Influence of Curing Agent Amount on Properties of Dynamic Vulcanized Phenyl Silicone Rubber-SEBS-SBS System
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.3. Scanning Electron Microscope Test
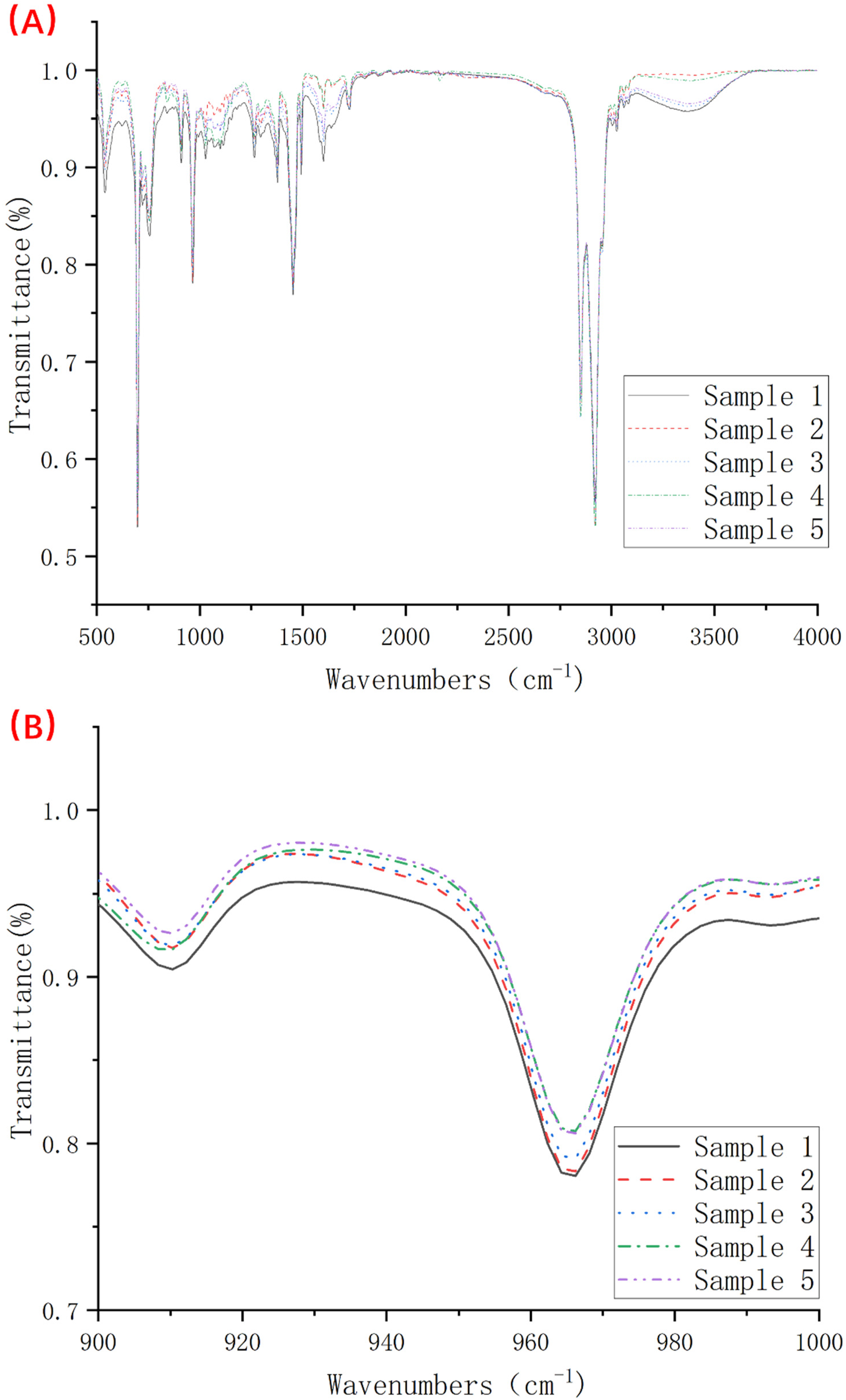
2.4. Fourier Transform Infrared Spectroscopy Test
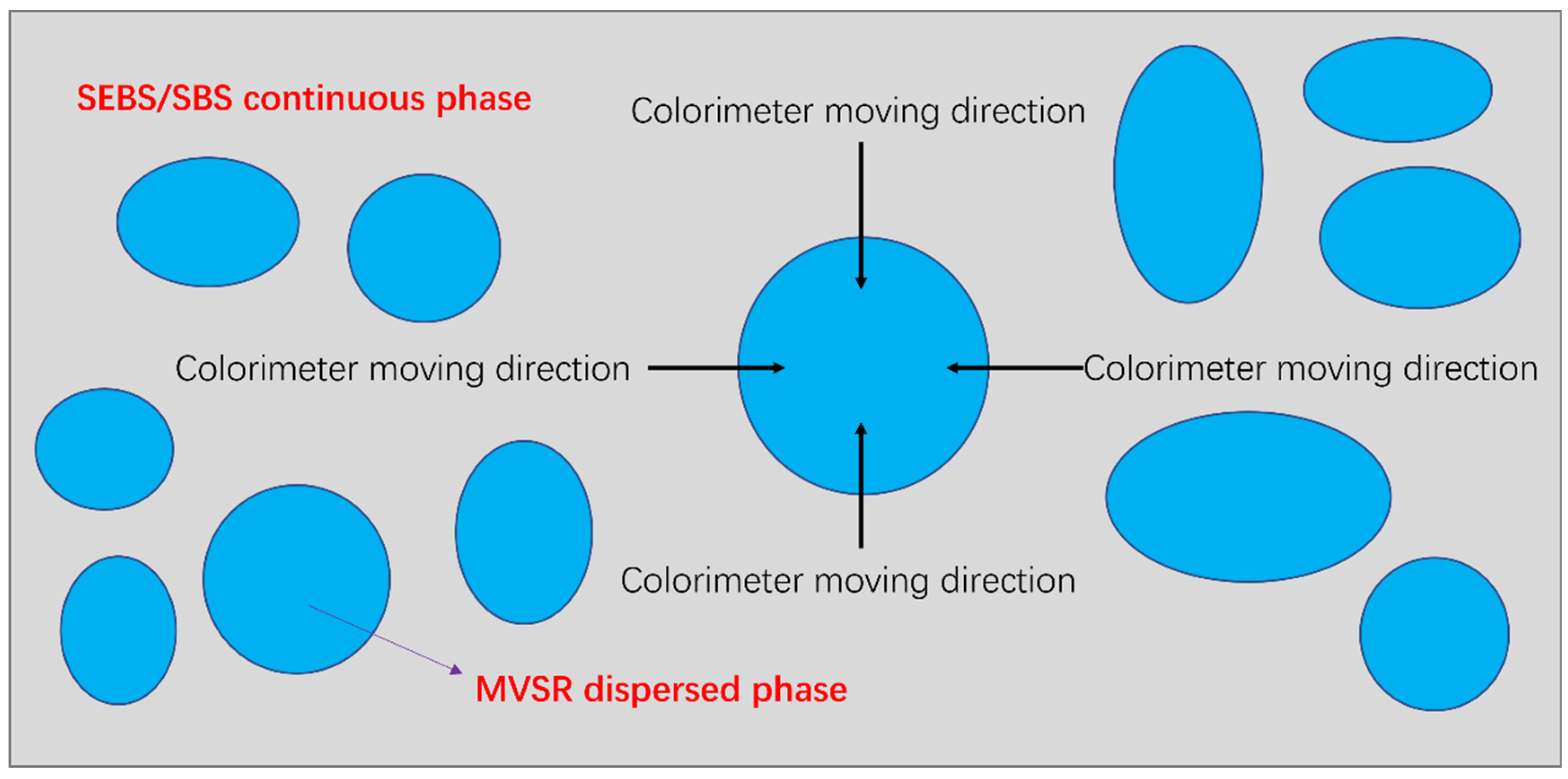
2.5. Compatibility Layer Quantitative Characterization Test
2.6. Mechanical Property Test
2.7. Dynamic Mechanical Properties Test
2.8. Low-Temperature Hardness Test
2.9. Melt Index Test
3. Results and Discussions
3.1. Torque Variation Diagram and Infrared Spectrum Analysis of Modified TPV Made of PSR and SEBS-SBS with Different Vulcanizing Agent Contents
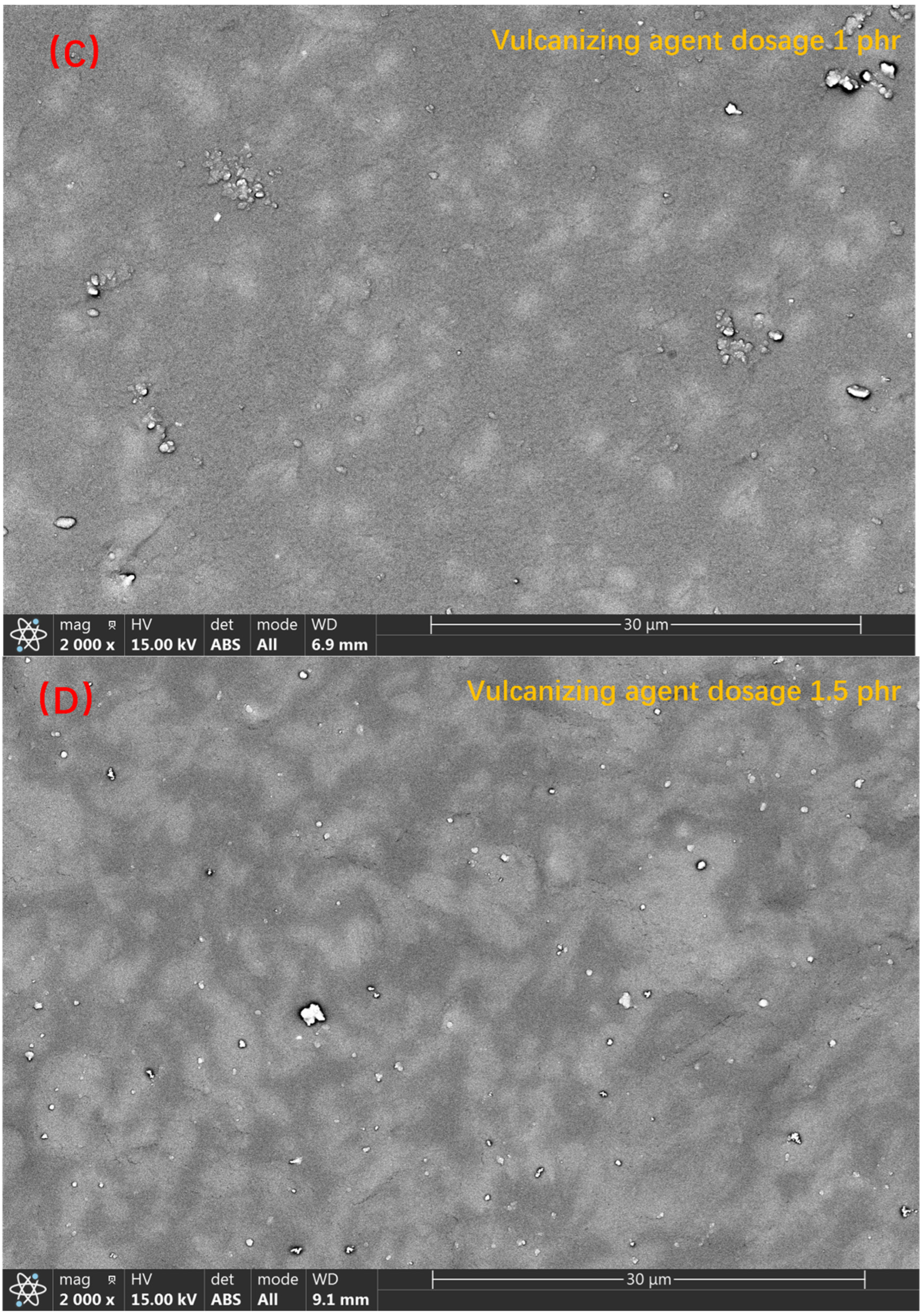
3.2. The Backscattered Electron Images of TPV Made of PSR and SEBS-SBS with Different Vulcanizing Agent Contents and Analysis of the Characterization Results of the Vulcanization Process
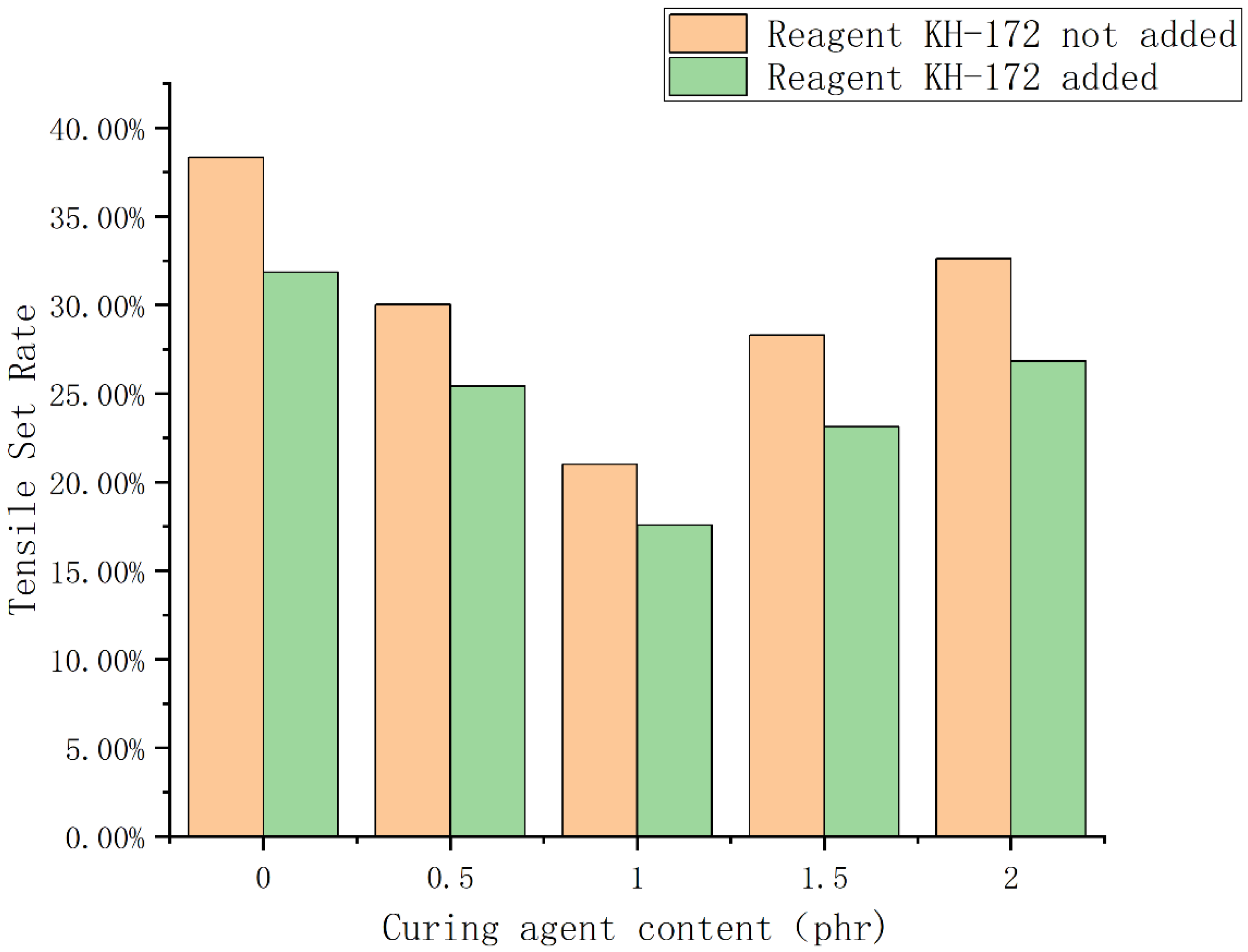
3.3. Analysis of the Results of the Size Chart of the Compatibility Layer and Mechanical Property Test Results
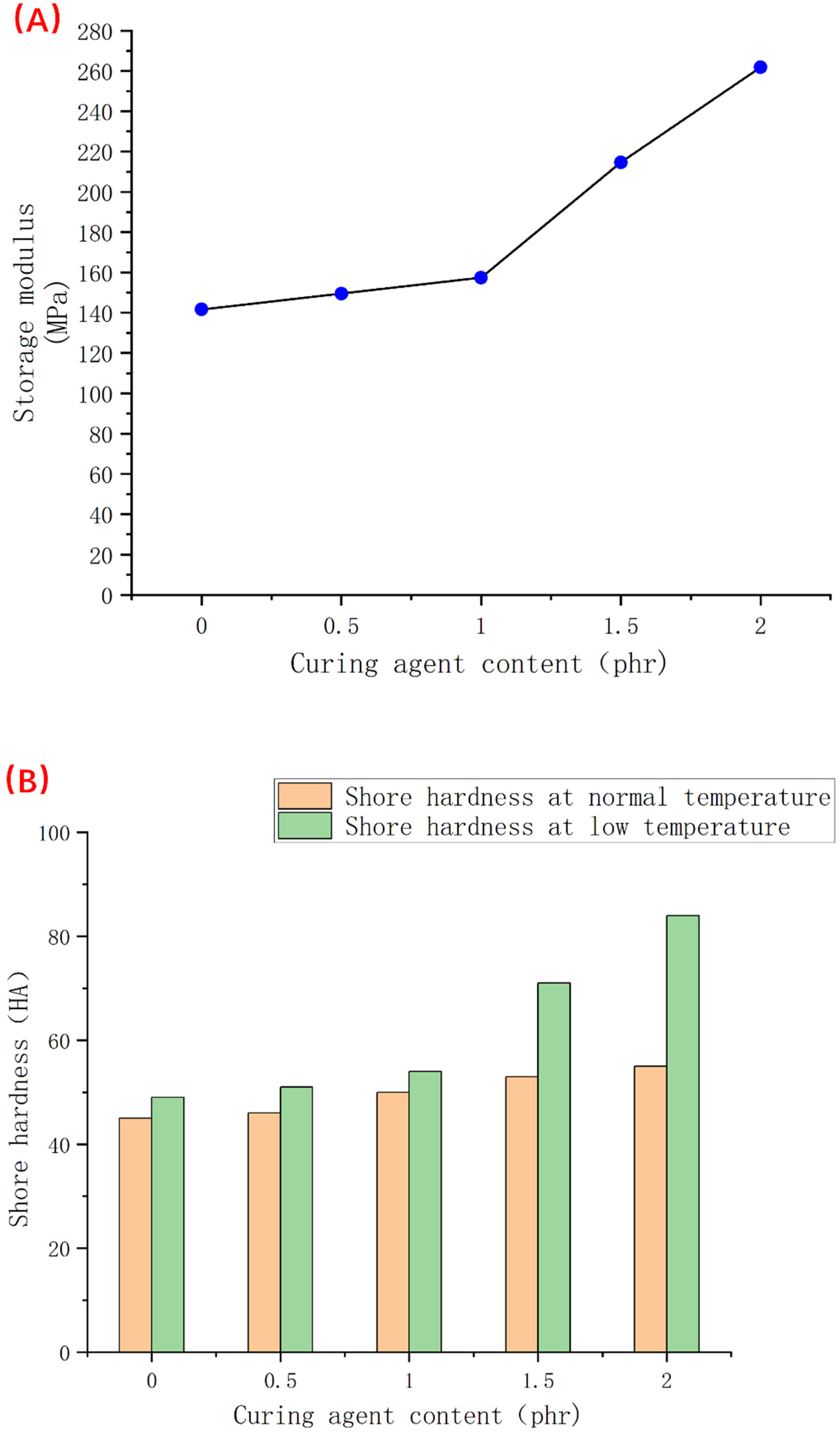
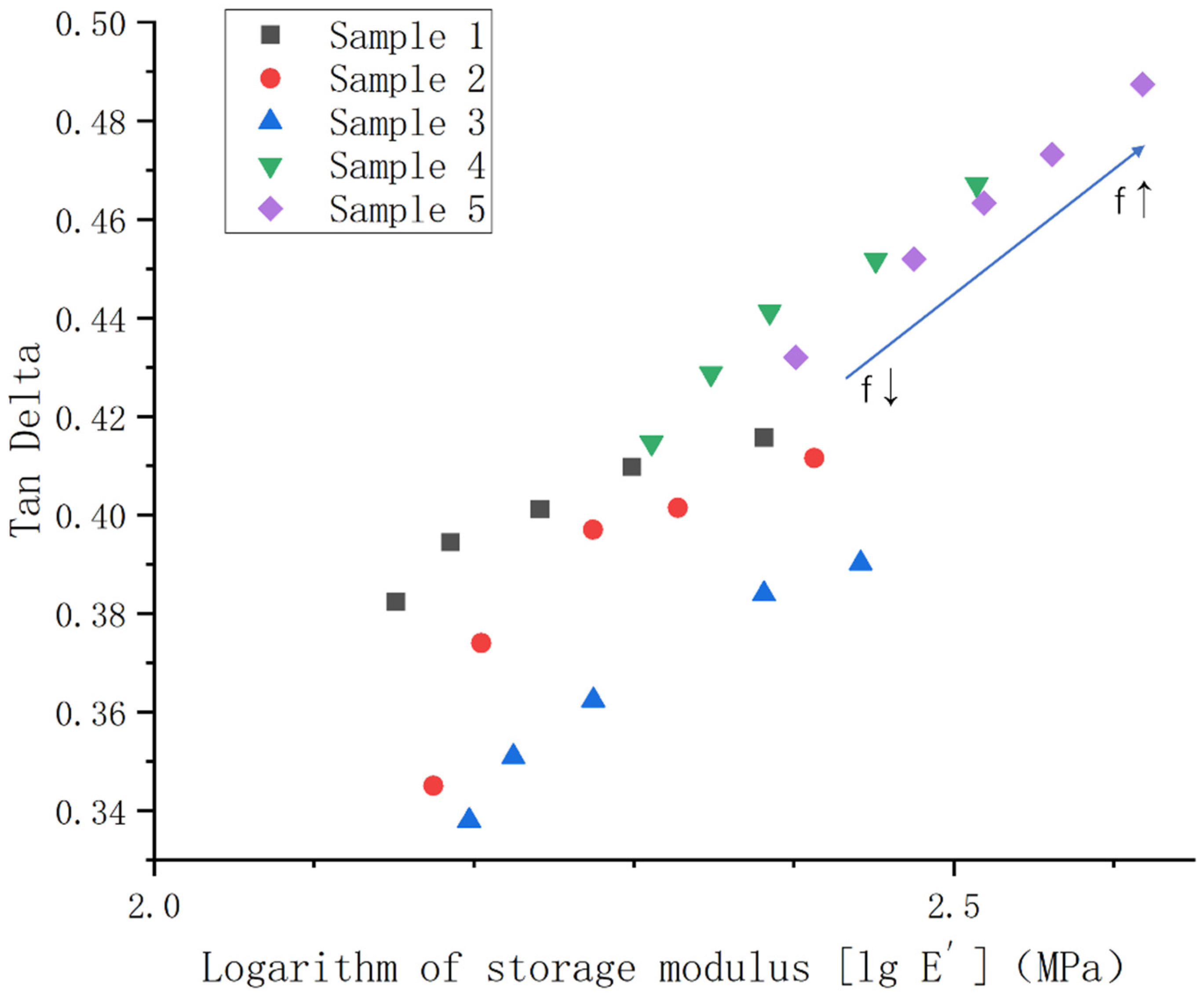
3.4. Analysis of the Experimental Results of Dynamic Mechanical Test and Low-Temperature Hardness
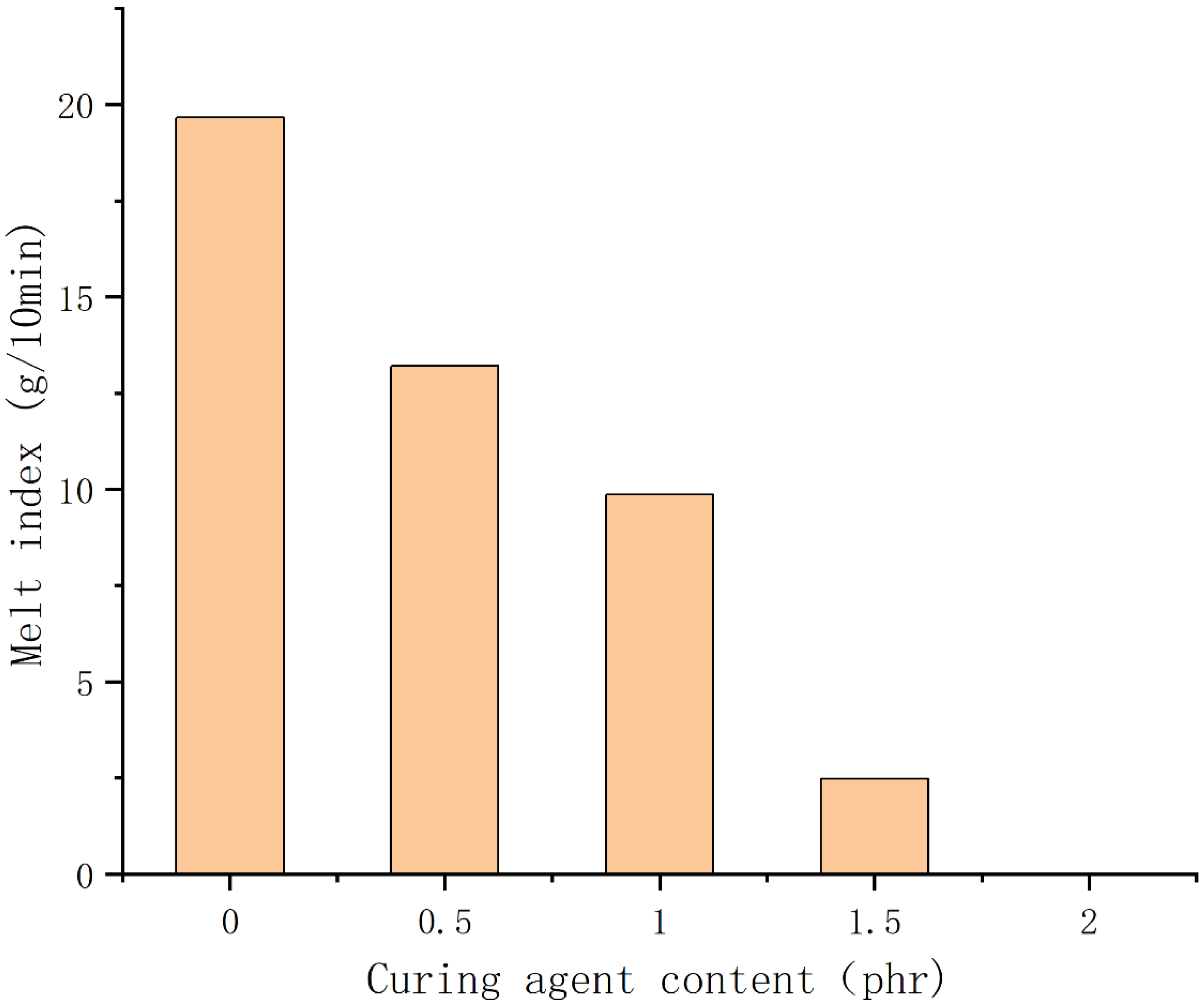
3.5. Analysis of Melt Index Test Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shit, S.C.; Shah, P. A review on silicone rubber. Natl. Acad. Sci. Lett. India 2013, 36, 355–365. [Google Scholar] [CrossRef]
- Deshpande, G.; Rezac, M.E. The effect of phenyl content on the degradation of poly(dimethyl diphenyl) siloxane copolymers. Polym. Degrad. Stab. 2001, 74, 363–370. [Google Scholar] [CrossRef]
- Lee, J.H.; Bae, J.W.; Choi, M.C. Effect of phenyl vinyl methyl silicone (PVMQ) on low temperature sealing performance of fluorosilicone composites. Elastomers Compos. 2021, 56, 209–216. [Google Scholar]
- Huang, Y.H.; Mu, Q.H.; Su, Z.T. High and Low Temperature Resistance of Phenyl silicone rubber. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1048, 012001. [Google Scholar] [CrossRef]
- Aksut, D.; Sen, M.; Demeter, M. Effect of radiation on vinyl-methyl-polysiloxane and phenyl-vinyl-methyl-polysiloxane elastomers cured with different co-agents: Comparative study of mechanical and relaxation properties. Radiat. Phys. Chem. 2019, 158, 87–93. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Fan, M.S.; Li, X.P. Silicon-containing functionalized polyolefin: Synthesis and application. Prog. Chem. 2020, 32, 84–92. [Google Scholar]
- Shi, C.R.; Zong, C.Z.; Pan, H.W. A method to essentially increase the compatibility of thermoplastic polyurethane/methyl vinyl silicone rubber based thermoplastic vulcanizate. J. Appl. Polym. Sci. 2022, 139, e52219. [Google Scholar] [CrossRef]
- Zhao, G.J.; Tian, K.; Pan, Q.; Zhang, Q.; Deng, H.; Fu, Q. A Novel Method to Incorporate Functional Filler into TPSiV for Balanced Physical Properties. Compos. Sci. Technol. 2021, 213, 108925. [Google Scholar] [CrossRef]
- Radusch, H.J. Dynamic vulcanizates on the basis of polypropylene/rubber mixtures. Kautsch Gummi Kunstst 1990, 43, 767–769. [Google Scholar]
- School, R. TPE production and use in the 1990s. Rubber World 1990, 201, 15. [Google Scholar]
- Choudhury, N.R.; Bhowmick, A.K. Strength of thermoplastic elastomers from rubber-polyolefin blends. J. Mater. Sci. 1990, 25, 161–167. [Google Scholar] [CrossRef]
- Radusch, H.J. Dynamic vulcanization of EPDM-polypropylene mixtures. Kautsch Gummi Kunstst 1991, 44, 1125–1128. [Google Scholar]
- Islam, S.K.S.; Singh, S.K.; Ransinchung, R.N.G.D. Performance Deterioration of SBS-Modified Asphalt Mix: Impact of Elevated Storage Temperature and SBS Concentration of Modified Binder. J. Mater. Civ. Eng. 2022, 34, 04021475. [Google Scholar] [CrossRef]
- Kok, B.V.; Erkus, Y.; Yilmaz, M. Effects of triple modification on the mechanical properties and the cost of asphalt mixtures. Environ. Eng. Manag. J. 2022, 21, 603–613. [Google Scholar] [CrossRef]
- Murthe, S.S.; Sreekantan, S. Study on the Physical, Thermal and Mechanical Properties of SEBS/PP (Styrene-Ethylene-Butylene-Styrene/Polypropylene) Blend as a Medical Fluid Bag. Polymers 2022, 14, 3267. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Yu, B.; Li, S.; Chen, S.; Zhu, Y.; Zhang, B.; Zhang, Y.; Cai, H.; Han, B. Preparation and properties of reinforced SEBS-based thermoplastic elastomers modified by PA6. Polym. Eng. Sci. 2022, 62, 1052–1060. [Google Scholar] [CrossRef]
- Zhao, H.; Gao, W.-C.; Li, Q.; Khan, M.R.; Hu, G.-H.; Liu, Y.; Wu, W.; Huang, C.-X.; Li, R.K. Recent advances in superhydrophobic polyurethane: Preparations and applications. Adv. Colloid Interface Sci. 2022, 303, 102644. [Google Scholar] [CrossRef]
- He, C.; Li, B.; Ren, Y.; Lu, W.; Zeng, Y.; He, W.; Feng, A. How the Crosslinking Agent Influences the Thermal Stability of RTV Phenyl Silicone Rubber. Materials 2019, 12, 88. [Google Scholar] [CrossRef]
- Shiva, M.; Dallakeh, M.K.; Ahmadi, M.; Lakhi, M. Effects of silicon carbide as a heat conductive filler in butyl rubber for bladder tire curing applications. Mater. Today Commun. 2021, 29, 102773. [Google Scholar] [CrossRef]
- Nurhajati, D.W.; Lestari, U.R.; Setyorini, I.; Mayasari, H.E. Effect of carbon black/Bi(2)O(3)ratio on the vulcanization characteristic and mechanical properties of rubber compound. Macromol. Symp. 2020, 391, 1900171. [Google Scholar] [CrossRef]
- Awasthi, P.; Banerjee, S.S. Design of ultrastretchable and super-elastic tailorable hydrophilic thermoplastic elastomeric materials. Polymer 2022, 252, 124914. [Google Scholar] [CrossRef]
- Shahroodi, Z.; Katbab, A.A. Preparation and characterization of peroxide-based dynamically vulcanized thermoplastic elastomer of poly (lactic acid)/chloroprene rubber. Polym. Eng. Sci. 2022, 62, 1485–1495. [Google Scholar] [CrossRef]
- Kana, M.; Saito, H. Tensile properties and interfacial adhesion of silicone rubber/polyethylene blends by reactive blending. J. Appl. Polym. Sci. 2018, 135, 46192. [Google Scholar]
- Yao, N.Q.; Wang, H.B.; Zhang, L.Q. One-pot Hydrothermal Synthesis of Silane-Functionalized Carbon Nanodots as Compatibilizers for the Immiscible TPU/MVQ blends. Appl. Surf. Sci. 2020, 530, 147124. [Google Scholar] [CrossRef]
- Toshio, I. Selective crosslinking in polymer blends I. Novel selective crosslink systems for polypropylene/unsaturated elastomer blends. J. Appl. Polym. Sci. 1994, 54, 709. [Google Scholar]
- Chambers, S.A. Elastic-scattering and interference of backscattered primary, auger and X-ray photoelectrons at high kinetic-energy-principles and applications. Surf. Sci. Rep. 1992, 16, 261–331. [Google Scholar] [CrossRef]
- Autrata, R. Backscattered electron imaging using single-crystal scintillator detectors. Scanning Microsc. 1989, 3, 739–763. [Google Scholar]
- Yoo, H.-S.; Kim, Y.-H.; Son, H.-T. The Effects of TiC Content on Microstructure of Modified A6013-3wt.%Si Alloy Powder Compact. J. Korean Powder Met. Inst. 2022, 29, 28–33. [Google Scholar] [CrossRef]
- Brackmann, L.; Schuppener, J.; Röttger, A.; Weber, S. Influence of a Partial Substitution of Co by Fe on the Phase Stability and Fatigue Behavior of a CoCrWC Hard Alloy at Room Temperature. Met. Mater. Trans. A 2022, 53, 2708–2723. [Google Scholar] [CrossRef]
- Rößler, C.; Zimmer, D.; Trimby, P.; Ludwig, H.-M. Chemical–crystallographic characterisation of cement clinkers by EBSD-EDS analysis in the SEM. Cem. Concr. Res. 2022, 154, 106721. [Google Scholar] [CrossRef]
- Chen, X.; Wang, G.; Dong, Q.; Zhao, X.; Wang, Y. Microscopic characterizations of pervious concrete using recycled Steel Slag Aggregate. J. Clean. Prod. 2020, 254, 120149. [Google Scholar] [CrossRef]
- Goizueta, G.; Chiba, T.; Inoue, T. Phase morphology of polymer blends: Scanning electron microscope observation by backscattering from a microtomed and stained surface. Polymer 1992, 33, 886. [Google Scholar] [CrossRef]
- Goizueta, G.; Chiba, T.; Inoue, T. Phase morphology of polymer blends: 2. SEM observation by secondary and backscattered electrons from microtomed and stained surface. Polymer 1993, 34, 253–256. [Google Scholar] [CrossRef]
- Masters, R.C.; Wan, Q.; Zhang, Y.; Dapor, M.; Sandu, A.M.; Jiao, C.; Zhou, Y.; Zhang, H.; Lidzey, D.G.; Rodenburg, C. Novel organic photovoltaic polymer blends: A rapid, 3-dimensional morphology analysis using backscattered electron imaging in the scanning electron microscope. Sol. Energy Mater. Sol. Cells 2017, 160, 182–192. [Google Scholar] [CrossRef]
- Wang, W.; Huang, J.; Gong, Z.; Fan, J.; Cao, L.; Chen, Y. Biobased PLA/NR-PMMA TPV with balanced stiffness-toughness: In-situ interfacial compatibilization, performance and toughening model. Polym. Test. 2019, 81, 106268. [Google Scholar] [CrossRef]
- Khanra, S.; Sreenivasan, P.; Das, S.; Hore, R.; Ganguly, D.; Chattopadhyay, S. Immobilization of a biobased process aid at the interface for binary silicone and fluoroelastomer based super specialty blends with silica for enhanced compatibility. J. Mater. Sci. 2022, 57, 13974–13990. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R. Scanning Electron Microscopy and X-ray Microanalysis; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Çakmak, U.; Fischlschweiger, M.; Graz, I.; Major, Z. Adherence Kinetics of a PDMS Gripper with Inherent Surface Tackiness. Polymers 2020, 12, 2440. [Google Scholar] [CrossRef]
- Tschoegl, N.W.; Knauss, W.G.; Emri, I. The Effect of Temperature and Pressure on the Mechanical Properties of Thermo- and/or Piezorheologically Simple Polymeric Materials in Thermodynamic Equilibrium—A Critical Review. Mech. Time Depend. Mater. 2002, 6, 53–99. [Google Scholar] [CrossRef]
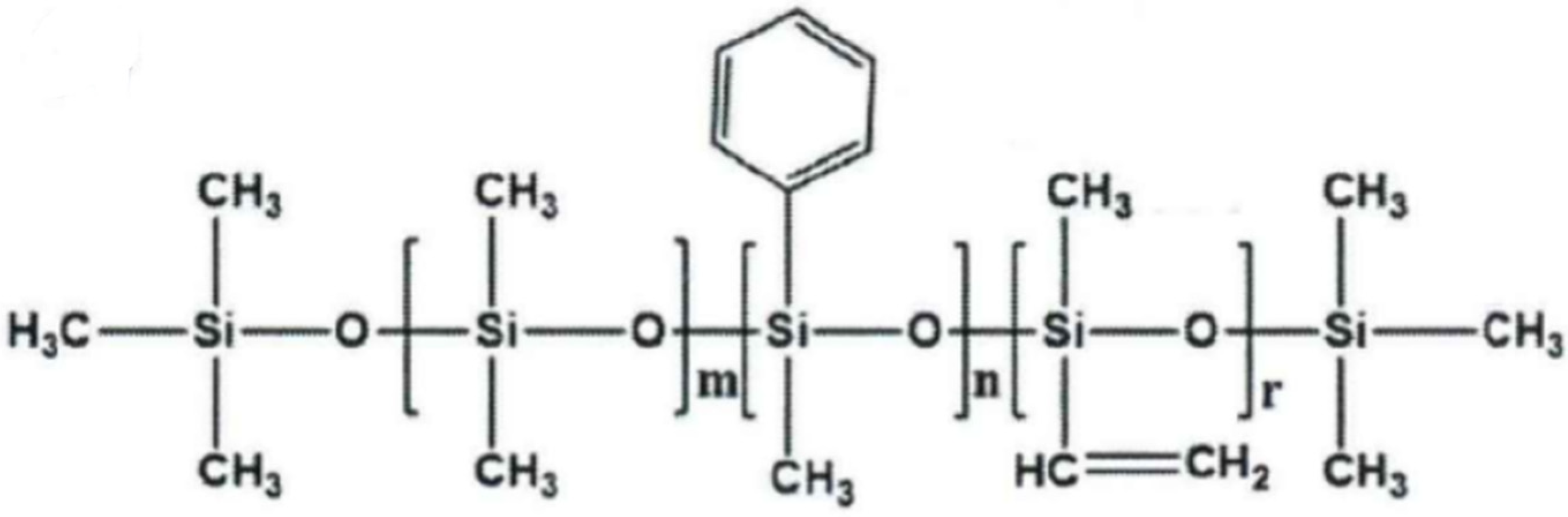









| Entry | PSR | SEBS | SBS | 250N | KH-172 | Platinum Catalyst | Hydrogen-Containing Silicone Oil |
|---|---|---|---|---|---|---|---|
| 1 | 50 | 25 | 25 | 10 | 1 | 0.5 | 0 |
| 2 | 50 | 25 | 25 | 10 | 1 | 0.5 | 0.5 |
| 3 | 50 | 25 | 25 | 10 | 1 | 0.5 | 1 |
| 4 | 50 | 25 | 25 | 10 | 1 | 0.5 | 1.5 |
| 5 | 50 | 25 | 25 | 10 | 1 | 0.5 | 2 |
| Wavenumber (cm−1) | Designation of IR Spectra |
|---|---|
| 541 | Styrene block, deformations of the aromatic ring |
| 700, 756 | Out of plane deformations of the aromatic ring |
| 720 | -(CH2)- rocking vibration, -(CH2)n-, n, number of consecutive -CH2- groups are more than 4 in SEBS, the same as in polyethylene |
| 738 | Out of plane deformation of cis double bonds overlapped by peak 756 cm−1 from an aromatic ring |
| 910 | δ-c-H, =C-H, out of plane deformations of vinyl units |
| 966 | Out of plane deformation of trans-unit |
| 1379 | -CH(CH2CH3)CH2- unit, -CH2 for hydrogenated products SEBS |
| 1453 | Deformation of aromatic ring |
| 1470 | Asymmetric vibrations and deformations of groups -CH2, -CH3 in SEBS |
| 1492, 1602 | Stretching vibration of the double bond of the aromatic ring |
| 1641 | Stretching vibration of the double bond of vinyl units |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; He, B.; Chen, X. Influence of Curing Agent Amount on Properties of Dynamic Vulcanized Phenyl Silicone Rubber-SEBS-SBS System. Polymers 2022, 14, 5443. https://doi.org/10.3390/polym14245443
Zhao C, He B, Chen X. Influence of Curing Agent Amount on Properties of Dynamic Vulcanized Phenyl Silicone Rubber-SEBS-SBS System. Polymers. 2022; 14(24):5443. https://doi.org/10.3390/polym14245443
Chicago/Turabian StyleZhao, Chunxu, Bobing He, and Xian Chen. 2022. "Influence of Curing Agent Amount on Properties of Dynamic Vulcanized Phenyl Silicone Rubber-SEBS-SBS System" Polymers 14, no. 24: 5443. https://doi.org/10.3390/polym14245443
APA StyleZhao, C., He, B., & Chen, X. (2022). Influence of Curing Agent Amount on Properties of Dynamic Vulcanized Phenyl Silicone Rubber-SEBS-SBS System. Polymers, 14(24), 5443. https://doi.org/10.3390/polym14245443





