Porous Membranes of Polysulfone and Graphene Oxide Nanohybrids for Vanadium Redox Flow Battery
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Graphene Oxide
2.2. Preparation of Pristine PSF and PSF/GO Nanocomposite Membrane
2.3. Material Characterization
2.4. Proton Conductivity
2.5. Permeability of Vanadium Ions
2.6. VRFB Single-Cell Test
3. Results and Discussion
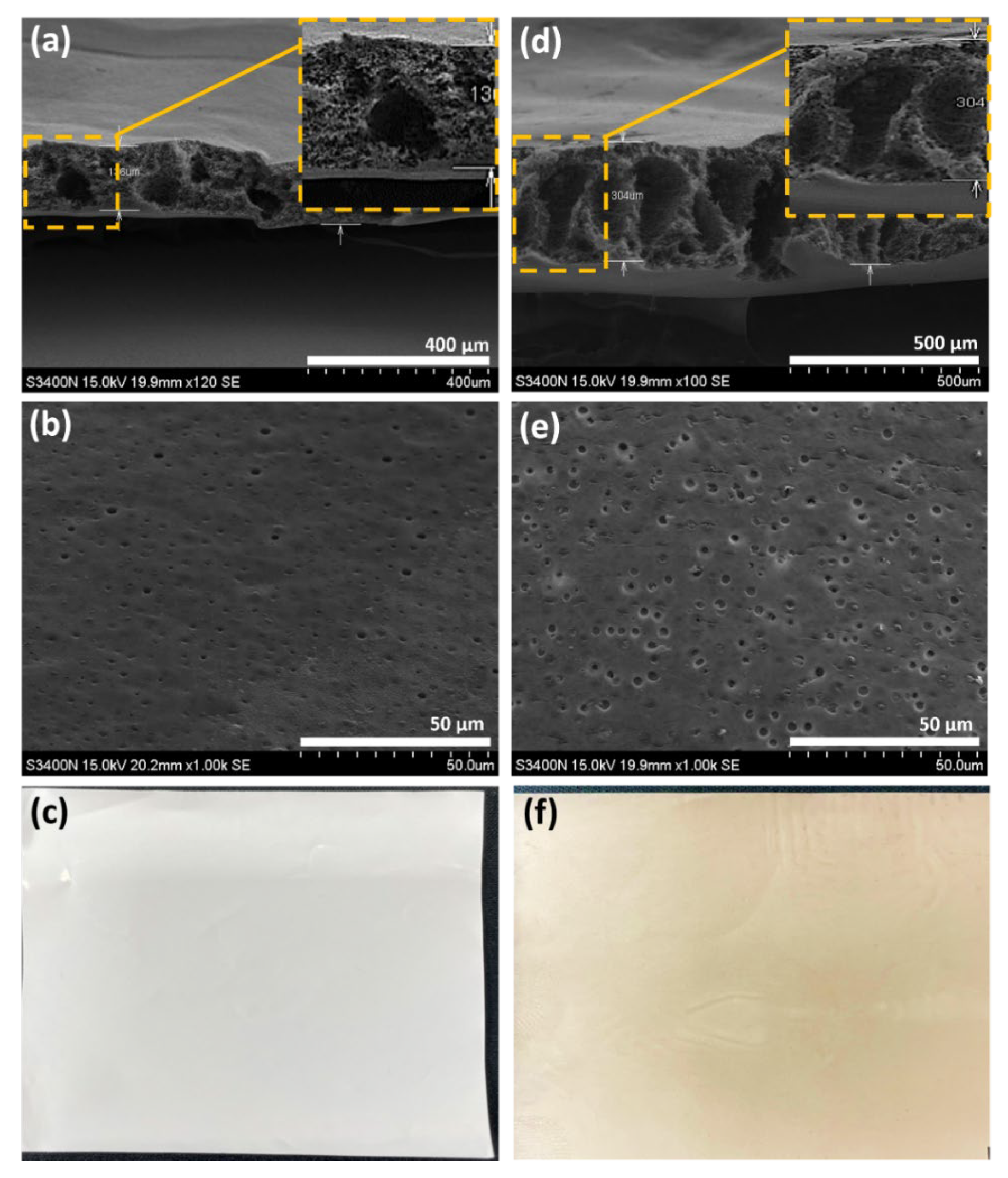
3.1. Morphology
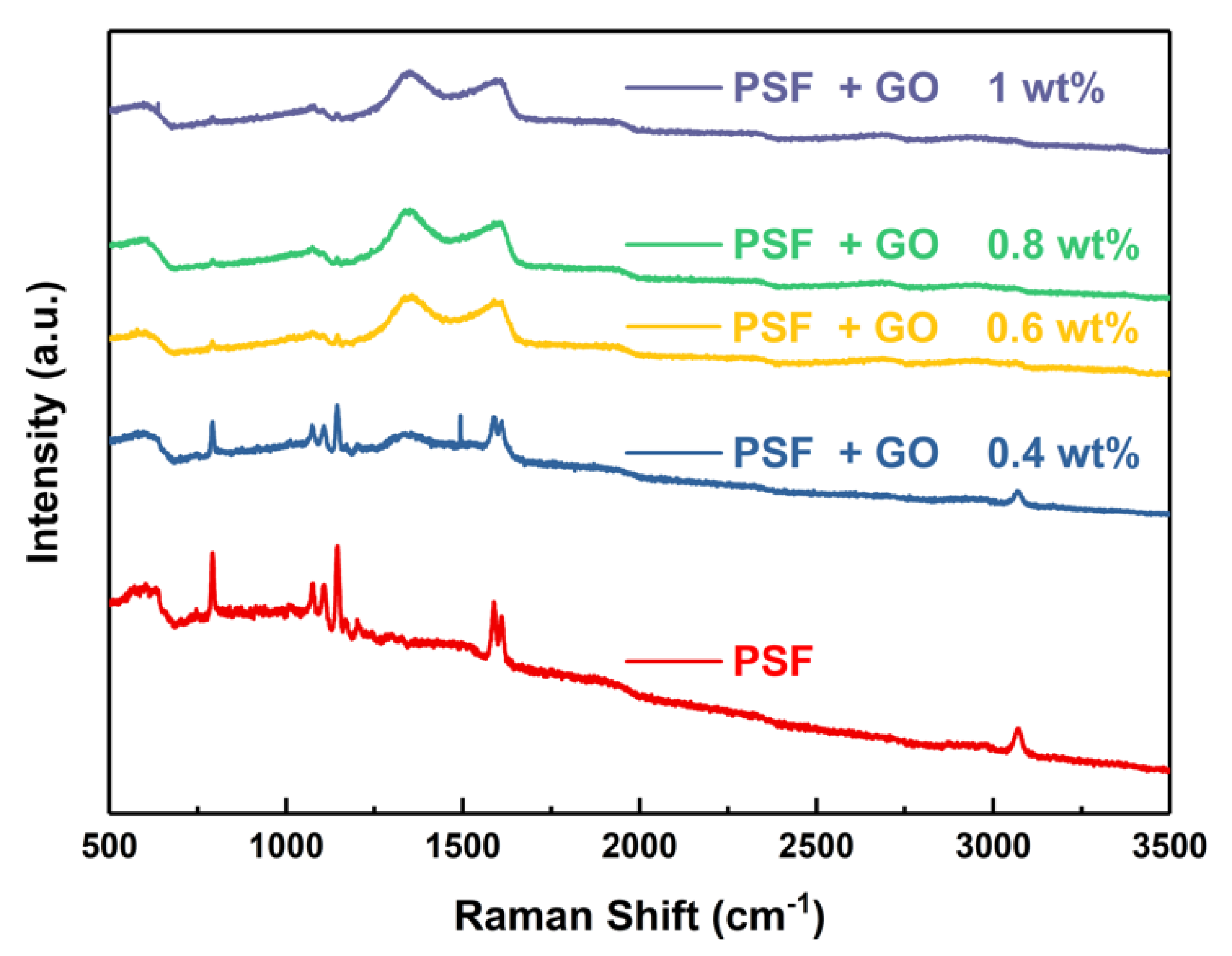
3.2. Raman Spectroscopy
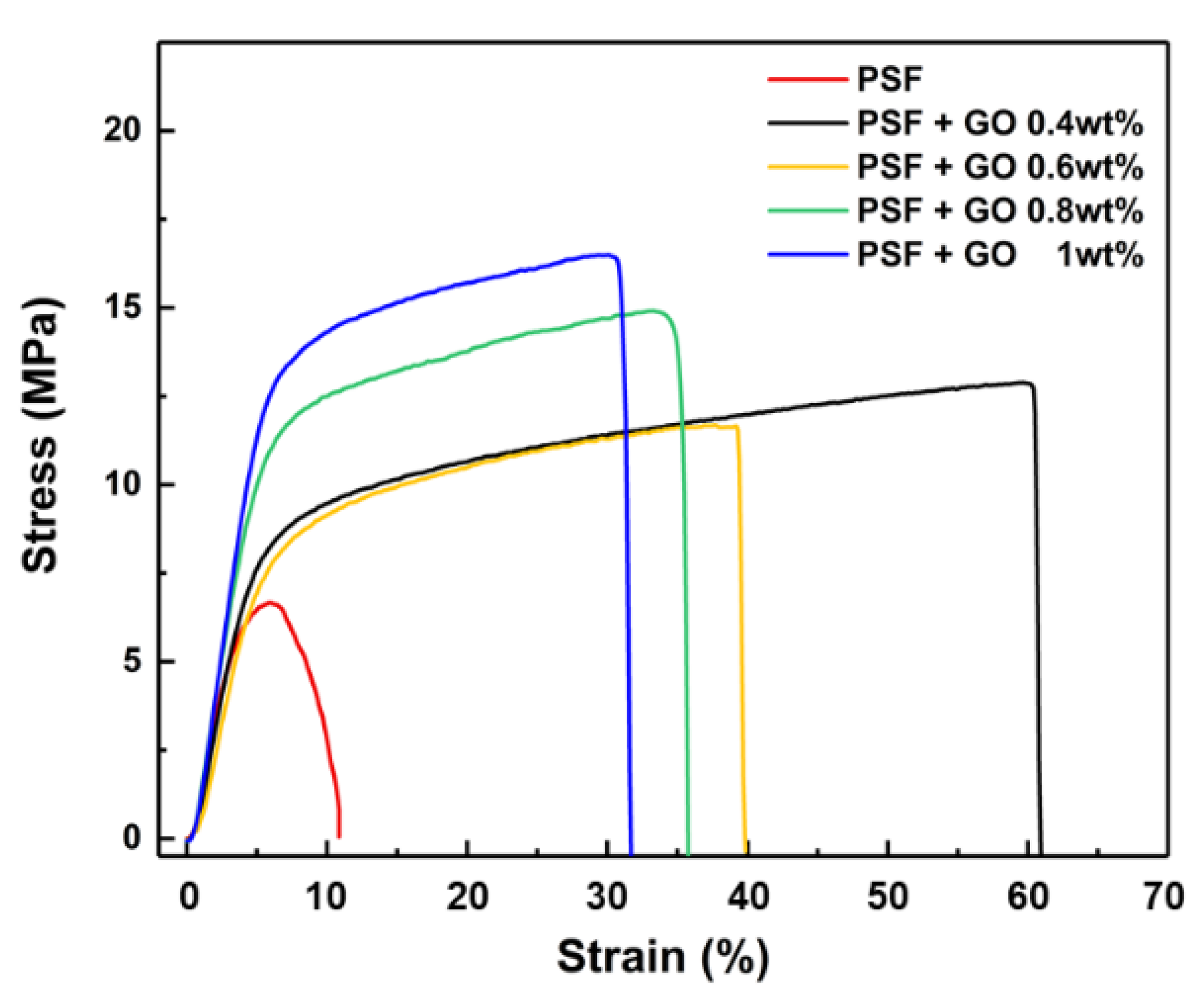
3.3. Tensile Strength
3.4. Thermogravimetric Analysis (TGA)
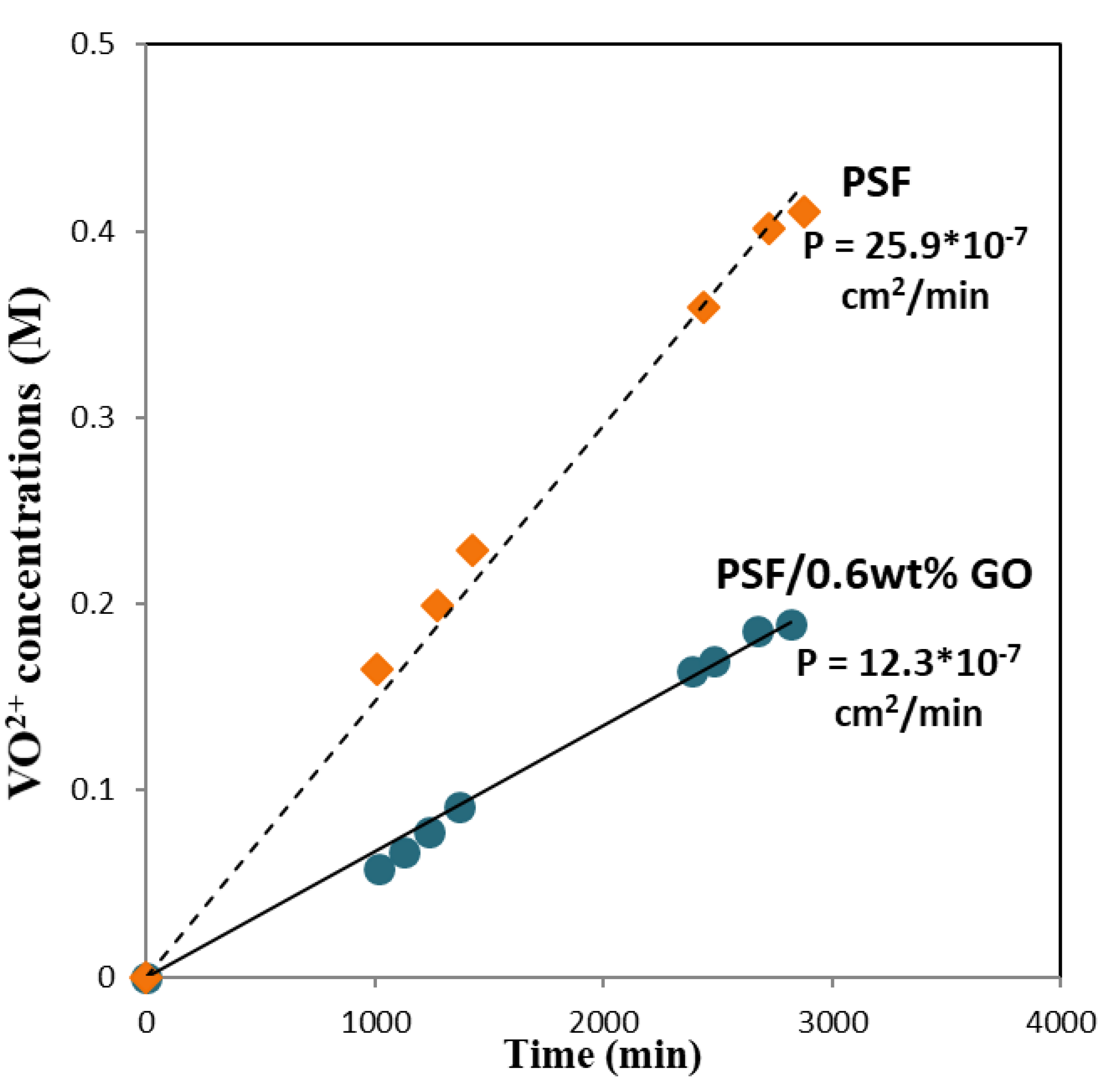
3.5. Permeability of Vanadium Ions
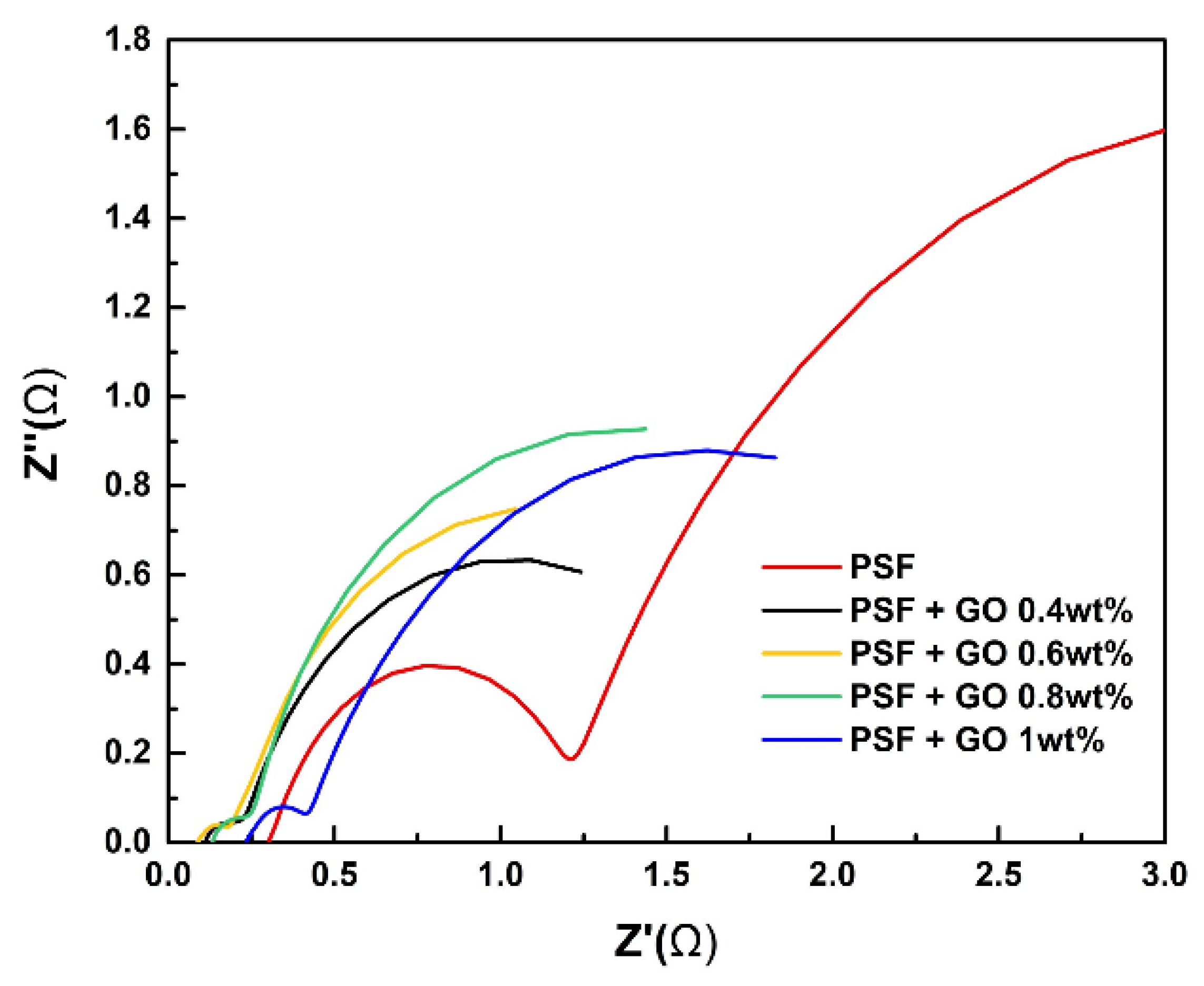
3.6. Electrochemical Impedance Spectroscopy (EIS)
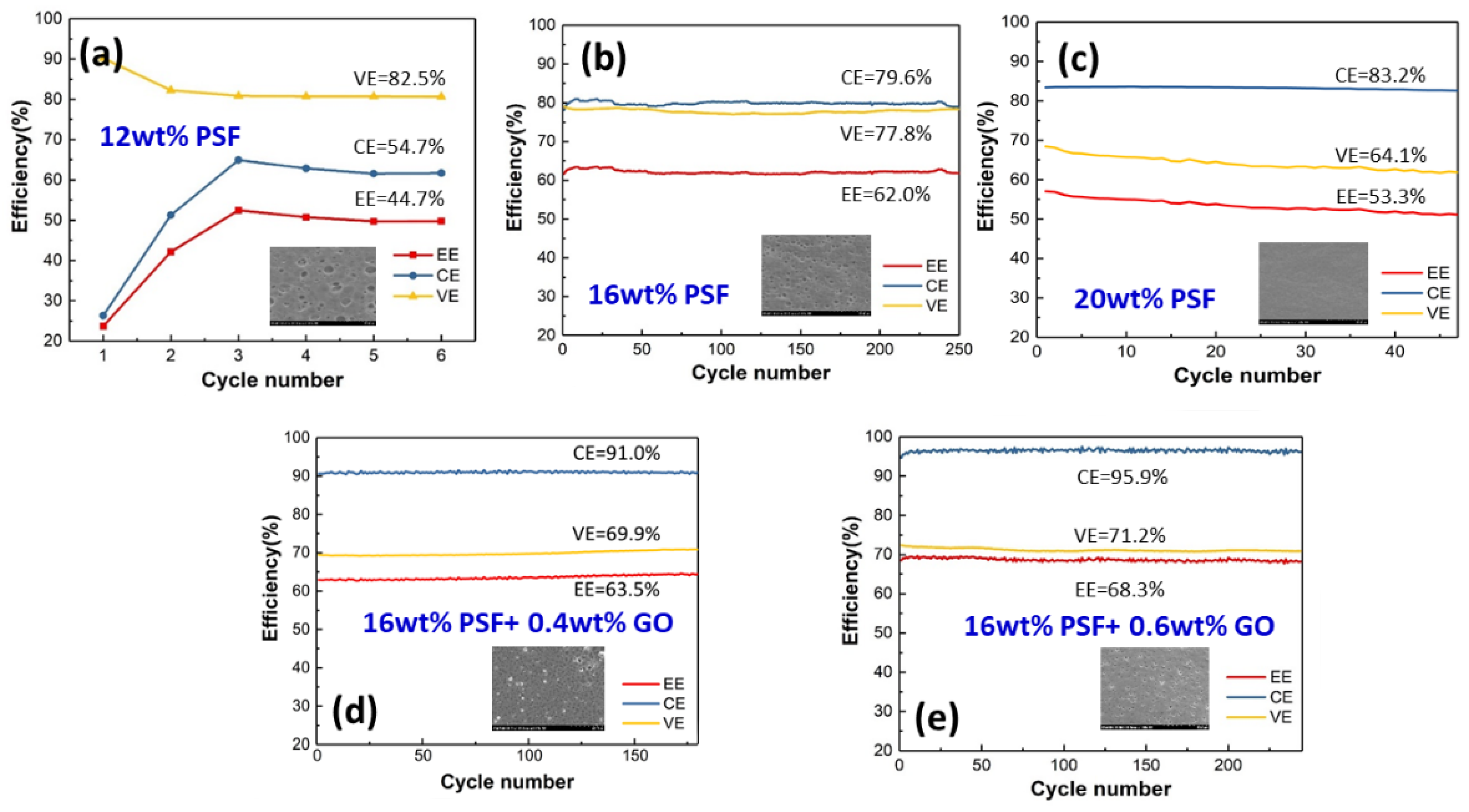
3.7. Charge/Discharge Efficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Thiam, B.G.; Vaudreuil, S. Recent membranes for vanadium redox flow batteries. J. Electrochem. Soc. 2021, 168, 070553. [Google Scholar]
- Thiam, B.G.; El Magri, A.; Vaudreuil, S. An overview on the progress and development of modified sulfonated polyether ether ketone membranes for vanadium redox flow battery applications. High Perform. Polym. 2022, 34, 131–148. [Google Scholar]
- Palanisamy, G.; Oh, T.H. TiO2 Containing Hybrid Composite Polymer Membranes for Vanadium Redox Flow Batteries. Polymers 2022, 14, 1617. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Zhuang, Y.-D.; Tsai, D.-G.; Wei, H.-J.; Liu, T.-Y. Performance enhancement of vanadium redox flow battery by treated carbon felt electrodes of polyacrylonitrile using atmospheric pressure plasma. Polymers 2020, 12, 1372. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.M.; Jeong, H.Y.; Jang, J.-K.; Lee, J.Y.; So, S.; Kim, Y.J.; Hong, Y.T.; Kim, T.-H. Polybenzimidazole/Nafion hybrid membrane with improved chemical stability for vanadium redox flow battery application. RSC Adv. 2018, 8, 25304–25312. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dai, W.; Yu, L.; Xi, J.; Qiu, X.; Chen, L. Sulfonated poly (ether ether ketone)/mesoporous silica hybrid membrane for high performance vanadium redox flow battery. J. Power Sources 2014, 257, 221–229. [Google Scholar] [CrossRef]
- Perry, M.L.; Weber, A.Z. Advanced redox-flow batteries: A perspective. J. Electrochem. Soc. 2015, 163, A5064. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Mai, Z.; Zhang, H.; Vankelecom, I. Ion exchange membranes for vanadium redox flow battery (VRB) applications. Energy Environ. Sci. 2011, 4, 1147–1160. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, H.; Li, X.; Liu, T.; Xing, F. Vanadium flow battery for energy storage: Prospects and challenges. J. Phys. Chem. Lett. 2013, 4, 1281–1294. [Google Scholar]
- Oriji, G.; Katayama, Y.; Miura, T. Investigations on V (IV)/V (V) and V (II)/V (III) redox reactions by various electrochemical methods. J. Power Sources 2005, 139, 321–324. [Google Scholar] [CrossRef]
- Kim, K.J.; Park, M.-S.; Kim, Y.-J.; Kim, J.H.; Dou, S.X.; Skyllas-Kazacos, M. A technology review of electrodes and reaction mechanisms in vanadium redox flow batteries. J. Mater. Chem. A 2015, 3, 16913–16933. [Google Scholar] [CrossRef]
- Tian, B.; Yan, C.; Wang, F. Proton conducting composite membrane from Daramic/Nafion for vanadium redox flow battery. J. Membr. Sci. 2004, 234, 51–54. [Google Scholar]
- Dai, W.; Yu, L.; Li, Z.; Yan, J.; Liu, L.; Xi, J.; Qiu, X. Sulfonated poly (ether ether ketone)/graphene composite membrane for vanadium redox flow battery. Electrochim. Acta 2014, 132, 200–207. [Google Scholar] [CrossRef]
- Chromik, A.; dos Santos, A.R.; Turek, T.; Kunz, U.; Häring, T.; Kerres, J. Stability of acid-excess acid–base blend membranes in all-vanadium redox-flow batteries. J. Membr. Sci. 2015, 476, 148–155. [Google Scholar] [CrossRef]
- Reed, D.; Thomsen, E.; Li, B.; Wang, W.; Nie, Z.; Koeppel, B.; Sprenkle, V. Performance of a low cost interdigitated flow design on a 1 kW class all vanadium mixed acid redox flow battery. J. Power Sources 2016, 306, 24–31. [Google Scholar] [CrossRef]
- Hoang, T.K.; Chen, P. Recent development of polymer membranes as separators for all-vanadium redox flow batteries. RSC Adv. 2015, 5, 72805–72815. [Google Scholar]
- Wang, W.; Luo, Q.; Li, B.; Wei, X.; Li, L.; Yang, Z. Recent progress in redox flow battery research and development. Advanced Funct. Mater. 2013, 23, 970–986. [Google Scholar] [CrossRef]
- Parasuraman, A.; Lim, T.M.; Menictas, C.; Skyllas-Kazacos, M. Review of material research and development for vanadium redox flow battery applications. Electrochim. Acta 2013, 101, 27–40. [Google Scholar] [CrossRef]
- Winardi, S.; Raghu, S.C.; Oo, M.O.; Yan, Q.; Wai, N.; Lim, T.M.; Skyllas-Kazacos, M. Sulfonated poly (ether ether ketone)-based proton exchange membranes for vanadium redox battery applications. J. Membr. Sci. 2014, 450, 313–322. [Google Scholar] [CrossRef]
- Cao, J.; Yuan, Z.; Li, X.; Xu, W.; Zhang, H. Hydrophilic poly (vinylidene fluoride) porous membrane with well connected ion transport networks for vanadium flow battery. J. Power Sources 2015, 298, 228–235. [Google Scholar]
- Xi, X.; Ding, C.; Zhang, H.; Li, X.; Cheng, Y.; Zhang, H. Solvent responsive silica composite nanofiltration membrane with controlled pores and improved ion selectivity for vanadium flow battery application. J. Power Sources 2015, 274, 1126–1134. [Google Scholar] [CrossRef]
- Leung, P.; Xu, Q.; Zhao, T.; Zeng, L.; Zhang, C. Preparation of silica nanocomposite anion-exchange membranes with low vanadium-ion crossover for vanadium redox flow batteries. Electrochim. Acta 2013, 105, 584–592. [Google Scholar]
- Kreuer, K.-D. Ion conducting membranes for fuel cells and other electrochemical devices. Chem. Mater. 2014, 26, 361–380. [Google Scholar] [CrossRef]
- Maurya, S.; Shin, S.-H.; Kim, Y.; Moon, S.-H. A review on recent developments of anion exchange membranes for fuel cells and redox flow batteries. RSC Adv. 2015, 5, 37206–37230. [Google Scholar] [CrossRef]
- Badrinezhad, L.; Ghasemi, S.; Azizian-Kalandaragh, Y.; Nematollahzadeh, A. Preparation and characterization of polysulfone/graphene oxide nanocomposite membranes for the separation of methylene blue from water. Polym. Bull. 2018, 75, 469–484. [Google Scholar]
- Sha’rani, S.; Jusoh, N.; Abouzari-Lotf, E.; Ahmad, A.; Ali, R. Enhanced proton conductivity of porous UHMWPE membrane with Graphene-based material for Vanadium Redox flow battery. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Novosibirsk, Russia, 22–27 May 2020; IOP: Bristol, UK, 2020; p. 012024. [Google Scholar]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, T. Fundamental models for flow batteries. Prog. Energy Combust. Sci. 2015, 49, 40–58. [Google Scholar]
- Ionita, M.; Pandele, A.M.; Crica, L.; Pilan, L. Improving the thermal and mechanical properties of polysulfone by incorporation of graphene oxide. Compos. Part B Eng. 2014, 59, 133–139. [Google Scholar] [CrossRef]
- Han, P.; Wang, H.; Liu, Z.; Chen, X.; Ma, W.; Yao, J.; Zhu, Y.; Cui, G. Graphene oxide nanoplatelets as excellent electrochemical active materials for VO2+/VO2+ and V2+/V3+ redox couples for a vanadium redox flow battery. Carbon 2011, 49, 693–700. [Google Scholar] [CrossRef]
- Sali, S.; Mackey, H.R.; Abdala, A.A. Effect of graphene oxide synthesis method on properties and performance of polysulfone-graphene oxide mixed matrix membranes. Nanomaterials 2019, 9, 769. [Google Scholar]
- González, Z.; Botas, C.; Blanco, C.; Santamaría, R.; Granda, M.; Álvarez, P.; Menéndez, R. Graphite oxide-based graphene materials as positive electrodes in vanadium redox flow batteries. J. Power Sources 2013, 241, 349–354. [Google Scholar]
- Jiang, Q.; Ren, Y.; Yang, Y.; Wang, L.; Dai, L.; He, Z. Recent advances in carbon-based electrocatalysts for vanadium redox flow battery: Mechanisms, properties, and perspectives. Compos. Part B Eng. 2022, 242, 110094. [Google Scholar] [CrossRef]
- Li, B.; Luo, Q.; Wei, X.; Nie, Z.; Thomsen, E.; Chen, B.; Sprenkle, V.; Wang, W. Capacity decay mechanism of microporous separator-based all-vanadium redox flow batteries and its recovery. ChemSusChem 2014, 7, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhang, H.; Li, X.; Zhang, H.; Li, Y.; Vankelecom, I. Hydrophobic asymmetric ultrafiltration PVDF membranes: An alternative separator for VFB with excellent stability. Phys. Chem. Chem. Phys. 2013, 15, 1766–1771. [Google Scholar] [PubMed]
- Wei, X.; Nie, Z.; Luo, Q.; Li, B.; Chen, B.; Simmons, K.; Sprenkle, V.; Wang, W. Nanoporous polytetrafluoroethylene/silica composite separator as a high-performance all-vanadium redox flow battery membrane. Adv. Energy Mater. 2013, 3, 1215–1220. [Google Scholar] [CrossRef]
- Che, X.; Zhao, H.; Ren, X.; Zhang, D.; Wei, H.; Liu, J.; Zhang, X.; Yang, J. Porous polybenzimidazole membranes with high ion selectivity for the vanadium redox flow battery. J. Membr. Sci. 2020, 611, 118359. [Google Scholar]
- Mu, T.; Leng, S.; Tang, W.; Shi, N.; Wang, G.; Yang, J. High-Performance and Low-Cost Membranes Based on Poly (vinylpyrrolidone) and Cardo-Poly (etherketone) Blends for Vanadium Redox Flow Battery Applications. Batteries 2022, 8, 230. [Google Scholar] [CrossRef]
- Pandit, B.; Rondiya, S.R.; Dzade, N.Y.; Shaikh, S.F.; Kumar, N.; Goda, E.S.; Al-Kahtani, A.A.; Mane, R.S.; Mathur, S.; Salunkhe, R.R. High stability and long cycle life of rechargeable sodium-ion battery using manganese oxide cathode: A combined density functional theory (DFT) and experimental study. ACS Appl. Mater. Interfaces 2021, 13, 11433–11441. [Google Scholar] [CrossRef]
- Pandit, B.; Goda, E.S.; Ubaidullah, M.; Shaikh, S.F.; Nakate, U.T.; Khedulkar, A.P.; Kumar, D.; Doong, R.-a. Hexagonal δ-MnO2 nanoplates as efficient cathode material for potassium-ion batteries. Ceram. Int. 2022, 48, 28856–28863. [Google Scholar]
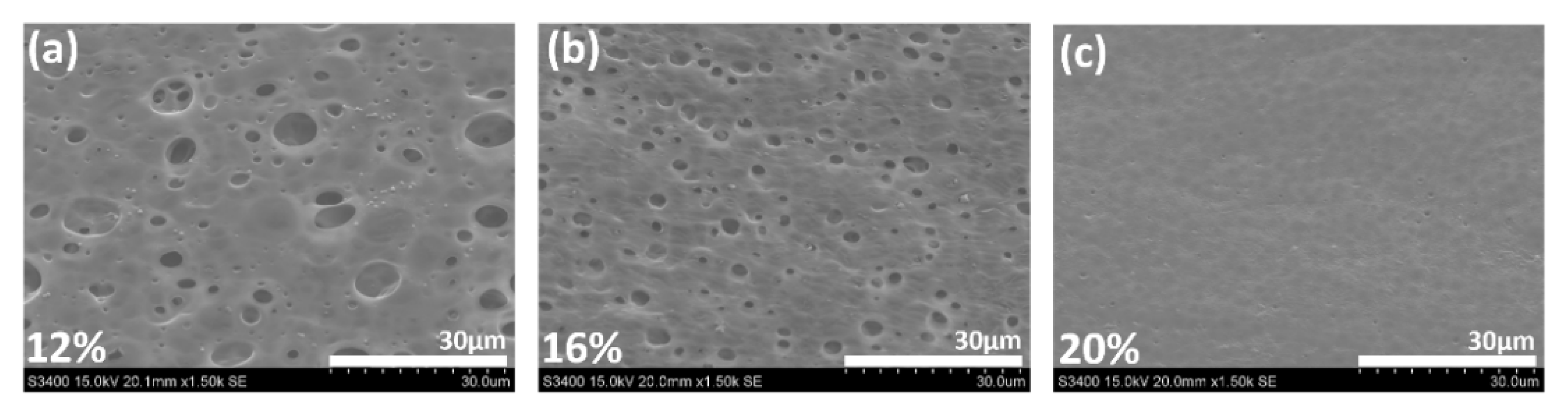
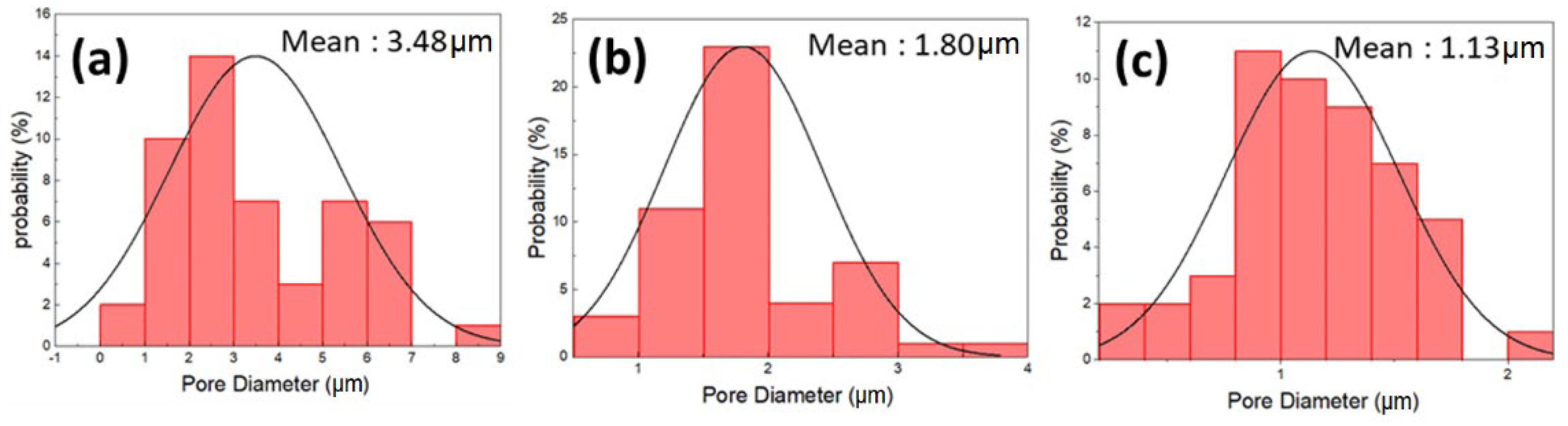

| Membranes | Average Surface Pore Size according to SEM (nm) | Inner Pore Size according to Pore Size Analyzer (nm) |
|---|---|---|
| 20 wt.% PSF | 1139 | 10 |
| 16 wt.% PSF | 1802 | 30 |
| 12 wt.% PSF | 3487 | 470 |
| Pristine PSF | PSF + 0.6 wt% GO | |
|---|---|---|
| Vanadium ion permeability (10−7 cm2 min−1) | 25.9 | 12.3 |
| Coulombic efficiency (CE) (%) | 79.6 | 95.9 |
| Voltage efficiency (VE) (%) | 77.8 | 71.2 |
| Energy efficiency (EE) (%) | 62.0 | 68.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-H.; Chien, M.-Y.; Chuang, Y.-C.; Lai, C.-C.; Sun, Y.-M.; Liu, T.-Y. Porous Membranes of Polysulfone and Graphene Oxide Nanohybrids for Vanadium Redox Flow Battery. Polymers 2022, 14, 5405. https://doi.org/10.3390/polym14245405
Lin C-H, Chien M-Y, Chuang Y-C, Lai C-C, Sun Y-M, Liu T-Y. Porous Membranes of Polysulfone and Graphene Oxide Nanohybrids for Vanadium Redox Flow Battery. Polymers. 2022; 14(24):5405. https://doi.org/10.3390/polym14245405
Chicago/Turabian StyleLin, Chien-Hong, Ming-Yen Chien, Yi-Cih Chuang, Chao-Chi Lai, Yi-Ming Sun, and Ting-Yu Liu. 2022. "Porous Membranes of Polysulfone and Graphene Oxide Nanohybrids for Vanadium Redox Flow Battery" Polymers 14, no. 24: 5405. https://doi.org/10.3390/polym14245405
APA StyleLin, C.-H., Chien, M.-Y., Chuang, Y.-C., Lai, C.-C., Sun, Y.-M., & Liu, T.-Y. (2022). Porous Membranes of Polysulfone and Graphene Oxide Nanohybrids for Vanadium Redox Flow Battery. Polymers, 14(24), 5405. https://doi.org/10.3390/polym14245405







