Synthesis and Characterization of Hybrid Materials Derived from Conjugated Copolymers and Reduced Graphene Oxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Synthesis of Hybrid Materials
2.4. Synthesis of Graphene Oxide and Reduction to the Desired rGO
2.5. Preparation of the Hybrid Dispersions
3. Results and Discussion
3.1. Raman and X-ray Diffraction Analysis
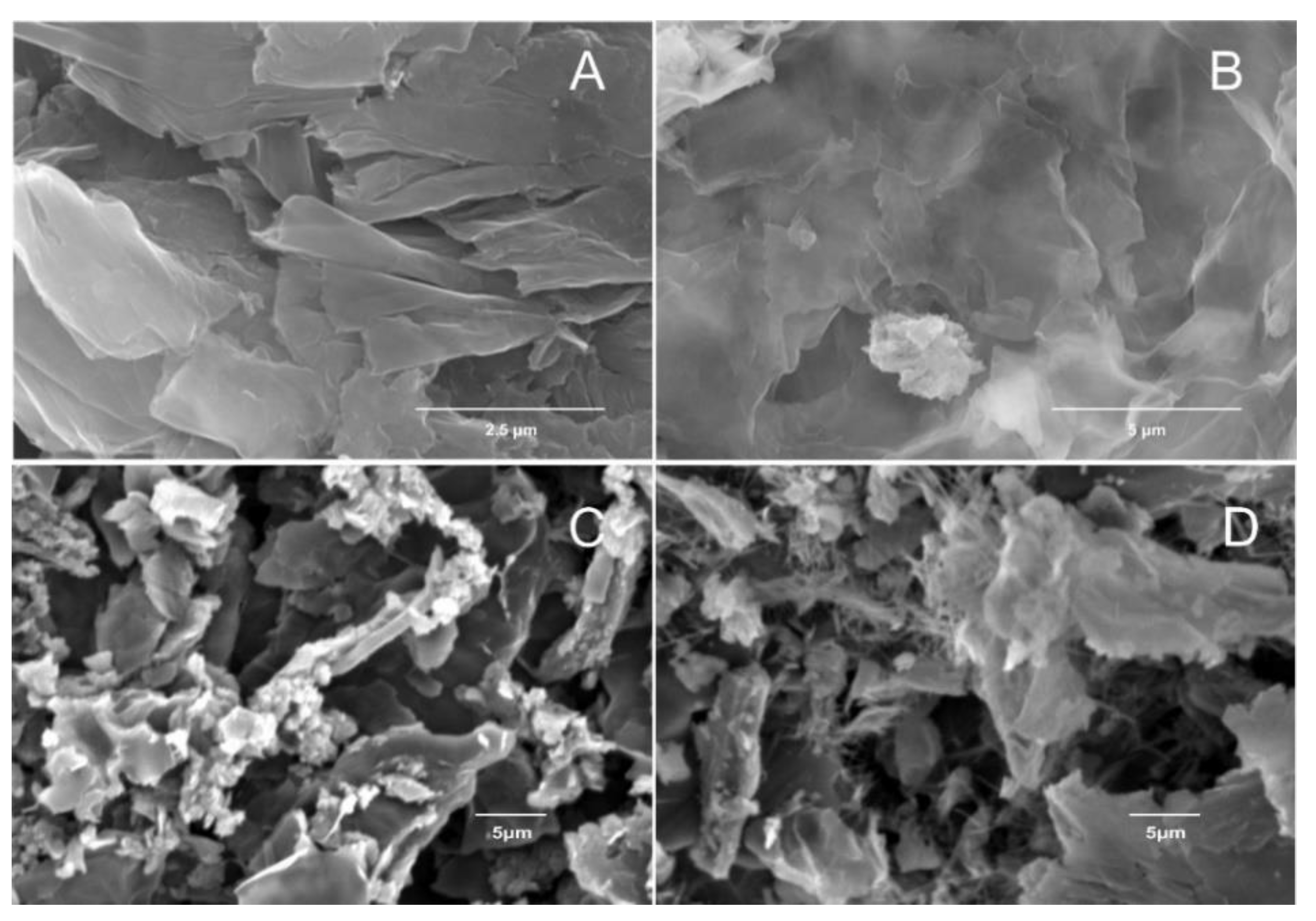
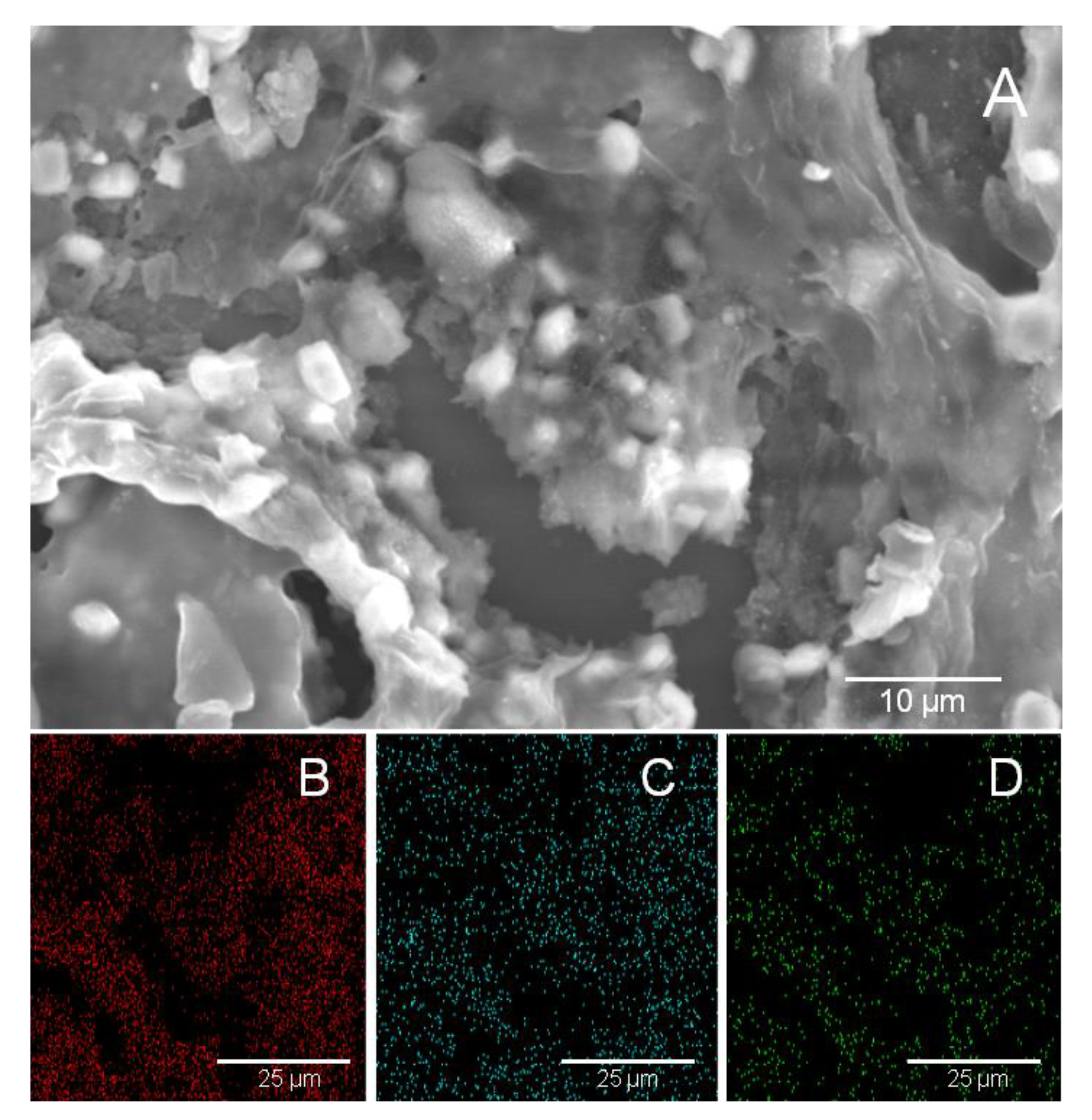
3.2. Morphological Characterization
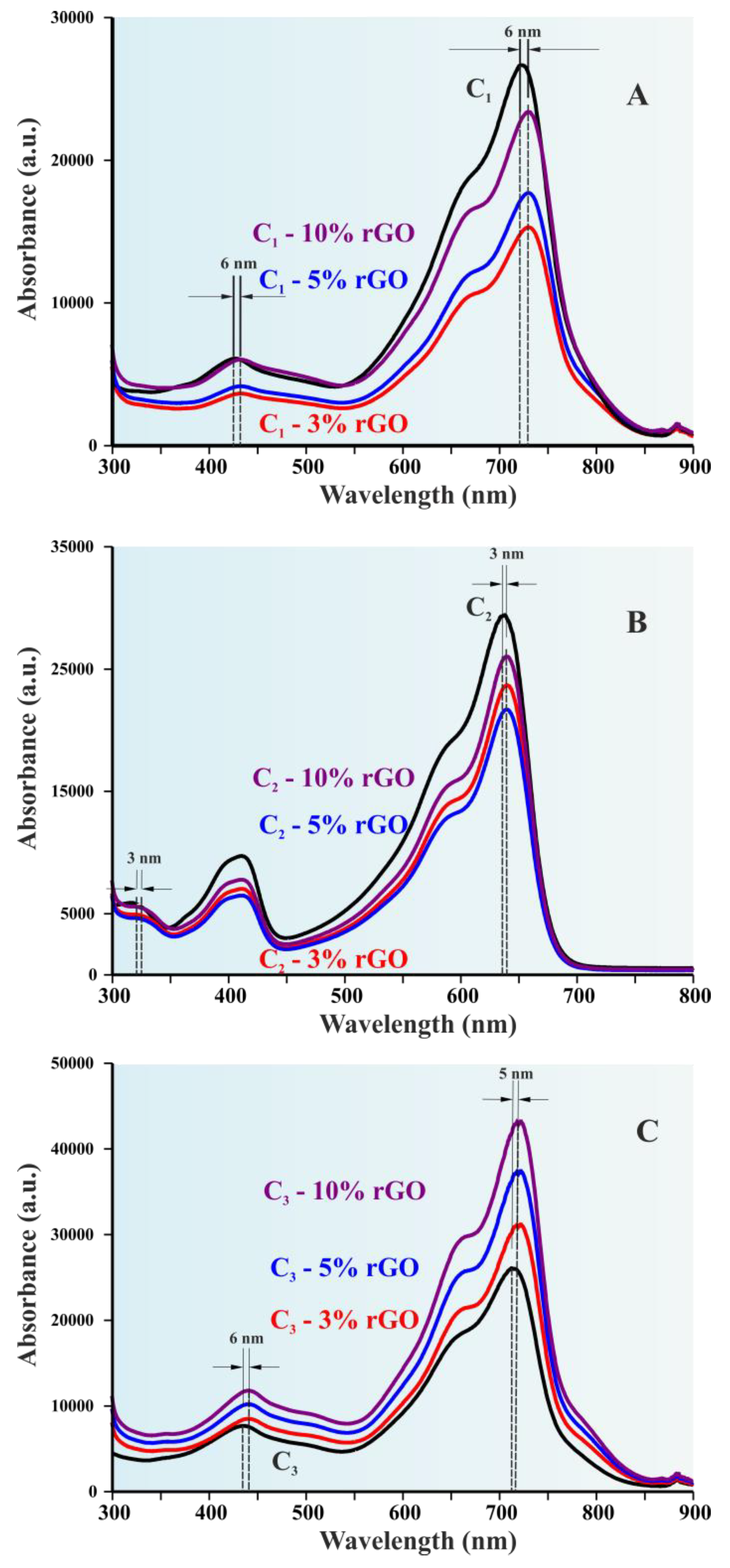
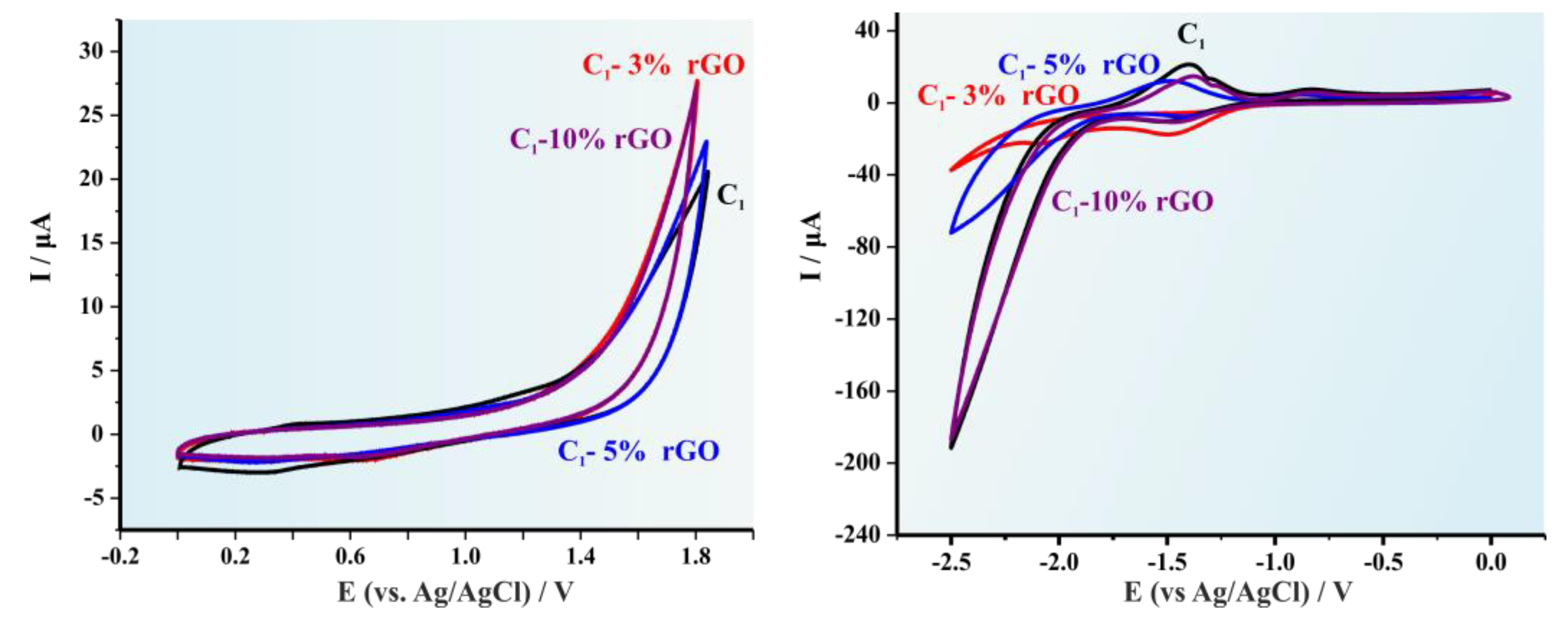
3.3. Optoelectronic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Staudenmaier, L. Method for the Preparation of the Graphite Acid. Eur. J. Inorg. Chem. 1898, 31, 1481–1487. [Google Scholar]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.B.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Politakos, N.; Liontos, G.; Karanastasis, A.; Zapsas, G.; Moschovas, D.; Avgeropoulos, A. Surface Initiated Polymerization from Graphene Oxide. Curr. Org. Chem. 2015, 19, 1757–1772. [Google Scholar] [CrossRef]
- Kern, K.; Gómez-Navarro, C.; Burghard, M.; Weitz, R.T.; Scolari, M.; Mews, A.; Bittner, A.M. Electronic Transport Properties of Individual Chemically Reduced Graphene Oxide Sheets. Nano Lett. 2007, 7, 3499–3503. [Google Scholar]
- Wu, J.; Becerril, H.A.; Bao, Z.; Liu, Z.; Chen, Y.; Peumans, P. Organic solar cells with solution-processed graphene transparent electrodes. Appl. Phys. Lett. 2008, 92, 263302. [Google Scholar] [CrossRef]
- Eda, G.; Lin, Y.-Y.; Miller, S.; Chen, C.-W.; Su, W.-F.; Chhowalla, M. Transparent and conducting electrodes for organic electronics from reduced graphene oxide. Appl. Phys. Lett. 2008, 92, 233305. [Google Scholar] [CrossRef]
- Zhou, M.; Zhai, Y.; Dong, S. Electrochemical sensing and biosensing platform based on chemically reduced graphene oxide. Anal. Chem. 2009, 81, 5603–5613. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Z.; Zhang, X.; Yang, L.; Zhang, N.; Pan, G.; Yin, S.; Chen, Y.; Wei, J. Polymer photovoltaic cells based on solytion-processable graphene and P3HT. Adv. Funct. Mater. 2009, 19, 894–904. [Google Scholar] [CrossRef]
- Zhuang, X.-D.; Chen, Y.; Liu, G.; Li, P.-P.; Zhu, C.-X.; Kang, E.-T.; Noeh, K.-G.; Zhang, B.; Zhu, J.-H.; Li, Y.-X. Conjugated-polymer-functionalized graphene oxide: Synthesis and nonvolatile rewritable memory effect. Adv. Mater. 2010, 22, 1731–1735. [Google Scholar] [CrossRef]
- Qi, X.; Tan, C.; Wei, J.; Zhang, H. Synthesis of graphene-conjugated polymer nanocomposites for electronic device applications. Nanoscale 2013, 5, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, Z.; Zhang, X.; Zhang, N.; Yang, L.; Yin, S.; Chen, Y. Organic photovoltaic cells based on an acceptor of soluble graphene. Appl. Phys. Lett. 2008, 92, 223303. [Google Scholar] [CrossRef]
- Liu, Z.; He, D.; Wang, Y.; Wu, H.; Wang, J. Solution-processable functionalized graphene in donor/acceptor-type organic photovoltaic cells. Sol. Energy Mater. Sol. Cells 2010, 94, 1196–1200. [Google Scholar] [CrossRef]
- Gupta, O.; Roy, S.; Mitra, S. Nanocarbon-immobilized membranes for separation of tetrahydrofuran from water via membrane distillation. ACS Appl. Nano Mater. 2020, 3, 6344–6353. [Google Scholar] [CrossRef]
- Sarapuu, A.; Kibena-Põldsepp, E.; Borghei, M.; Tammeveski, K. Electrocatalysis of oxygen reduction on heteroatom-doped nanocarbons and transition metal-nitrogen-carbon catalysts for alkaline membrane fuel cells. J. Mater. Chem. A 2018, 6, 776–804. [Google Scholar] [CrossRef]
- Tang, C.; Titirici, M.; Zhang, Q. A review of nanocarbons in energy electrocatalysis: Multifunctional substrates and highly active sites. J. Energy Chem. 2017, 26, 1077–1093. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.S. Interlayer Distance Controlled Graphene, Supercapacitor and Method of Producing the Same. U.S. Patent 10,214,422, 26 February 2019. [Google Scholar]
- Panwar, N.; Soehartono, A.M.; Chan, K.K.; Zeng, S.; Xu, G.; Qu, J.; Coquet, P.; Yong, K.-T.; Chen, X. Nanocarbons for biology and medicine: Sensing, imaging, and drug delivery. Chem. Rev. 2019, 119, 9559–9656. [Google Scholar] [CrossRef]
- Han, J.T.; Jang, J.I.; Cho, J.; Hwang, J.Y.; Woo, J.S.; Jeong, H.J.; Jeong, S.Y.; Seo, S.H.; Lee, G.-W. Synthesis of nanobelt-like 1-dimensional silver/nanocarbon hybrid materials for flexible and wearable electronics. Sci. Rep. 2017, 7, 4931. [Google Scholar] [CrossRef]
- Meng, L.; Watson, W.B.; Qin, Y. Hybrid conjugated polymer/magnetic nanoparticle composite nanofibers through cooperative non-covalent interactions. Nanoscale Adv. 2020, 2, 2462–2470. [Google Scholar] [CrossRef]
- Meng, L.; Fan, H.; Lane, J.M.D.; Qin, Y. Bottom-up approaches for precisely nanostructuring hybrid organic/inorganic multi-component composites for organic photovoltaics. MRS Adv. 2020, 5, 2055–2065. [Google Scholar] [CrossRef]
- Liang, L.; Xie, W.; Fang, S.; He, F.; Yin, B.; Tlili, C.; Wang, D.; Qiu, S.; Li, Q. High-efficiency dispersion and sorting of single-walled carbon nanotubes via non-covalent interactions. J. Mater. Chem. C 2017, 5, 11339–11368. [Google Scholar] [CrossRef]
- Girolamo, J.; Reiss, P.; Pron, A. Supramolecularly assembled hybrid materials via molecular recognition between diaminopyrimidine-functionalized poly(hexylthiophene) and thymine-capped CdSe nanocrystals. J. Phys. Chem. C 2007, 111, 14681–14688. [Google Scholar] [CrossRef]
- Carsten, B.; He, F.; Son, H.J.; Xu, T.; Yu, L. Stille polycondensation for synthesis of functional materials. Chem. Rev. 2011, 111, 1493–1528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Smith, J.; Watkins, S.E.; Gysel, R.; McGehee, M.; Salleo, A.; Kirkpatrick, J.; Ashraf, S.; Anthopoulos, T.; Heeney, M.; et al. Indacenodithiophene Semiconducting Polymers for High-Performance, Air-Stable Transistors. J. Am. Chem. Soc. 2010, 132, 11437–11439. [Google Scholar] [CrossRef]
- Chochos, C.L.; Drakopoulou, S.; Katsouras, A.; Squeo, B.M.; Sprau, C.; Colsmann, A.; Gregoriou, V.G.; Cando, A.P.; Allard, S.; Scherf, U.; et al. Beyond Donor–Acceptor (D–A) Approach: Structure–Optoelectronic Properties—Organic Photovoltaic Performance Correlation in New D–A1–D–A2 Low-Bandgap Conjugated Polymers. Macromol. Rapid Commun. 2017, 38, 1600720. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Guo, H.-L.; Tian, L.; Ning, S.-K. An efficient method of producing stable graphene suspensions with less toxicity using dimethyl ketoxime. Carbon 2012, 50, 5351–5358. [Google Scholar] [CrossRef]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef]
- Němeček, D.; Thomas, G.J. Raman Spectroscopy of Viruses and Viral Proteins. In Frontiers of Molecular Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2009; pp. 553–595. [Google Scholar]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-reduction of graphite- and graphene oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Tuncel, D. Non-covalent interactions between carbon nanotubes and conjugated polymers. Nanoscale 2011, 3, 3545–3554. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Gasparini, N.; Katsouras, A.; Prodromidis, M.I.; Avgeropoulos, A.; Baran, D.; Salvador, M.; Fladischer, S.; Spiecker, E.; Chochos, C.L.; Ameri, T.; et al. Photophysics of Molecular-Weight-Induced Losses in Indacenodithienothiophene-Based Solar Cells. Adv. Funct. Mater. 2015, 25, 4898–4907. [Google Scholar] [CrossRef]
- Kausar, A. Conjugated Polymer/Graphene Oxide Nanocomposites. J. Compos. Sci. 2021, 5, 292. [Google Scholar] [CrossRef]
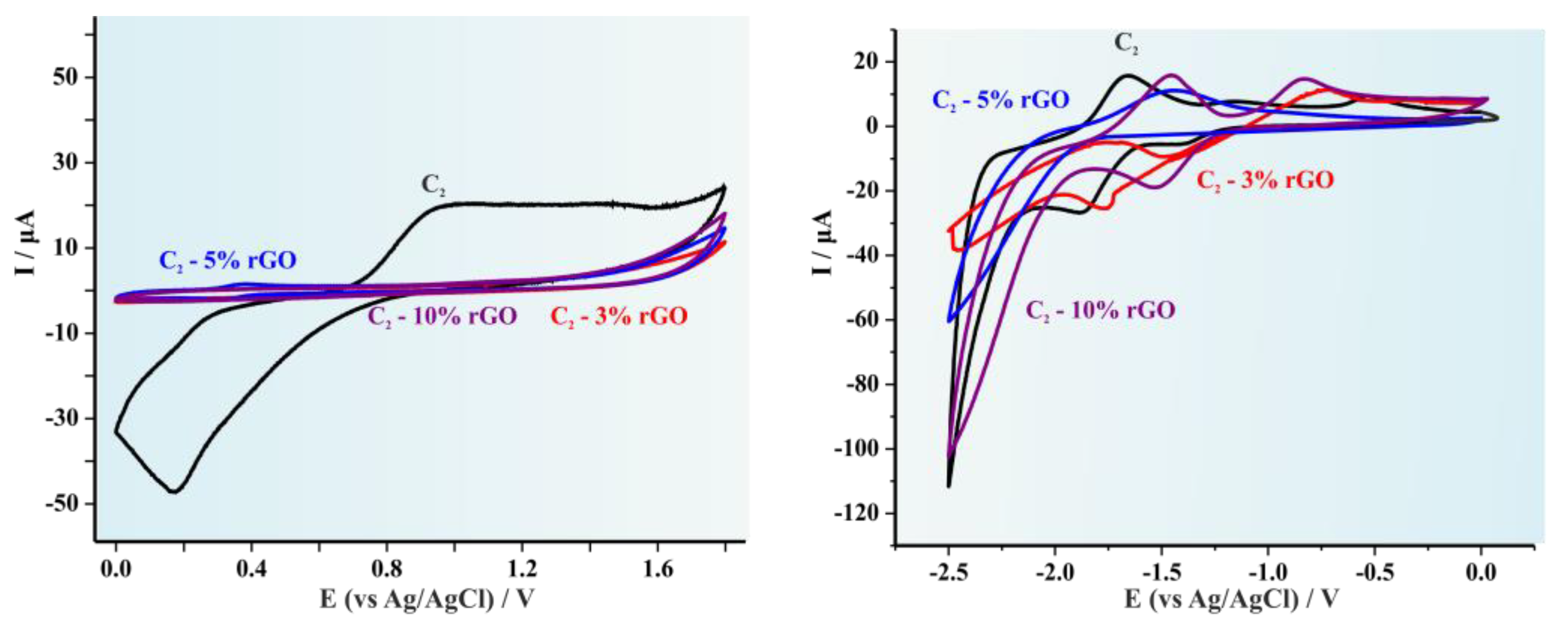
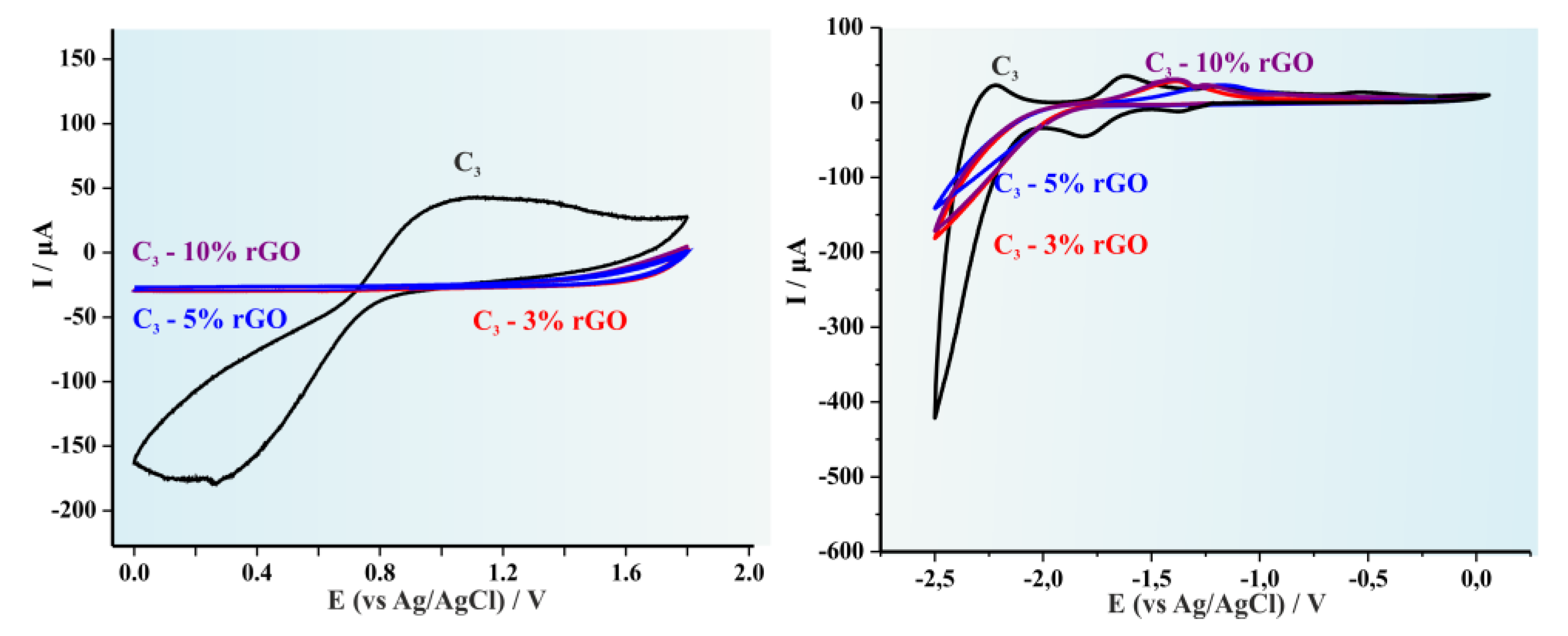
| Copolymer | (kDa) * | Eoxonset (V vs. Fc/Fc+) | Eredonset (V vs. Fc/Fc+) | EHOMO (eV) | ELUMO (eV) | Egec |
|---|---|---|---|---|---|---|
| 1 | 128 | 0.05 | −2.01 | −5.15 | −3.09 | 2.06 |
| 3 %rGO | −0.18 | −1.73 | −4.92 | −3.37 | 1.55 | |
| 5 %rGO | −0.13 | −2.09 | −4.88 | −3.01 | 1.87 | |
| 10 %rGO | 0.11 | −1.95 | −5.21 | −3.15 | 2.06 | |
| 2 | 153 | 0.13 | −2.35 | −5.23 | −2.75 | 2.48 |
| 3 %rGO | −0.16 | −1.88 | −4.94 | −3.22 | 1.72 | |
| 5 %rGO | −0.14 | −1.79 | −4.96 | −3.31 | 1.65 | |
| 10 %rGO | 0.44 | −2.07 | −5.54 | −3.03 | 2.51 | |
| 3 | 150 | 0.55 | −2.04 | −5.65 | −3.06 | 2.59 |
| 3 %rGO | 0.03 | −1.94 | −5.13 | −3.16 | 1.97 | |
| 5 %rGO | 0.01 | −1.59 | −5.11 | −3.51 | 1.60 | |
| 10 %rGO | 0.17 | −1.97 | −5.27 | −3.13 | 2.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazanas, A.C.; Katsouras, A.; Spanos, M.; Manesi, G.-M.; Moutsios, I.; Vashurkin, D.V.; Moschovas, D.; Gioti, C.; Karakassides, M.A.; Gregoriou, V.G.; et al. Synthesis and Characterization of Hybrid Materials Derived from Conjugated Copolymers and Reduced Graphene Oxide. Polymers 2022, 14, 5292. https://doi.org/10.3390/polym14235292
Lazanas AC, Katsouras A, Spanos M, Manesi G-M, Moutsios I, Vashurkin DV, Moschovas D, Gioti C, Karakassides MA, Gregoriou VG, et al. Synthesis and Characterization of Hybrid Materials Derived from Conjugated Copolymers and Reduced Graphene Oxide. Polymers. 2022; 14(23):5292. https://doi.org/10.3390/polym14235292
Chicago/Turabian StyleLazanas, Alexandros Ch., Athanasios Katsouras, Michael Spanos, Gkreti-Maria Manesi, Ioannis Moutsios, Dmitry V. Vashurkin, Dimitrios Moschovas, Christina Gioti, Michail A. Karakassides, Vasilis G. Gregoriou, and et al. 2022. "Synthesis and Characterization of Hybrid Materials Derived from Conjugated Copolymers and Reduced Graphene Oxide" Polymers 14, no. 23: 5292. https://doi.org/10.3390/polym14235292
APA StyleLazanas, A. C., Katsouras, A., Spanos, M., Manesi, G.-M., Moutsios, I., Vashurkin, D. V., Moschovas, D., Gioti, C., Karakassides, M. A., Gregoriou, V. G., Ivanov, D. A., Chochos, C. L., & Avgeropoulos, A. (2022). Synthesis and Characterization of Hybrid Materials Derived from Conjugated Copolymers and Reduced Graphene Oxide. Polymers, 14(23), 5292. https://doi.org/10.3390/polym14235292










