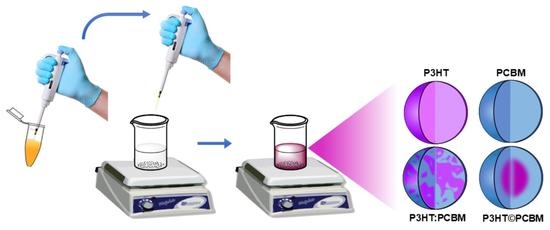
Synthesis of Organic Semiconductor Nanoparticles with Different Conformations Using the Nanoprecipitation Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
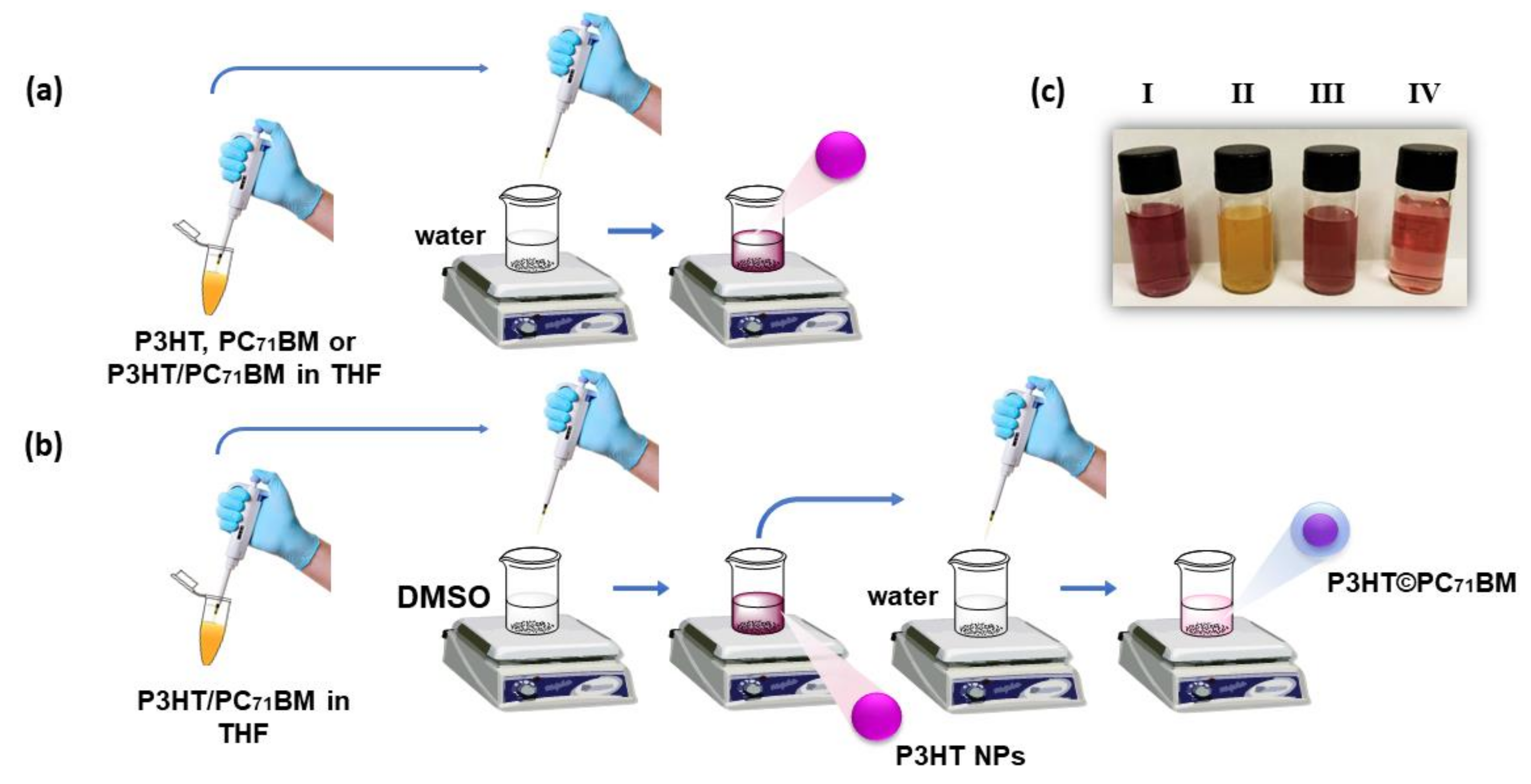
2.2. Synthesis of the Organic Nanoparticles
2.3. Characterization Methods
3. Results and Discussion
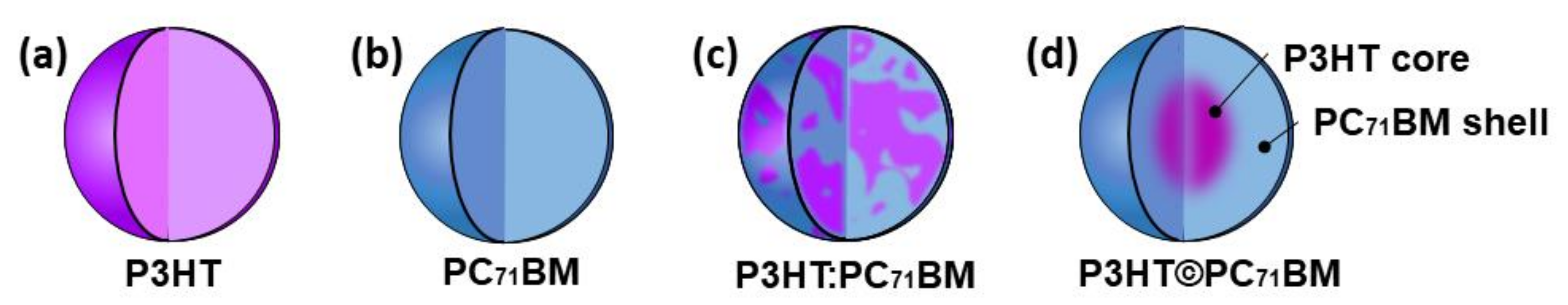
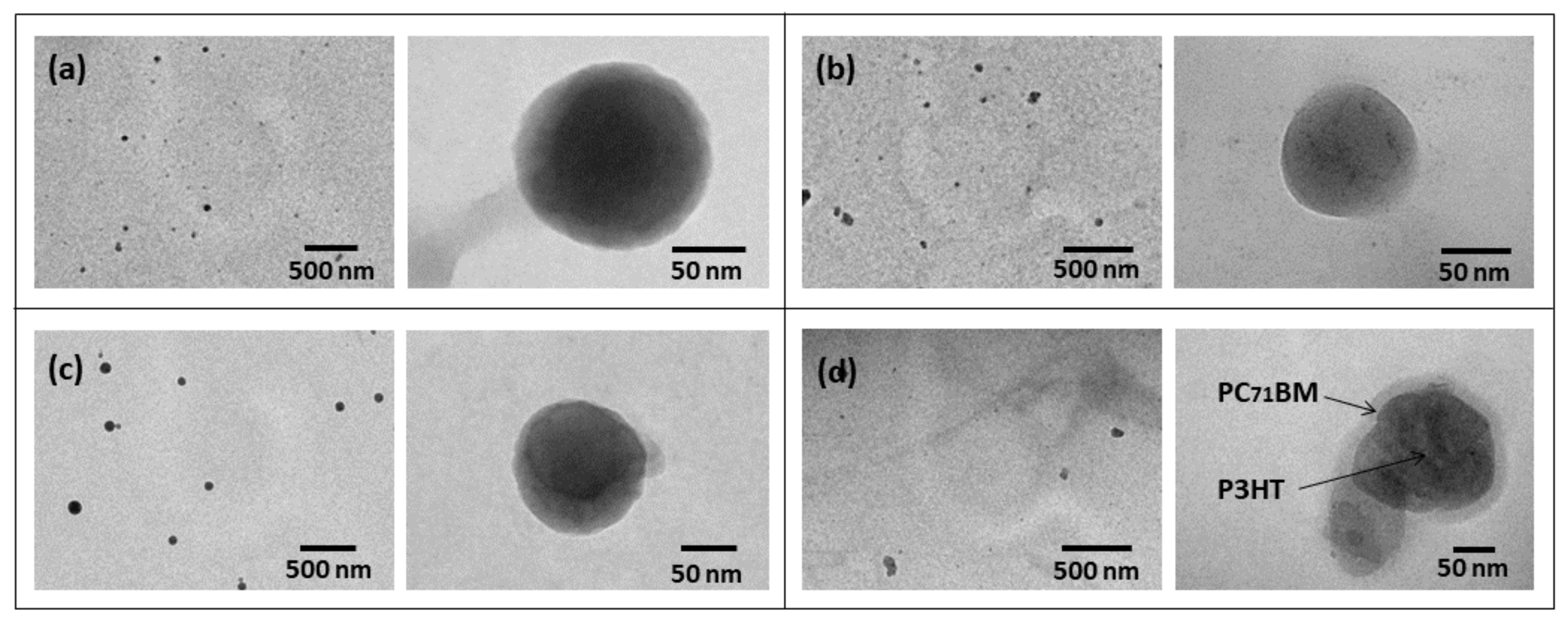
3.1. Structural Analysis
3.2. Optical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ballif, C.; Haug, F.-J.; Boccard, M.; Verlinden, P.J.; Hahn, G. Status and Perspectives of Crystalline Silicon Photovoltaics in Research and Industry. Nat. Rev. Mater. 2022, 7, 597–616. [Google Scholar] [CrossRef]
- Sampaio, P.G.V.; González, M.O.A. A Review on Organic Photovoltaic Cell. Int. J. Energy Res. 2022, 46, 17813–17828. [Google Scholar] [CrossRef]
- Yang, F.; Huang, Y.; Li, Y.; Li, Y. Large-Area Flexible Organic Solar Cells. Npj Flex. Electron. 2021, 5, 30. [Google Scholar] [CrossRef]
- Chang, J.K.; Huang, Y.Y.; Lin, D.L.; Tau, J.I.; Chen, T.H.; Chen, M.H. Solution-Processed, Semitransparent Organic Photovoltaics Integrated with Solution-Doped Graphene Electrodes. Sci. Rep. 2020, 10, 20010. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Pan, F.; Bin, H.; Zhang, J.; Xue, L.; Qiu, B.; Wei, Z.; Zhang, Z.-G.; Li, Y. A Low Cost and High Performance Polymer Donor Material for Polymer Solar Cells. Nat. Commun. 2018, 9, 743. [Google Scholar] [CrossRef]
- Kaltenbrunner, M.; White, M.S.; Głowacki, E.D.; Sekitani, T.; Someya, T.; Sariciftci, N.S.; Bauer, S. Ultrathin and Lightweight Organic Solar Cells with High Flexibility. Nat. Commun. 2012, 3, 770. [Google Scholar] [CrossRef]
- Chen, D.; Nakahara, A.; Wei, D.; Nordlund, D.; Russell, T.P. P3HT/PCBM Bulk Heterojunction Organic Photovoltaics: Correlating Efficiency and Morphology. Nano Lett. 2011, 11, 561–567. [Google Scholar] [CrossRef]
- Bi, S.; Ouyang, Z.; Shaik, S.; Li, D. Effect of Donor-Acceptor Vertical Composition Profile on Performance of Organic Bulk Heterojunction Solar Cells. Sci. Rep. 2018, 8, 9574. [Google Scholar] [CrossRef]
- Ghosekar, I.C.; Patil, G.C. Review on Performance Analysis of P3HT:PCBM-Based Bulk Heterojunction Organic Solar Cells. Semicond. Sci. Technol. 2021, 36, 045005. [Google Scholar] [CrossRef]
- Chandrasekaran, N.; Kumar, A.; Thomsen, L.; Kabra, D.; McNeill, C.R. High Performance As-Cast P3HT:PCBM Devices: Understanding the Role of Molecular Weight in High Regioregularity P3HT. Mater. Adv. 2021, 2, 2045–2054. [Google Scholar] [CrossRef]
- Garg, M.; Padmanabhan, V. Addition of P3HT-Grafted Silica Nanoparticles Improves Bulk-Heterojunction Morphology in P3HT-PCBM Blends. Sci. Rep. 2016, 6, 33219. [Google Scholar] [CrossRef] [PubMed]
- Kymakis, E.; Spyropoulos, G.D.; Fernandes, R.; Kakavelakis, G.; Kanaras, A.G.; Stratakis, E. Plasmonic Bulk Heterojunction Solar Cells: The Role of Nanoparticle Ligand Coating. ACS Photonics 2015, 2, 714–723. [Google Scholar] [CrossRef]
- Yang, P.; Zeigler, D.F.; Bryant, K.C.; Martin, T.R.; Gamelin, D.R.; Luscombe, C.K. Identifying Effects of TiO 2 Nanowires inside Bulk Heterojunction Organic Photovoltaics on Charge Diffusion and Recombination. J. Mater. Chem. C 2014, 2, 4922–4927. [Google Scholar] [CrossRef]
- Oh, S.H.; Heo, S.J.; Yang, J.S.; Kim, H.J. Effects of ZnO Nanoparticles on P3HT:PCBM Organic Solar Cells with DMF-Modulated PEDOT:PSS Buffer Layers. ACS Appl. Mater. Interfaces 2013, 5, 11530–11534. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H.; Cha, S.-H.; Kim, S.C.; Song, M.; Lee, J.; Shin, W.S.; Moon, S.-J.; Bahng, J.H.; Kotov, N.A.; Jin, S.-H. Silver Nanowire Embedded in P3HT:PCBM for High-Efficiency Hybrid Photovoltaic Device Applications. ACS Nano 2011, 5, 3319–3325. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Zhang, Z.; Li, L.; Agbolaghi, S.; Mousavi, S. Improved Stability in P3HT:PCBM Photovoltaics by Incorporation of Well-Designed Polythiophene/Graphene Compositions. Polym. Int. 2020, 69, 833–846. [Google Scholar] [CrossRef]
- Kymakis, E.; Kornilios, N.; Koudoumas, E. Carbon Nanotube Doping of P3HT: PCBM Photovoltaic Devices. J. Phys. D Appl. Phys. 2008, 41, 165110. [Google Scholar] [CrossRef]
- Wang, H.S.; Lin, L.H.; Chen, S.Y.; Wang, Y.L.; Wei, K.H. Ordered Polythiophene/Fullerene Composite Core-Shell Nanorod Arrays for Solar Cell Applications. Nanotechnology 2009, 20, 18–23. [Google Scholar] [CrossRef]
- Bag, M.; Gehan, T.S.; Renna, L.A.; Algaier, D.D.; Lahti, P.M.; Venkataraman, D.; Cao, W.; Xue, J.; Andersen, T.R.; Dam, H.F.; et al. Fabrication Conditions for Efficient Organic Photovoltaic Cells from Aqueous Dispersions of Nanoparticles. RSC Adv. 2014, 4, 45325–45331. [Google Scholar] [CrossRef]
- Ghezzi, D.; Antognazza, M.R.; Maccarone, R.; Bellani, S.; Lanzarini, E.; Martino, N.; Mete, M.; Pertile, G.; Bisti, S.; Lanzani, G.; et al. A Polymer Optoelectronic Interface Restores Light Sensitivity in Blind Rat Retinas. Nat. Photonics 2013, 7, 400–406. [Google Scholar] [CrossRef]
- Meng, L.; Fan, H.; Lane, J.M.D.; Qin, Y. Bottom-up approaches for precisely nanostructuring hybrid organic/inorganic multi-component composites for organic photovoltaics. MRS Adv. 2020, 5, 2055–2065. [Google Scholar] [CrossRef]
- Synthesis of IrO2 decorated core–shell PS@PPyNH2 microspheres for bio-interface application. Nanotechnology 2020, 31, 375605. [CrossRef] [PubMed]
- Holmes, N.P.; Burke, K.B.; Sista, P.; Barr, M.; Magurudeniya, H.D.; Stefan, M.C.; Kilcoyne, A.L.D.; Zhou, X.; Dastoor, P.C.; Belcher, W.J. Nano-domain behaviour in P3HT:PCBM nanoparticles, relating material properties to morphological changes. Sol. Energy Mater. Sol. Cells 2013, 117, 437–445. [Google Scholar] [CrossRef]
- Antognazza, M.R.; Di Paolo, M.; Ghezzi, D.; Mete, M.; Di Marco, S.; Maya-Vetencourt, J.F.; Maccarone, R.; Desii, A.; Di Fonzo, F.; Bramini, M.; et al. Characterization of a Polymer-Based, Fully Organic Prosthesis for Implantation into the Subretinal Space of the Rat. Adv. Healthc. Mater. 2016, 5, 2271–2282. [Google Scholar] [CrossRef] [PubMed]
- Zucchetti, E.; Zangoli, M.; Bargigia, I.; Bossio, C.; Di Maria, F.; Barbarella, G.; D’Andrea, C.; Lanzani, G.; Antognazza, M.R. Poly(3-Hexylthiophene) Nanoparticles for Biophotonics: Study of the Mutual Interaction with Living Cells. J. Mater. Chem. B 2017, 5, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Francia, S.; Shmal, D.; Di Marco, S.; Chiaravalli, G.; Maya-Vetencourt, J.F.; Mantero, G.; Michetti, C.; Cupini, S.; Manfredi, G.; DiFrancesco, M.L.; et al. Light-Induced Charge Generation in Polymeric Nanoparticles Restores Vision in Advanced-Stage Retinitis Pigmentosa Rats. Nat. Commun. 2022, 13, 3677. [Google Scholar] [CrossRef] [PubMed]
- Levi, S.R.; Ryu, J.; Liu, P.-K.; Tsang, S.H. Precision Medicine Trials in Retinal Degenerations. Annu. Rev. Vis. Sci. 2021, 7, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Chambon, S.; Schatz, C.; Sébire, V.; Pavageau, B.; Wantz, G.; Hirsch, L. Organic Semiconductor Core-Shell Nanoparticles Designed through Successive Solvent Displacements. Mater. Horiz. 2014, 1, 431–438. [Google Scholar] [CrossRef]
- Schwarz, K.N.; Farley, S.B.; Smith, T.A.; Ghiggino, K.P. Charge Generation and Morphology in P3HT: PCBM Nanoparticles Prepared by Mini-Emulsion and Reprecipitation Methods. Nanoscale 2015, 7, 19899–19904. [Google Scholar] [CrossRef] [PubMed]
- Gehan, T.S.; Bag, M.; Renna, L.A.; Shen, X.; Algaier, D.D.; Lahti, P.M.; Russell, T.P.; Venkataraman, D. Multiscale Active Layer Morphologies for Organic Photovoltaics through Self-Assembly of Nanospheres. Nano Lett. 2014, 14, 5238–5243. [Google Scholar] [CrossRef]
- Holmes, N.P.; Nicolaidis, N.; Feron, K.; Barr, M.; Burke, K.B.; Al-Mudhaffer, M.; Sista, P.; Kilcoyne, A.L.D.; Stefan, M.C.; Zhou, X.; et al. Probing the Origin of Photocurrent in Nanoparticulate Organic Photovoltaics. Sol. Energy Mater. Sol. Cells 2015, 140, 412–421. [Google Scholar] [CrossRef]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Influence of Process and Formulation Parameters on the Formation of Submicron Particles by Solvent Displacement and Emulsification-Diffusion Methods: Critical Comparison. Adv. Colloid Interface Sci. 2011, 163, 90–122. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Guan, C.; Xue, J.; Malesky, T.; Luo, Y.; Lin, Y.; Qin, Y.; He, J. Facile synthesis of water-dispersible poly (3-hexylthiophene) nanoparticles with high yield and excellent colloidal stability. Iscience 2022, 25, 104220, 2022. [Google Scholar] [CrossRef]
- Wohnhaas, C.T.; Leparc, G.G.; Fernandez-Albert, F.; Kind, D.; Gantner, F.; Viollet, C.; Hildebrandt, T.; Baum, P. DMSO Cryopreservation Is the Method of Choice to Preserve Cells for Droplet-Based Single-Cell RNA Sequencing. Sci. Rep. 2019, 9, 10699. [Google Scholar] [CrossRef] [PubMed]
- ISO 22412:2017; Particle Size Analysis—Dynamic Light Scattering (DLS). International Standard Organization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/65410.html (accessed on 10 December 2021).
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Knappett, B.R.; Abdulkin, P.; Ringe, E.; Jefferson, D.A.; Lozano-Perez, S.; Rojas, T.C.; Fernández, A.; Wheatley, A.E.H. Characterisation of Co@Fe3O4 Core@shell Nanoparticles Using Advanced Electron Microscopy. Nanoscale 2013, 5, 5765. [Google Scholar] [CrossRef]
- Browning, N.D.; Chisholm, M.F.; Pennycook, S.J. Atomic-Resolution Chemical Analysis Using a Scanning Transmission Electron Microscope. Nature 1993, 366, 143–146. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Ho, J.J.; Wang, C.K.; Lee, W.; Lu, C.C.; Yau, B.S.; Nain, J.L.; Chang, S.H.; Chang, C.C.; Wang, K.L. Improvement of Organic Solar Cells by Flexible Substrate and ITO Surface Treatments. Appl. Surf. Sci. 2010, 256, 7606–7611. [Google Scholar] [CrossRef]
- Tuna, O.; Selamet, Y.; Aygun, G.; Ozyuzer, L. High Quality ITO Thin Films Grown by Dc and RF Sputtering without Oxygen. J. Phys. D Appl. Phys. 2010, 43, 055402. [Google Scholar] [CrossRef]
- Aziz, F.; Ismail, A.F. Spray Coating Methods for Polymer Solar Cells Fabrication: A Review. Mater. Sci. Semicond. Process. 2015, 39, 416–425. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Alford, T.L.; Honsberg, C.B. Solvent-Controlled Spin-Coating Method for Large-Scale Area Deposition of Two-Dimensional Silica Nanosphere Assembled Layers. Langmuir 2014, 30, 5732–5738. [Google Scholar] [CrossRef] [PubMed]
- Otep, S.; Lin, Y.C.; Matsumoto, H.; Mori, T.; Wei, K.H.; Michinobu, T. Diketopyrrolopyrrole–thiophene–methoxythiophene based random copolymers for organic field effect transistor applications. Org. Electron. 2020, 87, 105986. [Google Scholar] [CrossRef]
- Farouil, L.; Alary, F.; Bedel-Pereira, E.; Heully, J.L. Revisiting the vibrational and optical properties of P3HT: A combined experimental and theoretical study. J. Phys. Chem. A 2018, 122, 6532–6545, 2018. [Google Scholar] [CrossRef]
- Pan, H.; Tan, B.; Yazdani, A.; Budhlall, B.; Sobkowicz, M.J. Controlling the Particle Size of Aqueous Conjugated Polymer Colloids and Impact on Transistor Performance. Colloids Surf. A Physicochem. Eng. Asp. 2020, 594, 124633. [Google Scholar] [CrossRef]
- Park, Y.D.; Lee, H.S.; Choi, Y.J.; Kwak, D.; Cho, J.H.; Lee, S.; Cho, K. Solubility-Induced Ordered Polythiophene Precursors for High-Performance Organic Thin-Film Transistors. Adv. Funct. Mater. 2009, 19, 1200–1206. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, Y.B.; Park, D.H.; Kim, M.S.; Cho, E.H.; Joo, J. Tuning Optical Properties of Poly(3-Hexylthiophene) Nanoparticles through Hydrothermal Processing. Sci. Technol. Adv. Mater. 2011, 12, 025002. [Google Scholar] [CrossRef]
- Khlaifia, D.; Ewels, C.P.; Massuyeau, F.; Chemek, M.; Faulques, E.; Duvail, J.L.; Alimi, K. Unraveling the Real Structures of Solution-Based and Surface-Bound Poly(3-Hexylthiophene) (P3HT) Oligomers: A Combined Theoretical and Experimental Study. RSC Adv. 2016, 6, 56174–56182. [Google Scholar] [CrossRef]
- Trotzky, S.; Hoyer, T.; Tuszynski, W.; Lienau, C.; Parisi, J. Femtosecond Up-Conversion Technique for Probing the Charge Transfer in a P3HT: PCBM Blend via Photoluminescence Quenching. J. Phys. D Appl. Phys. 2009, 42, 055105. [Google Scholar] [CrossRef]
- Brabec, C.J.; Sariciftci, N.S.; Hummelen, J.C. Plastic Solar Cells. Adv. Funct. Mater. 2001, 11, 15–26. [Google Scholar] [CrossRef]
- Kurpiers, J.; Ferron, T.; Roland, S.; Jakoby, M.; Thiede, T.; Jaiser, F.; Albrecht, S.; Janietz, S.; Collins, B.A.; Howard, I.A.; et al. Probing the Pathways of Free Charge Generation in Organic Bulk Heterojunction Solar Cells. Nat. Commun. 2018, 9, 2038. [Google Scholar] [CrossRef]
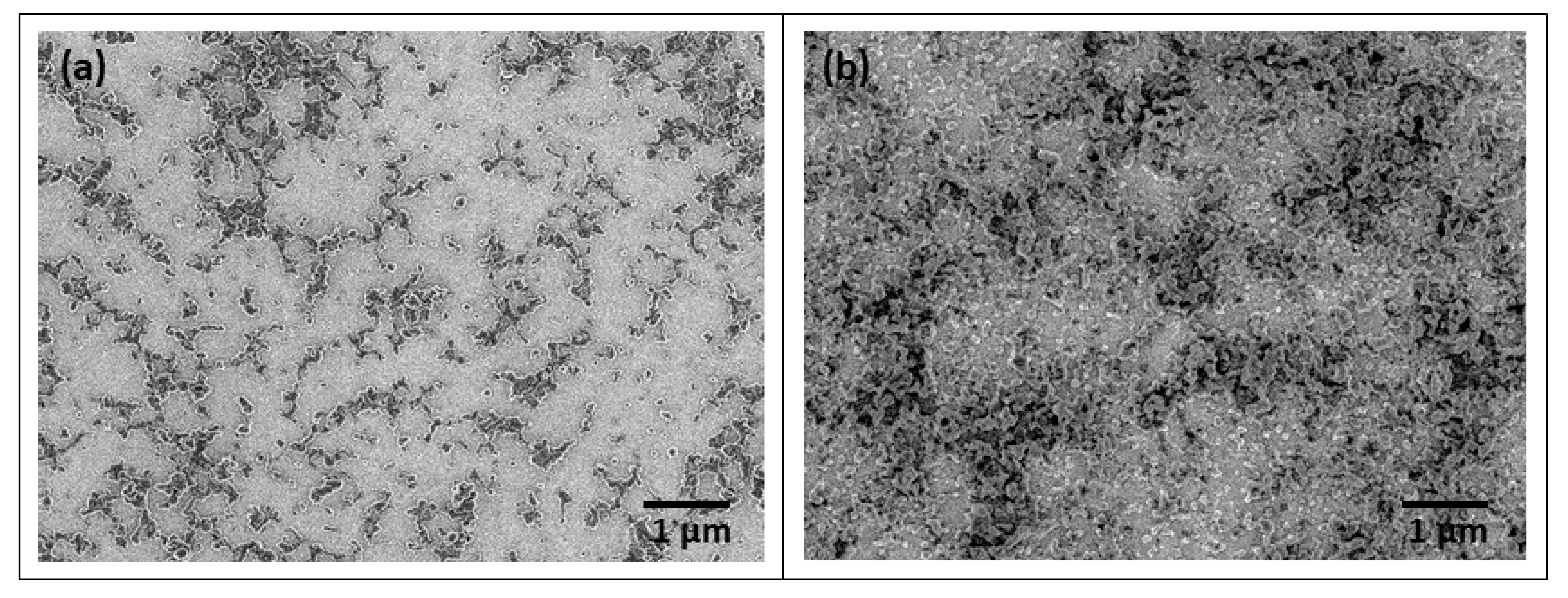
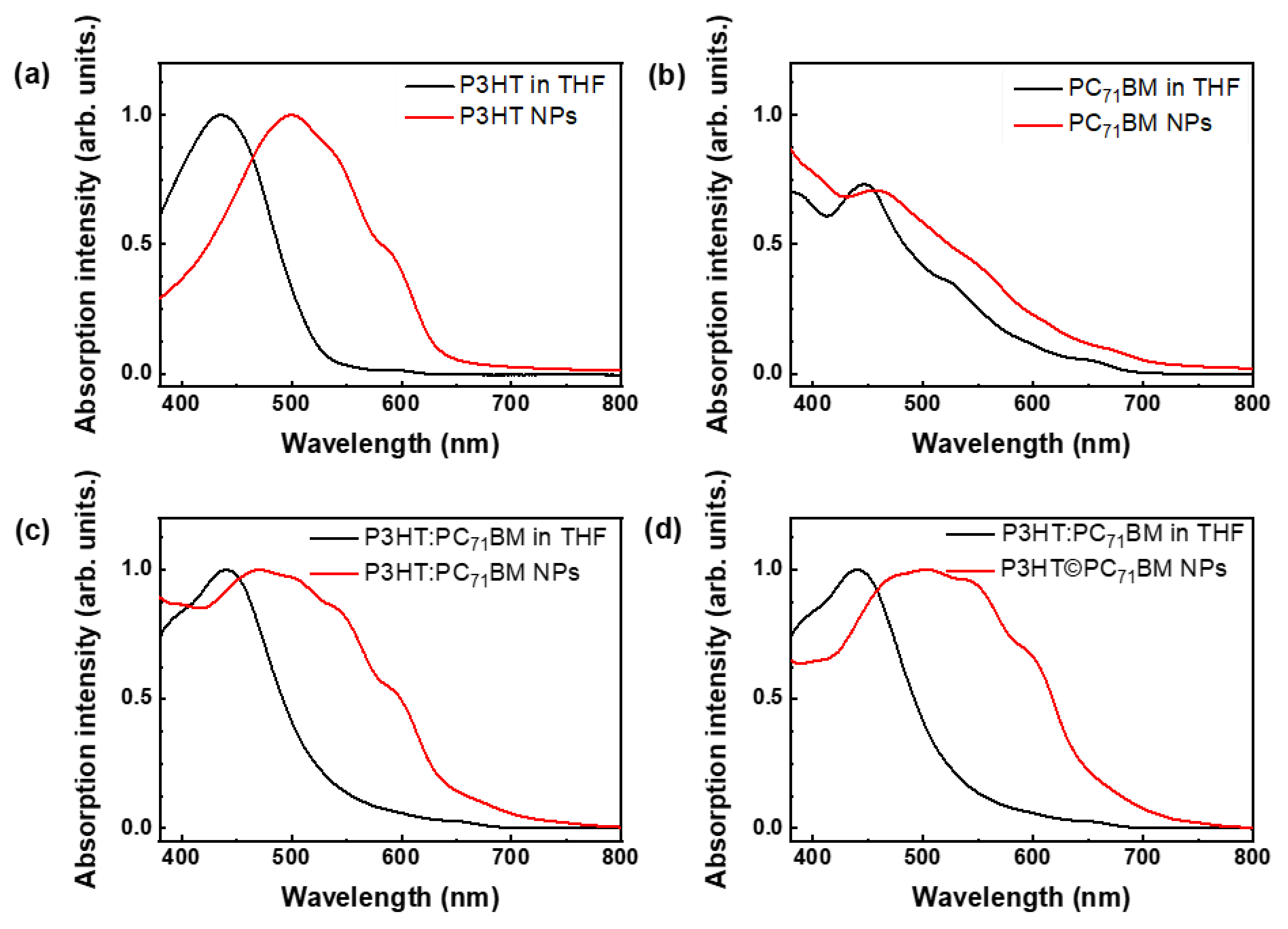


| Nanoparticles | Average Diameter (nm) | PDI |
|---|---|---|
| P3HT | (1.4 ± 0.6) × 102 | 0.15 |
| PC71BM | (1.3 ± 0.8) × 102 | 0.23 |
| P3HT:PC71BM | (1.4 ± 0.5) × 102 | 0.09 |
| P3HT©PC71BM | (2.0 ± 1.0) × 102 | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshioka, N.A.; Faraco, T.A.; Barud, H.S.; Ribeiro, S.J.L.; Cremona, M.; Fragneaud, B.; Maciel, I.O.; Quirino, W.G.; Legnani, C. Synthesis of Organic Semiconductor Nanoparticles with Different Conformations Using the Nanoprecipitation Method. Polymers 2022, 14, 5336. https://doi.org/10.3390/polym14245336
Yoshioka NA, Faraco TA, Barud HS, Ribeiro SJL, Cremona M, Fragneaud B, Maciel IO, Quirino WG, Legnani C. Synthesis of Organic Semiconductor Nanoparticles with Different Conformations Using the Nanoprecipitation Method. Polymers. 2022; 14(24):5336. https://doi.org/10.3390/polym14245336
Chicago/Turabian StyleYoshioka, Nathalia A., Thales A. Faraco, Hernane S. Barud, Sidney J. L. Ribeiro, Marco Cremona, Benjamin Fragneaud, Indhira O. Maciel, Welber G. Quirino, and Cristiano Legnani. 2022. "Synthesis of Organic Semiconductor Nanoparticles with Different Conformations Using the Nanoprecipitation Method" Polymers 14, no. 24: 5336. https://doi.org/10.3390/polym14245336
APA StyleYoshioka, N. A., Faraco, T. A., Barud, H. S., Ribeiro, S. J. L., Cremona, M., Fragneaud, B., Maciel, I. O., Quirino, W. G., & Legnani, C. (2022). Synthesis of Organic Semiconductor Nanoparticles with Different Conformations Using the Nanoprecipitation Method. Polymers, 14(24), 5336. https://doi.org/10.3390/polym14245336