Average | 4133 | 51 | 6423 | 209 | 9588 | 351 |
| MAX/MIN | 1.02 | 1.01 | 1.55 | 2.04 | 1.73 | 1.54 |
| Injection Volume (µL) | ST-1 Polysaccharide | ST-4 Polysaccharide | ||
|---|---|---|---|---|
| Mw (kDa) | Rz (nm) | Mw (kDa) | Rz (nm) | |
| 50 | 281 | 38 | 247 | 40 |
| 100 | 275 | 37 | 240 | 39 |
| 150 | 269 | 38 | 234 | 40 |
| Average | 275 | 38 | 240 | 39 |
| MAX/MIN | 1.05 | 1.04 | 1.06 | 1.03 |
| MALS Fit Model | Zimm 1st Degree | Berry 2nd Degree | Zimm 1st Degree | Berry 2nd Degree |
|---|---|---|---|---|
| MVC Injection (µL) | ST-1 MVC Mw (kDa) | ST-4 MVC Mw (kDa) | ||
| 50 | 5059 | 4857 | 6986 | 5904 |
| 100 | 6345 | 5558 | 9713 | 7130 |
| 150 | 7863 | 6129 | 12,066 | 8159 |
| Avg | 6423 | 5515 | 9588 | 7064 |
| MAX/MIN | 1.55 | 1.26 | 1.73 | 1.38 |
| Injections | ST-1 MVC | ST-4 MVC | ||
|---|---|---|---|---|
| Mw (kDa) | Rz (nm) | Mw (kDa) | Rz (nm) | |
| Injection-1 | 18,910 | 160 | 40,303 | 184 |
| Injection-2 | 19,135 | 163 | 62,532 | 211 |
| Average | 19,022 | 161 | 51,418 | 198 |
| MAX/MIN | 1.01 | 1.02 | 1.55 | 1.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.Z.; Lin, J.; Chen, M.; Lancaster, C.; Zhuang, P. Characterization of High Molecular Weight Pneumococcal Conjugate by SEC-MALS and AF4-MALS. Polymers 2022, 14, 3769. https://doi.org/10.3390/polym14183769
Deng JZ, Lin J, Chen M, Lancaster C, Zhuang P. Characterization of High Molecular Weight Pneumococcal Conjugate by SEC-MALS and AF4-MALS. Polymers. 2022; 14(18):3769. https://doi.org/10.3390/polym14183769
Chicago/Turabian StyleDeng, James Z., Jason Lin, Michelle Chen, Catherine Lancaster, and Ping Zhuang. 2022. "Characterization of High Molecular Weight Pneumococcal Conjugate by SEC-MALS and AF4-MALS" Polymers 14, no. 18: 3769. https://doi.org/10.3390/polym14183769
APA StyleDeng, J. Z., Lin, J., Chen, M., Lancaster, C., & Zhuang, P. (2022). Characterization of High Molecular Weight Pneumococcal Conjugate by SEC-MALS and AF4-MALS. Polymers, 14(18), 3769. https://doi.org/10.3390/polym14183769







