Analysis of PLA/PHB Biopolymer Material with Admixture of Hydroxyapatite and Tricalcium Phosphate for Clinical Use
Abstract
1. Introduction
2. Methodology
2.1. Characteristics of the Material
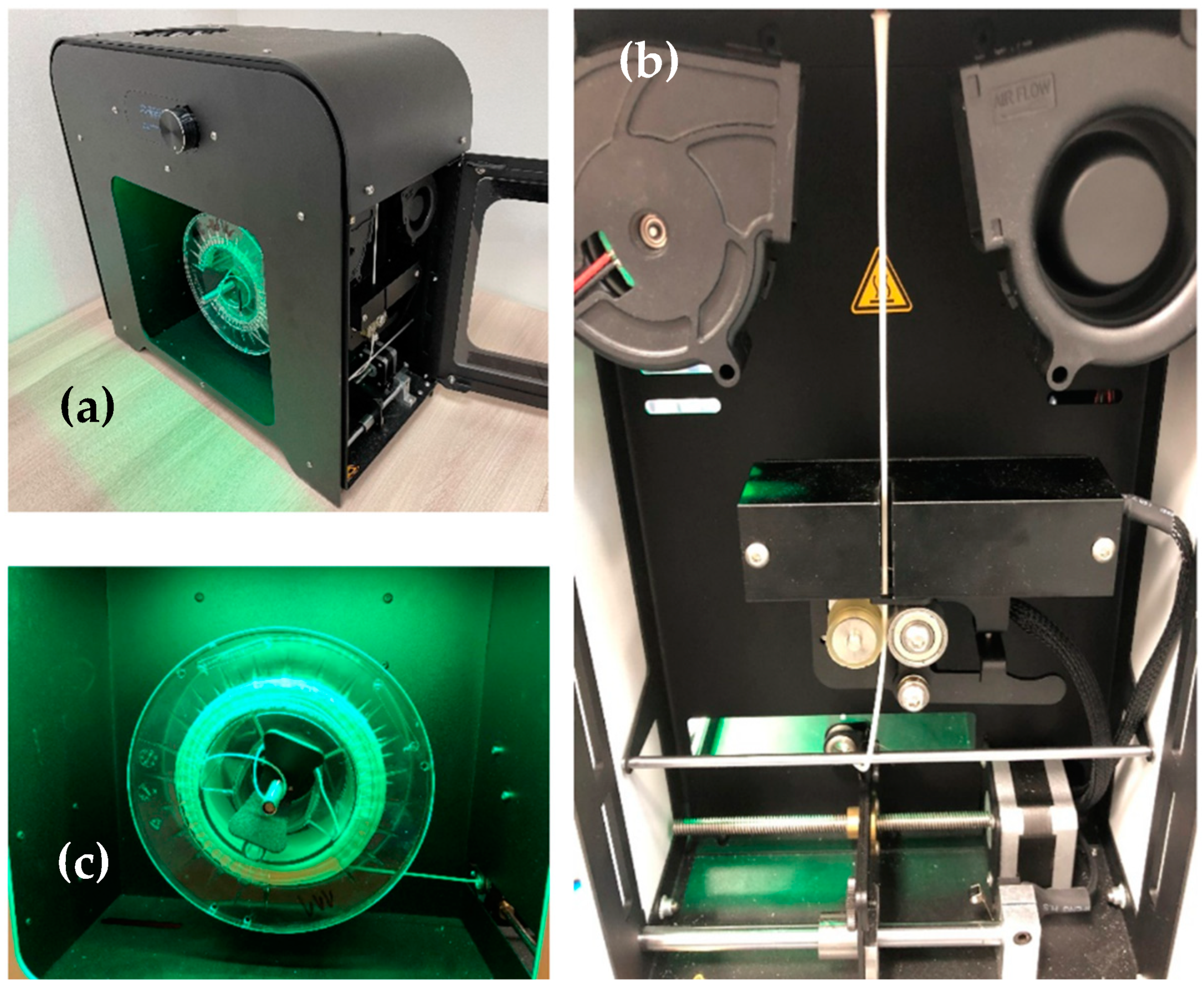
2.2. Filament Production
2.3. Microscopic Analysis
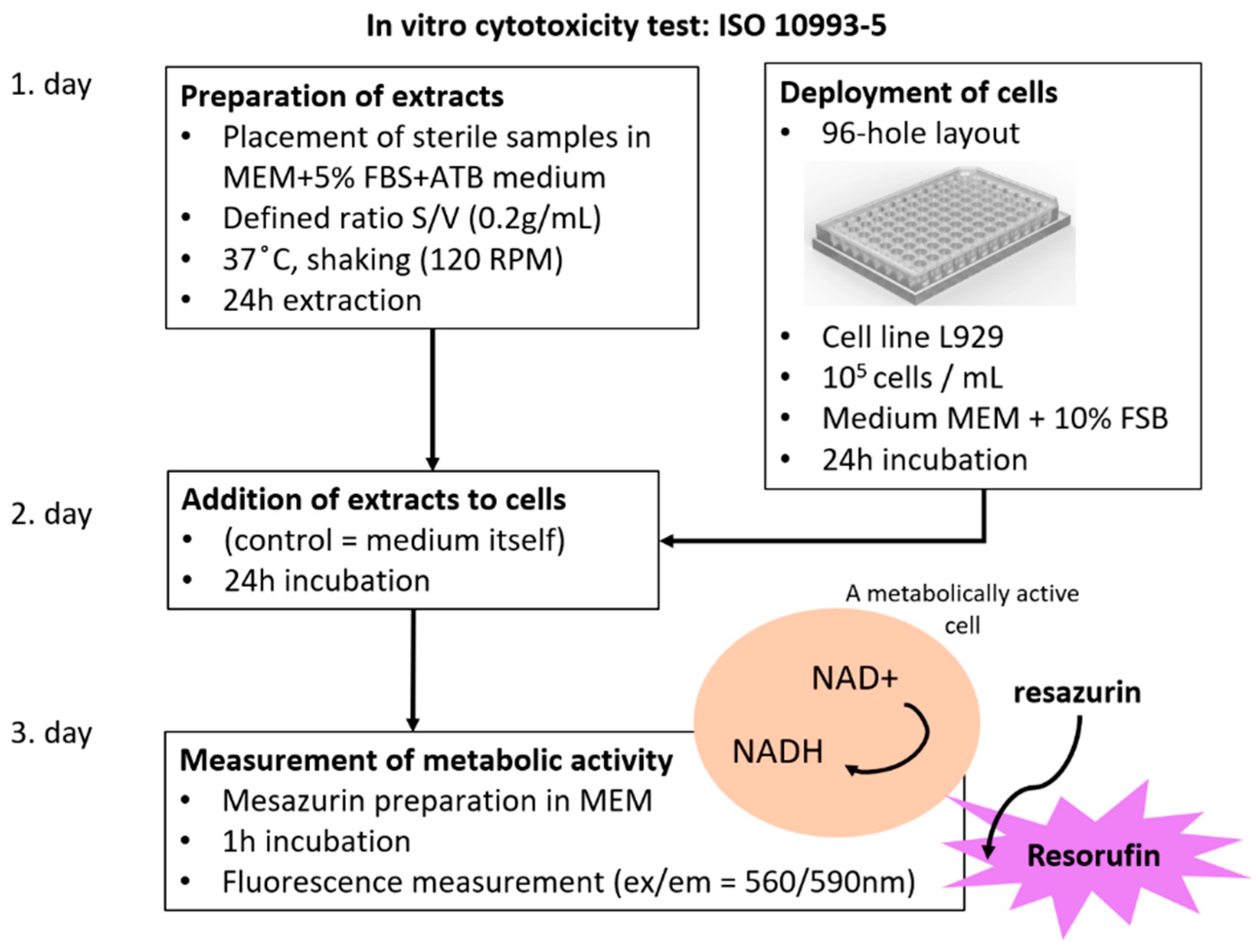
2.4. Cytotoxicity Test
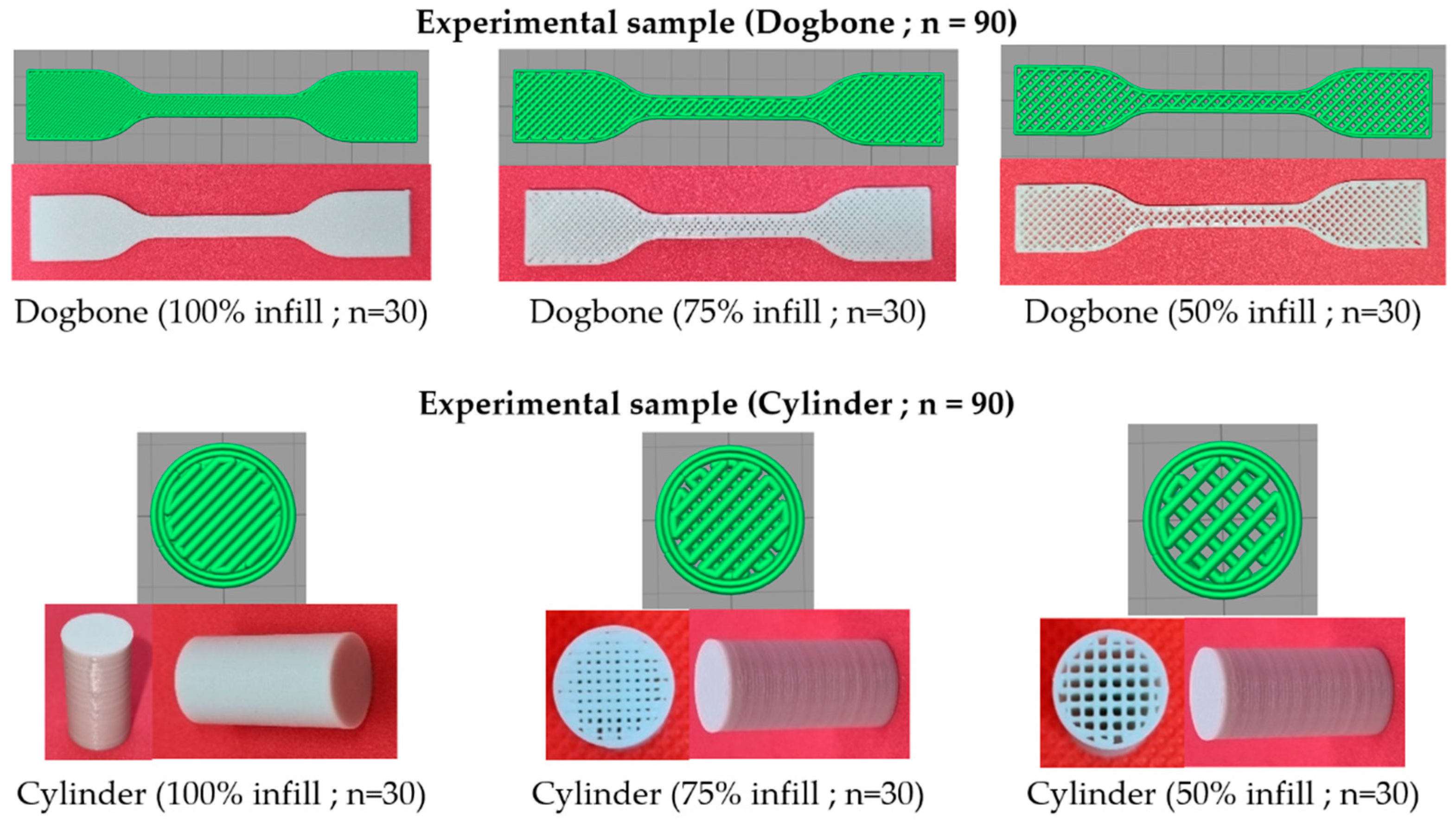
2.5. Production of Experimental Samples
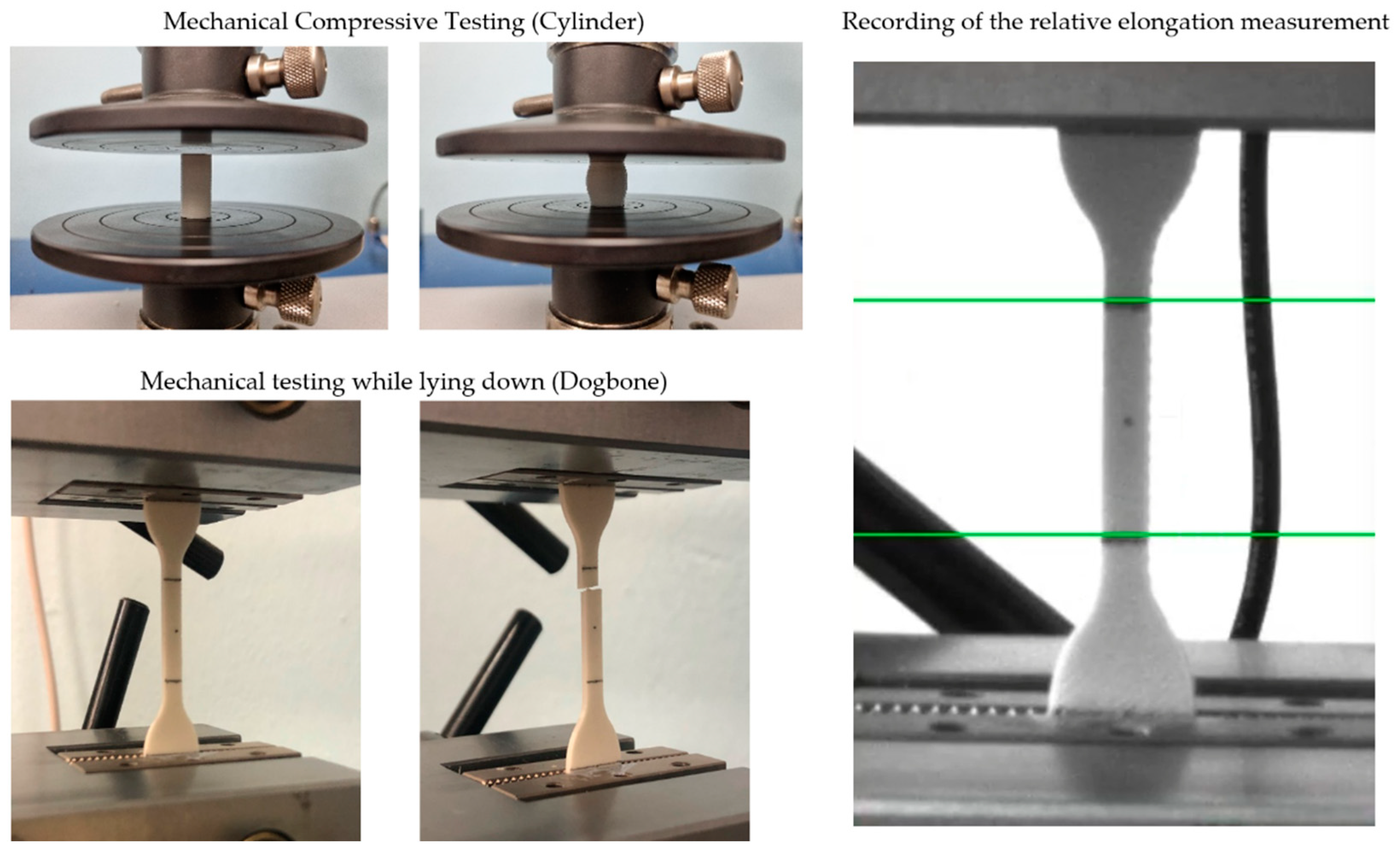
2.6. Mechanical Testing
3. Results and Discussion
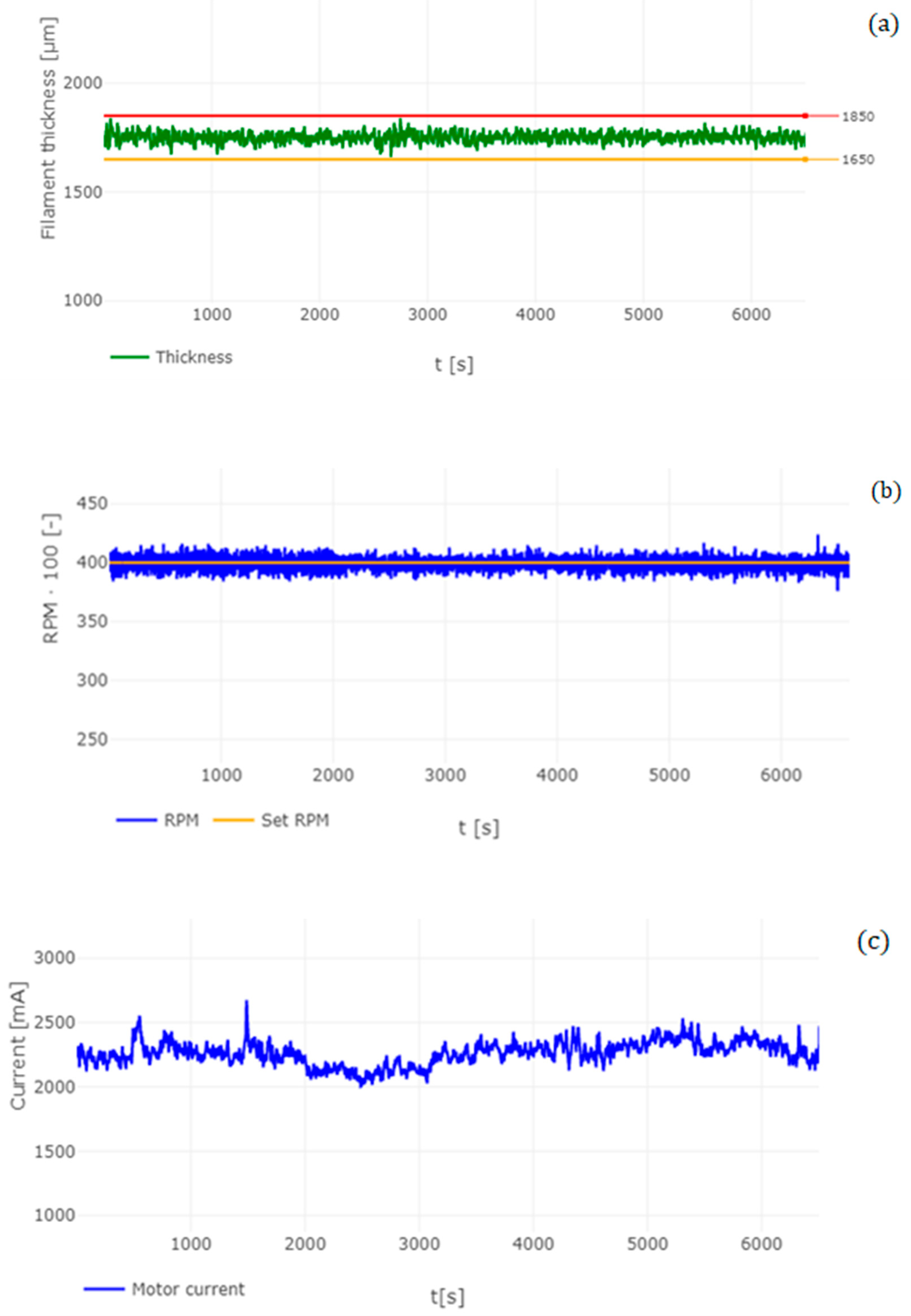
3.1. Analysis of Manufactured Filament
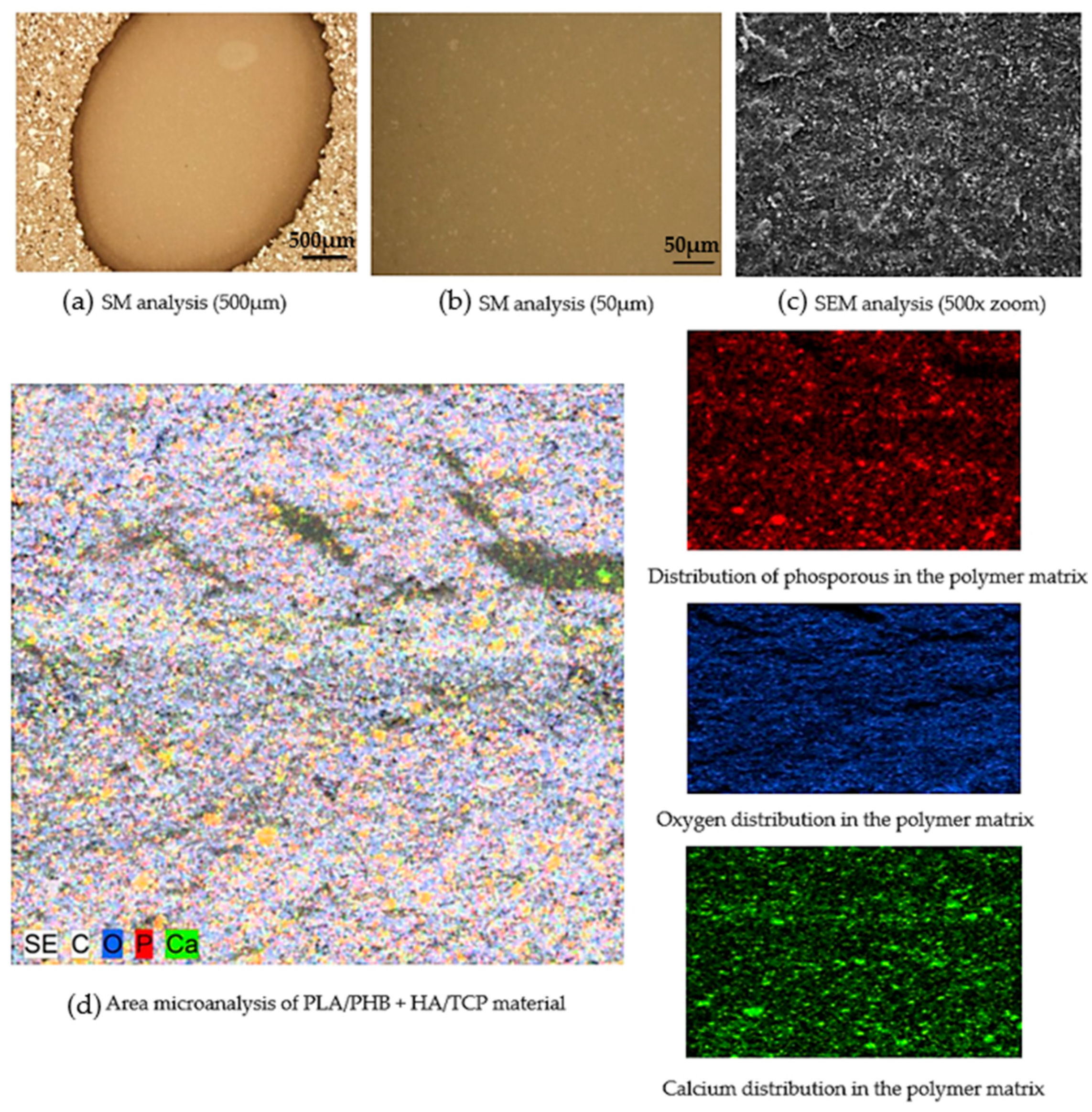
3.2. Microscopic Analysis of the Filament Produced
3.3. Test of Cytotoxicity
3.4. Mechanical Testing Analysis
3.4.1. Tensile Mechanical Testing
3.4.2. Compressive Mechanical Testing
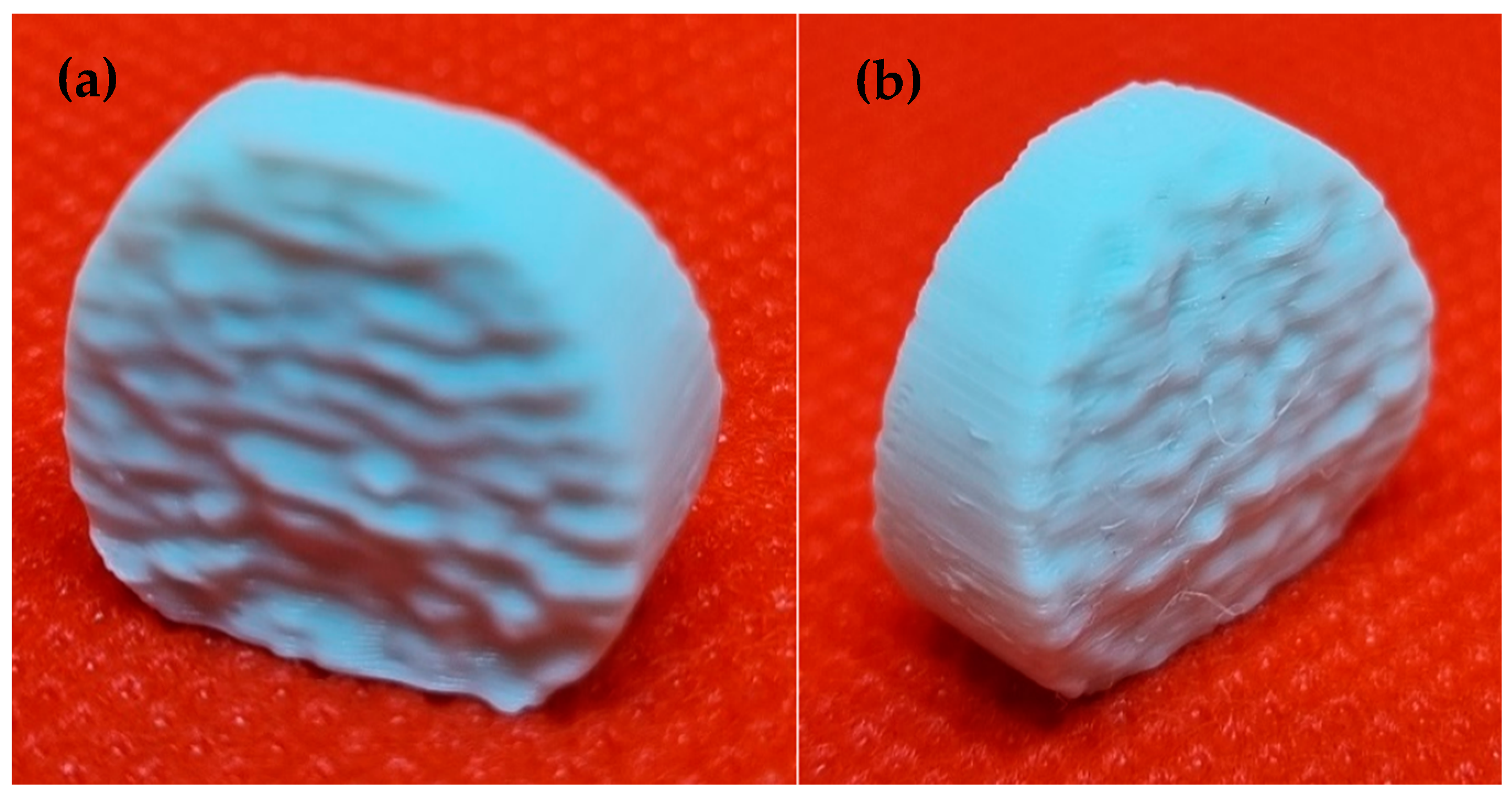
3.5. Analysis of the Dimensions of the Printed Implant
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wickramasinghe, S.; Do, T.; Tran, P. FDM-Based 3D Printing of Polymer and Associated Composite: A Review on Mechanical Properties, Defects and Treatments. Polymers 2020, 12, 1529. [Google Scholar] [CrossRef] [PubMed]
- Vasco, J.C. Additive Manufacturing for the Automotive Industry. In Additive Manufacturing; Elsevier: Amsterdam, The Netherlands, 2021; pp. 505–530. [Google Scholar]
- Ghahfarokhi, P.S.; Podgornovs, A.; Kallaste, A.; Cardoso, A.J.M.; Belahcen, A.; Vaimann, T.; Tiismus, H.; Asad, B. Opportunities and Challenges of Utilizing Additive Manufacturing Approaches in Thermal Management of Electrical Machines. IEEE Access 2021, 9, 36368–36381. [Google Scholar] [CrossRef]
- Rohan, P.; Lukáč, F.; Kolaříková, M.; Krum, S.; Horník, J.; Lukeš, J.; Šepitka, J.; Kuchař, J. Pulsed Plasma Surfacing of Titanium Matrix Cermet Based on B4C. J. Therm. Spray Technol. 2022, 31, 1975–1984. [Google Scholar] [CrossRef]
- Levashkin, D.; Ogin, P.; Vasilyev, F. Efficiency of Hybrid Cyclic Processing with the Use of Additive Technologies on CNC Machines for the Manufacture of Composite Aviation Parts Due to the Reduction of Processing Errors. Mater. Sci. For. 2019, 946, 959–965. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Saxena, A. Additive Manufacturing Applications in Cardiology: A Review. Egypt. Heart J. 2018, 70, 433–441. [Google Scholar] [CrossRef]
- Ramola, M.; Yadav, V.; Jain, R. On the Adoption of Additive Manufacturing in Healthcare: A Literature Review. J. Manuf. Technol. Manag. 2019, 30, 48–69. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A. Additive Manufacturing Applications in Medical Cases: A Literature Based Review. Alex. J. Med. 2018, 54, 411–422. [Google Scholar] [CrossRef]
- Allied Market Research. Allied Market Research. 3D Printing Healthcare Market to Reach $3.69 Bn, Globally, by 2026 at 18.2% CAGR. Available online: https://www.prnewswire.com/news-releases/3d-printing-healthcare-market-to-reach-3-69-bn-globally-by-2026-at-18-2-cagr-allied-market-research-300944847.html (accessed on 31 October 2022).
- Kervran, M.; Vagner, C.; Cochez, M.; Ponçot, M.; Saeb, M.R.; Vahabi, H. Thermal Degradation of Polylactic Acid (PLA)/Polyhydroxybutyrate (PHB) Blends: A Systematic Review. Polym. Degrad. Stab. 2022, 201, 109995. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Perdiguero, M.; Fiori, S.; Kenny, J.M.; Peponi, L. Biodegradable Electrospun PLA-PHB Fibers Plasticized with Oligomeric Lactic Acid. Polym. Degrad. Stab. 2020, 179, 109226. [Google Scholar] [CrossRef]
- Aversa, C.; Barletta, M.; Puopolo, M.; Vesco, S. Cast Extrusion of Low Gas Permeability Bioplastic Sheets in PLA/PBS and PLA/PHB Binary Blends. Polym.-Plast. Technol. Mater. 2020, 59, 231–240. [Google Scholar] [CrossRef]
- Dawin, T.P.; Ahmadi, Z.; Taromi, F.A. Biocompatible PLA/PHB Coatings Obtained from Controlled Solid State Polymerization. Prog. Org. Coat. 2019, 132, 41–49. [Google Scholar] [CrossRef]
- Frone, A.N.; Batalu, D.; Chiulan, I.; Oprea, M.; Gabor, A.R.; Nicolae, C.-A.; Raditoiu, V.; Trusca, R.; Panaitescu, D.M. Morpho-Structural, Thermal and Mechanical Properties of PLA/PHB/Cellulose Biodegradable Nanocomposites Obtained by Compression Molding, Extrusion, and 3D Printing. Nanomaterials 2019, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Advanced Pharmaceutical Applications of Hot-Melt Extrusion Coupled with Fused Deposition Modelling (FDM) 3D Printing for Personalised Drug Delivery. Pharmaceutics 2018, 10, 203. [Google Scholar] [CrossRef]
- Kristiawan, R.B.; Imaduddin, F.; Ariawan, D.; Ubaidillah; Arifin, Z. A Review on the Fused Deposition Modeling (FDM) 3D Printing: Filament Processing, Materials, and Printing Parameters. Open Eng. 2021, 11, 639–649. [Google Scholar] [CrossRef]
- Wasti, S.; Adhikari, S. Use of Biomaterials for 3D Printing by Fused Deposition Modeling Technique: A Review. Front. Chem. 2020, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Koch, F.; Thaden, O.; Tröndle, K.; Zengerle, R.; Zimmermann, S.; Koltay, P. Open-Source Hybrid 3D-Bioprinter for Simultaneous Printing of Thermoplastics and Hydrogels. HardwareX 2021, 10, e00230. [Google Scholar] [CrossRef]
- Lovecchio, J.; Cortesi, M.; Zani, M.; Govoni, M.; Dallari, D.; Giordano, E. Fiber Thickness and Porosity Control in a Biopolymer Scaffold 3D Printed through a Converted Commercial FDM Device. Materials 2022, 15, 2394. [Google Scholar] [CrossRef]
- Park, S.; Fu, K. Polymer-Based Filament Feedstock for Additive Manufacturing. Compos. Sci. Technol. 2021, 213, 108876. [Google Scholar] [CrossRef]
- Rydz, J.; Šišková, A.; Andicsová Eckstein, A. Scanning Electron Microscopy and Atomic Force Microscopy: Topographic and Dynamical Surface Studies of Blends, Composites, and Hybrid Functional Materials for Sustainable Future. Adv. Mater. Sci. Eng. 2019, 2019, 6871785. [Google Scholar] [CrossRef]
- D’Anna, A.; Arrigo, R.; Frache, A. PLA/PHB Blends: Biocompatibilizer Effects. Polymers 2019, 11, 1416. [Google Scholar] [CrossRef]
- Liu, A.R.; Xu, B.Z.; Chen, C.C.; Huang, D.Y.; Liang, E.W.; Ge, F.X.; Ge, G.J. Effects of Modified SWCNT on the Mechanical and Thermal Properties of PLA/PHB Bio-Composites. AIP Adv. 2020, 10, 075122. [Google Scholar] [CrossRef]
- Farias, N.C.; Major, I.; Devine, D.; Brennan Fournet, M.; Pezzoli, R.; Farshbaf Taghinezhad, S.; Hesabi, M. Multiple Recycling of a PLA / PHB Biopolymer Blend for Sustainable Packaging Applications: Rheology-morphology, Thermal, and Mechanical Performance Analysis. Polym. Eng. Sci. 2022, 62, 1764–1774. [Google Scholar] [CrossRef]
- Arif, U.; Haider, S.; Haider, A.; Khan, N.; Alghyamah, A.A.; Jamila, N.; Khan, M.I.; Almasry, W.A.; Kang, I.-K. Biocompatible Polymers and Their Potential Biomedical Applications: A Review. Curr. Pharm. Des. 2019, 25, 3608–3619. [Google Scholar] [CrossRef] [PubMed]
- Surisaeng, J.; Kanabenja, W.; Passornraprasit, N.; Aumnate, C.; Potiyaraj’, P. Polyhydroxybutyrate/Polylactic Acid Blends: An Alternative Feedstock for 3D Printed Bone Scaffold Model. J. Phys. Conf. Ser. 2022, 2175, 012021. [Google Scholar] [CrossRef]
- Azami, M.; Moztarzadeh, F.; Tahriri, M. Preparation, Characterization and Mechanical Properties of Controlled Porous Gelatin/Hydroxyapatite Nanocomposite through Layer Solvent Casting Combined with Freeze-Drying and Lamination Techniques. J. Porous Mater. 2010, 17, 313–320. [Google Scholar] [CrossRef]
- Senatov, F.; Anisimova, N.; Kiselevskiy, M.; Kopylov, A.; Tcherdyntsev, V.; Maksimkin, A. Polyhydroxybutyrate/Hydroxyapatite Highly Porous Scaffold for Small Bone Defects Replacement in the Nonload-Bearing Parts. J. Bionic Eng. 2017, 14, 648–658. [Google Scholar] [CrossRef]
- Porter, M.M.; Lee, S.; Tanadchangsaeng, N.; Jaremko, M.J.; Yu, J.; Meyers, M.; McKittrick, J. Porous Hydroxyapatite-Polyhydroxybutyrate Composites Fabricated by a Novel Method via Centrifugation. In Mechanics of Biological Systems and Materials, Volume 5; Springer: New York, NY, USA, 2013; pp. 63–71. [Google Scholar]
- Grabowik, C.; Kalinowski, K.; Ćwikła, G.; Paprocka, I.; Kogut, P. Tensile Tests of Specimens Made of Selected Group of the Filament Materials Manufactured with FDM Method. MATEC Web Conf. 2017, 112, 04017. [Google Scholar] [CrossRef]
- Cardona, C.; Curdes, A.H.; Isaacs, A.J. Effects of Filament Diameter Tolerances in Fused Filament Fabrication. IU J. Undergrad. Res. 2016, 2, 44–47. [Google Scholar] [CrossRef]
- Mirón, V.; Ferrándiz, S.; Juárez, D.; Mengual, A. Manufacturing and Characterization of 3D Printer Filament Using Tailoring Materials. Procedia Manuf. 2017, 13, 888–894. [Google Scholar] [CrossRef]
- Mohammed, M.I.; Wilson, D.; Gomez-Kervin, E.; Tang, B.; Wang, J. Investigation of Closed-Loop Manufacturing with Acrylonitrile Butadiene Styrene over Multiple Generations Using Additive Manufacturing. ACS Sustain. Chem. Eng. 2019, 7, 13955–13969. [Google Scholar] [CrossRef]
- Spinelli, G.; Kotsilkova, R.; Ivanov, E.; Petrova-Doycheva, I.; Menseidov, D.; Georgiev, V.; Maio, R.D.; Silvestre, C. Effects of Filament Extrusion, 3D Printing and Hot-Pressing on Electrical and Tensile Properties of Poly(Lactic) Acid Composites Filled with Carbon Nanotubes and Graphene. Nanomaterials 2019, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Prasad, E.; Islam, M.T.; Goodwin, D.J.; Megarry, A.J.; Halbert, G.W.; Florence, A.J.; Robertson, J. Development of a Hot-Melt Extrusion (HME) Process to Produce Drug Loaded AffinisolTM 15LV Filaments for Fused Filament Fabrication (FFF) 3D Printing. Addit. Manuf. 2019, 29, 100776. [Google Scholar] [CrossRef]
- McIlroy, C.; Olmsted, P.D. Disentanglement Effects on Welding Behaviour of Polymer Melts during the Fused-Filament-Fabrication Method for Additive Manufacturing. Polymer 2017, 123, 376–391. [Google Scholar] [CrossRef]
- Anderegg, D.A.; Bryant, H.A.; Ruffin, D.C.; Skrip, S.M., Jr.; Fallon, J.J.; Gilmer, E.L.; Bortner, M.J. In-Situ Monitoring of Polymer Flow Temperature and Pressure in Extrusion Based Additive Manufacturing. Addit. Manuf. 2019, 26, 76–83. [Google Scholar] [CrossRef]
- Chen, J.; Smith, D.E. Filament Rheological Characterization for Fused Filament Fabrication Additive Manufacturing: A Low-Cost Approach. Addit. Manuf. 2021, 47, 102208. [Google Scholar] [CrossRef]
- Mackay, M.E. The Importance of Rheological Behavior in the Additive Manufacturing Technique Material Extrusion. J. Rheol. 2018, 62, 1549–1561. [Google Scholar] [CrossRef]
- Phan, D.D.; Horner, J.S.; Swain, Z.R.; Beris, A.N.; Mackay, M.E. Computational Fluid Dynamics Simulation of the Melting Process in the Fused Filament Fabrication Additive Manufacturing Technique. Addit. Manuf. 2020, 33, 101161. [Google Scholar] [CrossRef]
- Wagh, A.S.; Poeppel, R.B.; Singh, J.P. Open Pore Description of Mechanical Properties of Ceramics. J. Mater. Sci. 1991, 26, 3862–3868. [Google Scholar] [CrossRef]
- Maté-Sánchez de Val, J.E.; Calvo-Guirado, J.L.; Delgado-Ruiz, R.A.; Ramírez-Fernández, M.P.; Negri, B.; Abboud, M.; Martínez, I.M.; de Aza, P.N. Physical Properties, Mechanical Behavior, and Electron Microscopy Study of a New α-TCP Block Graft with Silicon in an Animal Model: New Block Graft of α-TCP with Silicon. J. Biomed. Mater. Res. A 2012, 100, 3446–3454. [Google Scholar] [CrossRef]
- Rh Owen, G.; Dard, M.; Larjava, H. Hydoxyapatite/Beta-Tricalcium Phosphate Biphasic Ceramics as Regenerative Material for the Repair of Complex Bone Defects. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2493–2512. [Google Scholar] [CrossRef]
- Spoerk, M.; Arbeiter, F.; Raguž, I.; Holzer, C.; Gonzalez-Gutierrez, J. Mechanical Recyclability of Polypropylene Composites Produced by Material Extrusion-Based Additive Manufacturing. Polymers 2019, 11, 1318. [Google Scholar] [CrossRef]
- Ramier, J.; Bouderlique, T.; Stoilova, O.; Manolova, N.; Rashkov, I.; Langlois, V.; Renard, E.; Albanese, P.; Grande, D. Biocomposite Scaffolds Based on Electrospun Poly(3-Hydroxybutyrate) Nanofibers and Electrosprayed Hydroxyapatite Nanoparticles for Bone Tissue Engineering Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 38, 161–169. [Google Scholar] [CrossRef]
- Danoux, C.B.; Barbieri, D.; Yuan, H.; de Bruijn, J.D.; van Blitterswijk, C.A.; Habibovic, P. In Vitro and in Vivo Bioactivity Assessment of a Polylactic Acid/Hydroxyapatite Composite for Bone Regeneration. Biomatter 2014, 4, e27664. [Google Scholar] [CrossRef]
- Chen, L.J.; Wang, M. Production and Evaluation of Biodegradable Composites Based on PHB–PHV Copolymer. Biomaterials 2002, 23, 2631–2639. [Google Scholar] [CrossRef]
- Kovalcik, A.; Sangroniz, L.; Kalina, M.; Skopalova, K.; Humpolíček, P.; Omastova, M.; Mundigler, N.; Müller, A.J. Properties of Scaffolds Prepared by Fused Deposition Modeling of Poly(Hydroxyalkanoates). Int. J. Biol. Macromol. 2020, 161, 364–376. [Google Scholar] [CrossRef]
- Shangguan, Y.-Y.; Wang, Y.-W.; Wu, Q.; Chen, G.-Q. The Mechanical Properties and in Vitro Biodegradation and Biocompatibility of UV-Treated Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate). Biomaterials 2006, 27, 2349–2357. [Google Scholar] [CrossRef]
- Moorkoth, D.; Nampoothiri, K.M. Production and Characterization of Poly(3-Hydroxy Butyrate-Co-3 Hydroxyvalerate) (PHBV) by a Novel Halotolerant Mangrove Isolate. Bioresour. Technol. 2016, 201, 253–260. [Google Scholar] [CrossRef]
- Maskery, I.; Sturm, L.; Aremu, A.O.; Panesar, A.; Williams, C.B.; Tuck, C.J.; Wildman, R.D.; Ashcroft, I.A.; Hague, R.J.M. Insights into the Mechanical Properties of Several Triply Periodic Minimal Surface Lattice Structures Made by Polymer Additive Manufacturing. Polymer 2017, 152, 62–71. [Google Scholar] [CrossRef]
- Borges, R.; Ribeiro, S.; Marchi, J.; Yoshimura, H.N. Mechanical Characterization of Tricalcium Phosphate Ceramics Doped with Magnesium. Mater. Sci. For. 2014, 798–799, 454–459. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine, 3rd ed.; Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Black, J. Biological Performance of Materials: Fundamentals of Biocompatibility, 3rd ed.; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Russias, J.; Saiz, E.; Nalla, R.K.; Gryn, K.; Ritchie, R.O.; Tomsia, A.P. Fabrication and Mechanical Properties of PLA/HA Composites: A Study of in Vitro Degradation. Mater. Sci. Eng. C Biomim. Supramol. Syst. 2006, 26, 1289–1295. [Google Scholar] [CrossRef]
- Kabir, S.M.F.; Mathur, K.; Seyam, A.-F.M. A Critical Review on 3D Printed Continuous Fiber-Reinforced Composites: History, Mechanism, Materials and Properties. Compos. Struct. 2020, 232, 111476. [Google Scholar] [CrossRef]
- Ingole, V.H.; Vuherer, T.; Maver, U.; Vinchurkar, A.; Ghule, A.V.; Kokol, V. Mechanical Properties and Cytotoxicity of Differently Structured Nanocellulose-Hydroxyapatite Based Composites for Bone Regeneration Application. Nanomaterials 2019, 10, 25. [Google Scholar] [CrossRef]
- Tehrani, A.H.; Zadhoush, A.; Karbasi, S. Preparing Nanocomposite Fibrous Scaffolds of P3HB/NHA for Bone Tissue Engineering. In In Proceedings of the 2010 17th Iranian Conference of Biomedical Engineering (ICBME), Isfahan, Iran, 3–4 November 2010; IEEE: Piscataway, NJ, USA. [Google Scholar]
- Lalegani Dezaki, M.; Mohd Ariffin, M.K.A. The Effects of Combined Infill Patterns on Mechanical Properties in FDM Process. Polymers 2020, 12, 2792. [Google Scholar] [CrossRef]
- Bakır, A.A.; Atik, R.; Özerinç, S. Mechanical Properties of Thermoplastic Parts Produced by Fused Deposition Modeling:A Review. Rapid Prototyp. J. 2021, 27, 537–561. [Google Scholar] [CrossRef]
- Kim, S.; Shin, Y.; Jung, H.; Hwang, C.; Baik, H.; Cha, J. Precision nad trueness of dental models manufactured with different 3-dimensional printing techniques. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 144–153. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Choi, J.-H.; Kim, N.-K.; Kim, Y.; Lee, J.-K.; Kim, M.-K.; Lee; J.-H.; Kim, M.-J. Analysis of errors in medical rapid prototyping models. Int. J. Oral Maxillofac. Surg. 2002, 31, 23–32. [Google Scholar] [CrossRef]
- Kim, T.; Lee, S.; Kim, G.B.; Hong, D.; Kwon, J.; Park, J.-W.; Kim, N. Accuracy of a simplified 3D-printed implant surgical guide. J. Prosthet. Dent. 2020, 124, 195–201. [Google Scholar] [CrossRef]
- El-Katatny, I.; Mosood, S.H.; Morsi, Y.S. Error analysis of FDM fabricated medical replicas. Rapid Prototyp. J. 2010, 16, 36–43. [Google Scholar] [CrossRef]






| PLA | PHB | HA | β-TCP | |
|---|---|---|---|---|
| Melting point temperature [°C] | 170–180 | 175–180 | 1100 °C | 1670 °C |
| Glass transition temperature [°C] | 50–80 °C | 5 °C | n.a. | n.a. |
| Molecular weight [g/mol] | 72.06 | 86 | 502.3 | 310.18 |
| Density [g/cm3] | 1.24 | 3.07 | 5.12 | 1.25 |
| Granulate size | 2–4 mm | 2–4 mm | 2–6 µm | 2–6 µm |
| Morphology | spherical | spherical | angular | spherical/ angular |
| Purity | Medical grade | Medical grade | >95% | >95% |
| Supplier | Purasorb® (Amsterdam, Netherlands) | Biomer P300 (Frankfurt, Germenny) | Captal® (Pune, India) | Captal® (Pune, India) |
| Filament Diameter (Average) | 1.773 mm |
| Standard deviation (SD) | 0.0223 mm |
| Max. value of filament | 1.84 mm |
| Min. value of filament | 1.663 mm |
| Variance (VAR) | −0.00049 |
| Skewness (SKS) | −0.1006 |
| 3D Model (Nominal) [mm] | Printed Implant [mm] | |
|---|---|---|
| Height | 14 | 13.967 |
| Width | 16 | 15.963 |
| Thickness | 8.144 | 8.198 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohan, M.; Lancoš, S.; Schnitzer, M.; Živčák, J.; Hudák, R. Analysis of PLA/PHB Biopolymer Material with Admixture of Hydroxyapatite and Tricalcium Phosphate for Clinical Use. Polymers 2022, 14, 5357. https://doi.org/10.3390/polym14245357
Kohan M, Lancoš S, Schnitzer M, Živčák J, Hudák R. Analysis of PLA/PHB Biopolymer Material with Admixture of Hydroxyapatite and Tricalcium Phosphate for Clinical Use. Polymers. 2022; 14(24):5357. https://doi.org/10.3390/polym14245357
Chicago/Turabian StyleKohan, Miroslav, Samuel Lancoš, Marek Schnitzer, Jozef Živčák, and Radovan Hudák. 2022. "Analysis of PLA/PHB Biopolymer Material with Admixture of Hydroxyapatite and Tricalcium Phosphate for Clinical Use" Polymers 14, no. 24: 5357. https://doi.org/10.3390/polym14245357
APA StyleKohan, M., Lancoš, S., Schnitzer, M., Živčák, J., & Hudák, R. (2022). Analysis of PLA/PHB Biopolymer Material with Admixture of Hydroxyapatite and Tricalcium Phosphate for Clinical Use. Polymers, 14(24), 5357. https://doi.org/10.3390/polym14245357






