Sunflower Oil as a Renewable Resource for Polyurethane Foams: Effects of Flame-Retardants
Abstract
1. Introduction
2. Experimental Details
2.1. Materials
2.2. Synthesis of Sunflower-Based Polyol
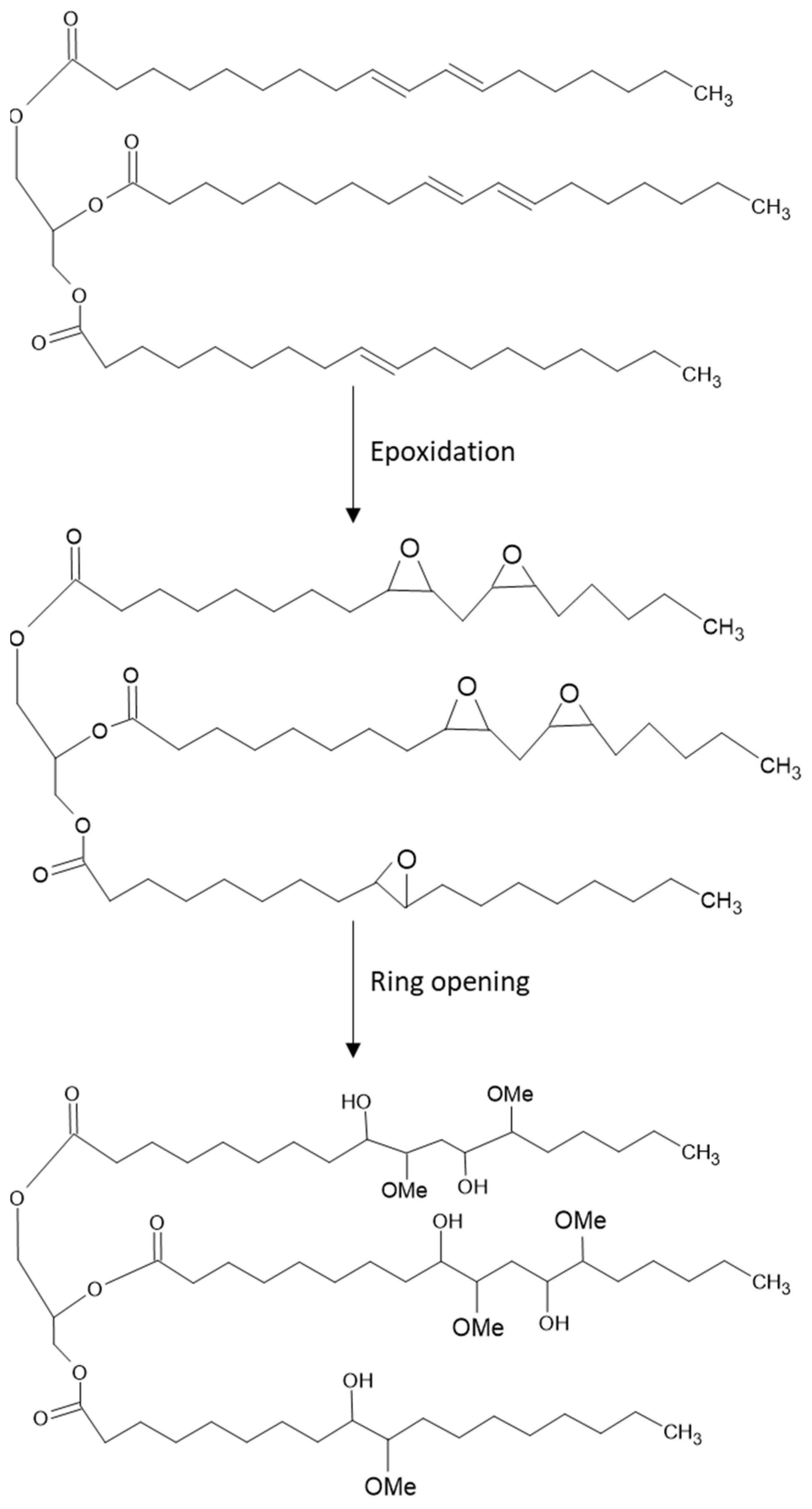
2.2.1. Epoxidation of SFO
2.2.2. Ring Opening of Epoxidized SFO
2.3. Characterization of Epoxide and Polyol
2.4. Preparation of Rigid Polyurethane Foams
2.5. Characterization of Rigid Polyurethane Foams
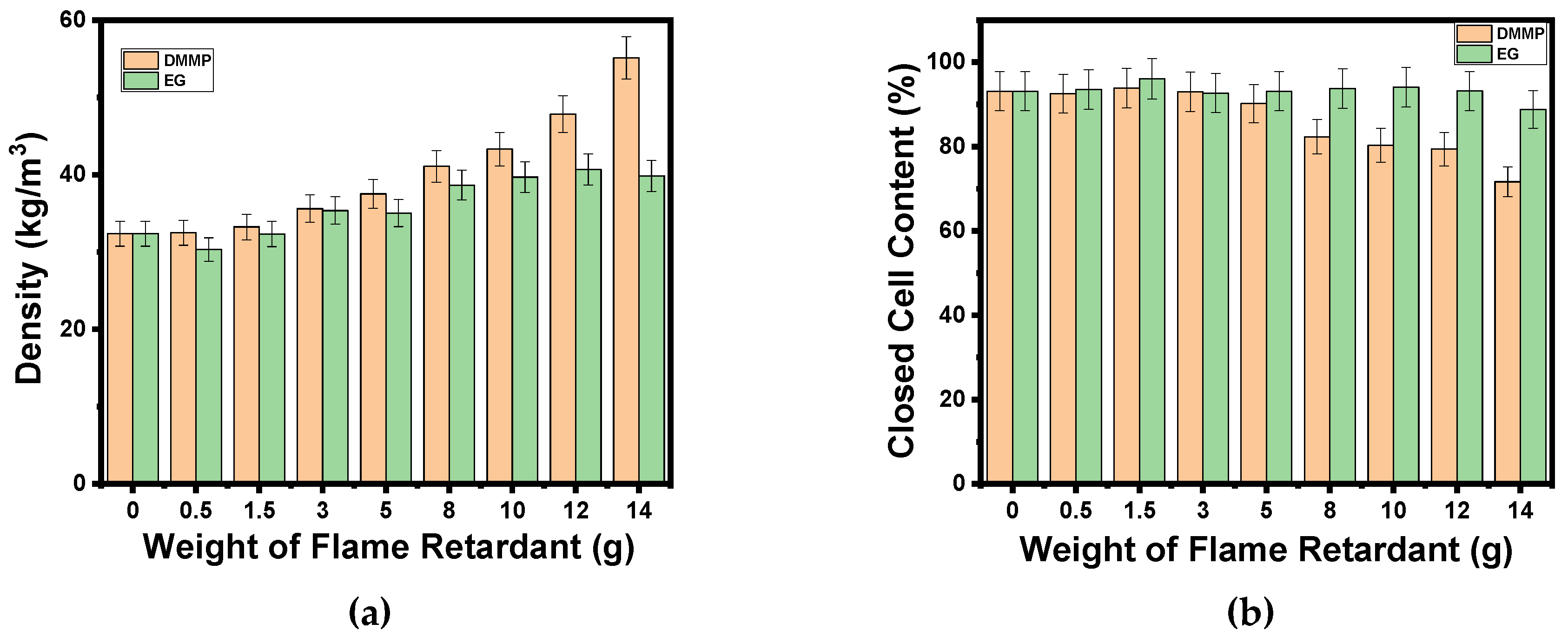
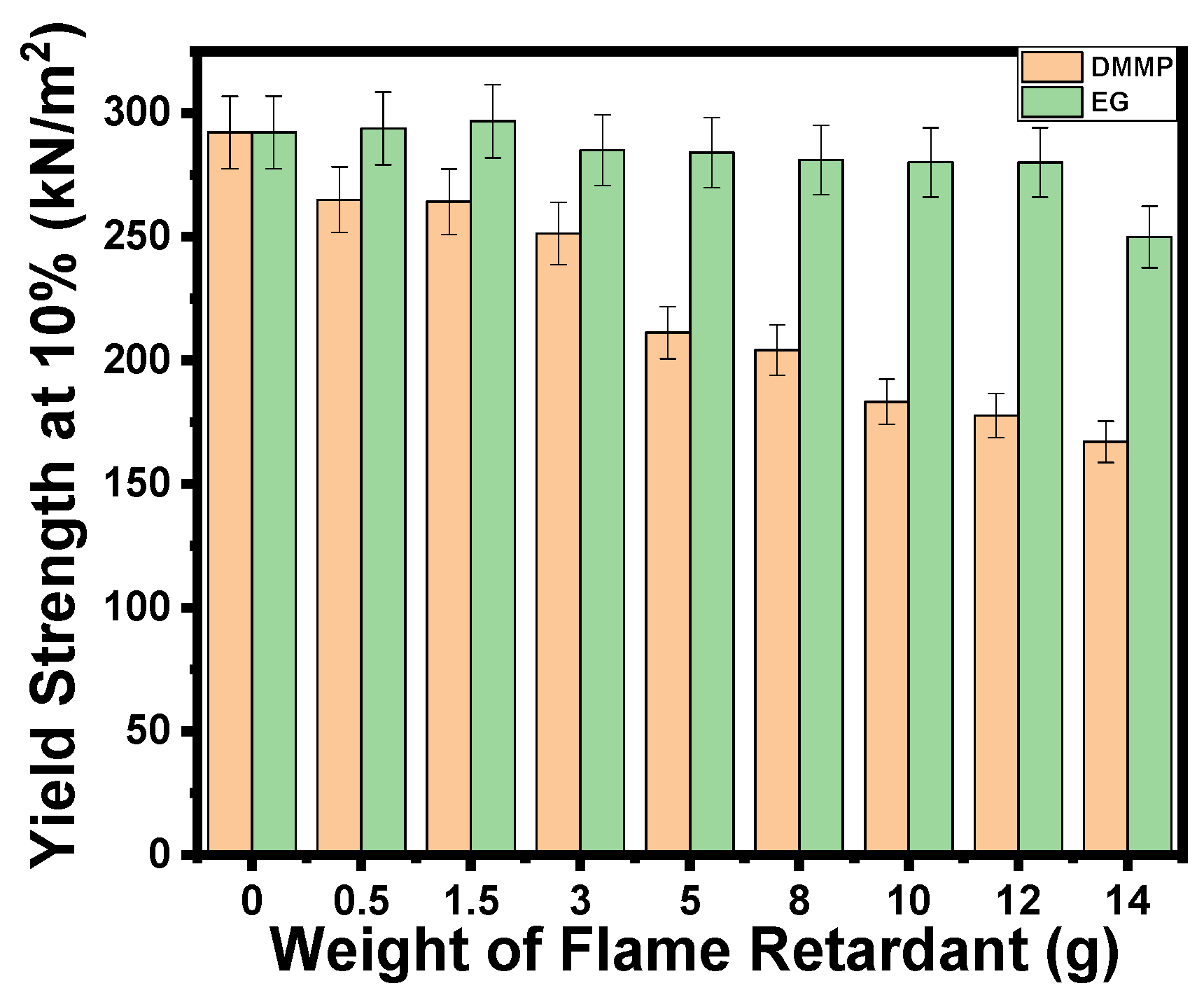
3. Results and Discussion
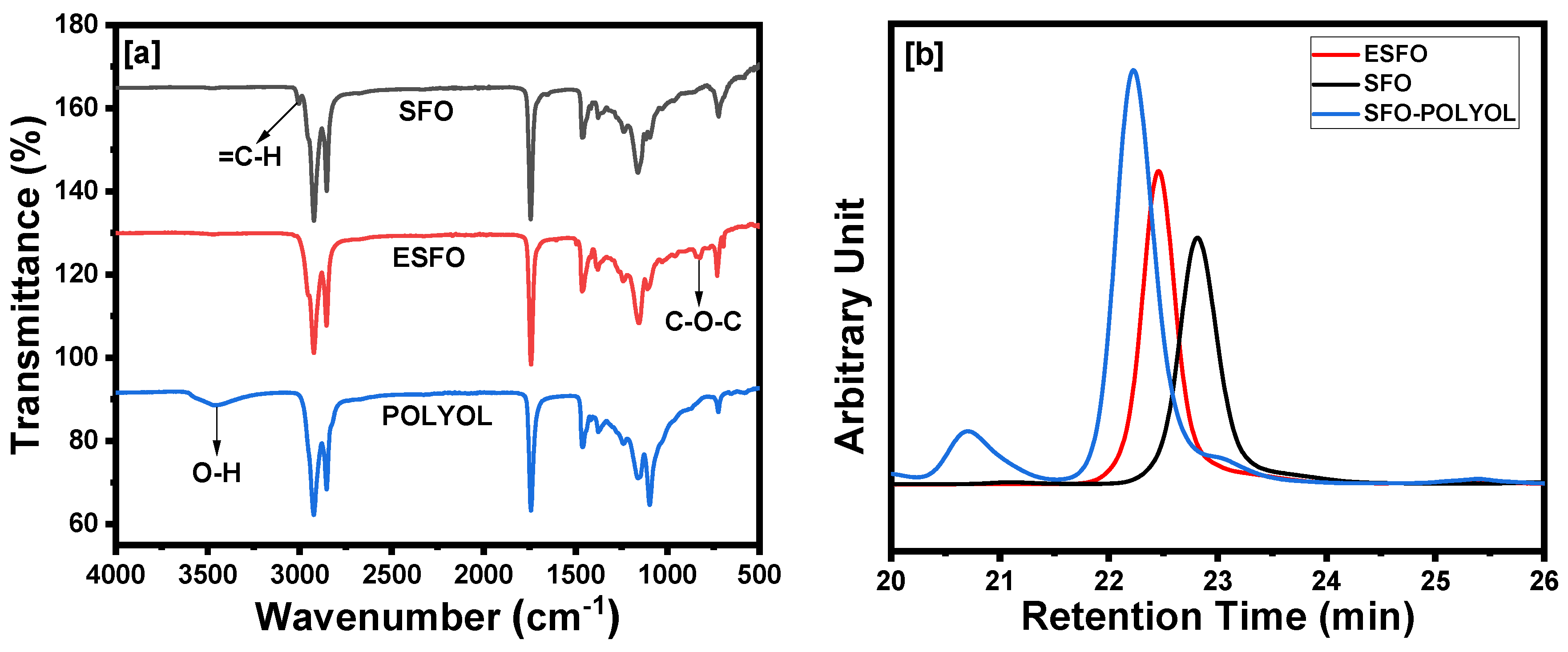
Characterization of Sunflower-Based Epoxide and Polyol
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ugarte, L.; Gómez-Fernández, S.; Peña-Rodríuez, C.; Prociak, A.; Corcuera, M.A.; Eceiza, A. Tailoring Mechanical Properties of Rigid Polyurethane Foams by Sorbitol and Corn Derived Biopolyol Mixtures. ACS Sustain. Chem. Eng. 2015, 3, 3382–3387. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, S. Preparation of Flame-Retardant Rigid Polyurethane Foams by Combining Modified Melamine–Formaldehyde Resin and Phosphorus Flame Retardants. ACS Omega. 2020, 5, 9658–9667. [Google Scholar] [CrossRef] [PubMed]
- Ranaweera, C.K.; Ionescu, M.; Bilic, N.; Wan, X.; Kahol, P.K.; Gupta, R.K. Biobased Polyols Using Thiol-Ene Chemistry for Rigid Polyurethane Foams with Enhanced Flame-Retardant Properties. J. Renew. Mater. 2017, 5, 1–12. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, J.; Hse, C.Y.; Shupe, T.F. Preparation of polyurethane foams using fractionated products in liquefied wood. J. Appl. Polym. Sci. 2014, 131, 1–7. [Google Scholar] [CrossRef]
- He, W.; Kang, P.; Fang, Z.; Hao, J.; Wu, H.; Zhu, Y.; Guo, K. Flow Reactor Synthesis of Bio-Based Polyol from Soybean Oil for the Production of Rigid Polyurethane Foam. Ind. Eng. Chem. Res. 2020, 59, 17513–17519. [Google Scholar] [CrossRef]
- Zhang, C.; Madbouly, S.A.; Kessler, M.R. Biobased polyurethanes prepared from different vegetable oils. ACS Appl. Mater. Interfaces. 2015, 7, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Li, Y.; Huang, S.; Huang, K.; Zeng, X.; Dong, Q.; Liu, C.; Feng, P.; Zhang, C. Tailoring the Performance of Vegetable Oil-Based Waterborne Polyurethanes through Incorporation of Rigid Cyclic Rings into Soft Polymer Networks. ACS Sustain. Chem. Eng. 2020, 8, 914–925. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, C.; Kessler, M.R. Polyols and polyurethanes prepared from epoxidized soybean oil ring-opened by polyhydroxy fatty acids with varying oh numbers. J. Appl. Polym. Sci. 2015, 132, 1–17. [Google Scholar] [CrossRef]
- de Souza, F.M.; Kahol, P.K.; Gupta, R.K. Polyols from Sustainable Resources. In Polyurethane Chemistry: Renewable Polyols and Isocyanates; American Chemical Society: Washington, DC, USA, 2021; pp. 2–25. [Google Scholar]
- Guo, A.; Cho, Y.; Petrović, Z.S. Structure and properties of halogenated and nonhalogenated soy-based polyols. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 3900–3910. [Google Scholar] [CrossRef]
- Ji, D.; Fang, Z.; He, W.; Zhang, K.; Luo, Z.; Wang, T.; Guo, K. Synthesis of soy-polyols using a continuous microflow system and preparation of soy-based polyurethane rigid foams. ACS Sustain. Chem. Eng. 2015, 3, 1197–1204. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Q. Synthesis of Cardanol-Based Polyols via Thiol-ene/Thiol-epoxy Dual Click-Reactions and Thermosetting Polyurethanes Therefrom. ACS Sustain. Chem. Eng. 2018, 6, 12088–12095. [Google Scholar] [CrossRef]
- Fang, Z.; Qiu, C.; Ji, D.; Yang, Z.; Zhu, N.; Meng, J.; Hu, X.; Guo, K. Development of High-Performance Biodegradable Rigid Polyurethane Foams Using Full Modified Soy-Based Polyols. J. Agric. Food Chem. 2019, 67, 2220–2226. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, M.R. Prediction of fatty acid composition of sunflower seeds by near-infrared reflectance spectroscopy. J. Food Sci. Technol. 2018, 55, 2318–2325. [Google Scholar] [CrossRef] [PubMed]
- Ayerdi, G.A.; Larbi, R. Effects of refining process on sunflower oil minor components: A review. OCL 2016, 23, D207. [Google Scholar]
- Liang, Q.; Cui, J.; Li, H.; Liu, J.; Zhao, G. Florets of sunflower (Helianthus annuus L.): Potential new sources of dietary fiber and phenolic acids. J. Agric. Food Chem. 2013, 61, 3435–3442. [Google Scholar] [CrossRef] [PubMed]
- Victor, E.I.; Okechukwu, J.O.; Femi, M.A.; Ogbonneya, O.M. Polyurethane Foam Production using Sunflower oil and Soybean oil as Polyol and Surfactant. Int. J. Mod. Sci. Technol. 2019, 4, 48–53. [Google Scholar]
- Redeuil, K.; Theurillat, X.; Nicolas, M.; Nagy, K. Recommendations for Oil Extraction and Refining Process to Prevent the Formation of Monochloropropane-diol Esters in Sunflower Oil. J. Agric. Food Chem. 2021, 69, 6043–6053. [Google Scholar] [CrossRef]
- Somidi, A.K.R.; Sharma, R.V.; Dalai, A.K. Synthesis of epoxidized canola oil using a sulfated-SnO2 Catalyst. Ind. Eng. Chem. Res. 2014, 53, 18668–18677. [Google Scholar] [CrossRef]
- Dinda, S.; Patwardhan, A.V.; Goud, V.V.; Pradhan, N.C. Epoxidation of cottonseed oil by aqueous hydrogen peroxide catalysed by liquid inorganic acids. Bioresour. Technol. 2008, 99, 3737–3744. [Google Scholar] [CrossRef]
- Aguilera, A.F.; Tolvanen, P.; Heredia, S.; Muñoz, M.G.; Samson, T.; Oger, A.; Verove, A.; Eränen, K.; Leveneur, S.; Mikkola, J.P.; et al. Epoxidation of Fatty Acids and Vegetable Oils Assisted by Microwaves Catalyzed by a Cation Exchange Resin. Ind. Eng. Chem. Res. 2018, 57, 3876–3886. [Google Scholar] [CrossRef]
- Wang, S.; Wang, S.; Shen, M.; Xu, X.; Liu, H.; Wang, D.; Wang, H.; Shang, S. Biobased Phosphorus Siloxane-Containing Polyurethane Foam with Flame-Retardant and Smoke-Suppressant Performances. ACS Sustain. Chem. Eng. 2021, 9, 8623–8634. [Google Scholar] [CrossRef]
- Chen, M.J.; Shao, Z.B.; Wang, X.L.; Chen, L.; Wang, Y.Z. Halogen-free flame-retardant flexible polyurethane foam with a novel nitrogen-phosphorus flame retardant. Ind. Eng. Chem. Res. 2012, 51, 9769–9776. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.; Yang, R. Effects of Dimethyl Methylphosphonate, Aluminum Hydroxide, Ammonium Polyphosphate, and Expandable Graphite on the Flame Retardancy and Thermal Properties of Polyisocyanurate-Polyurethane Foams. Ind. Eng. Chem. Res. 2015, 54, 5876–5884. [Google Scholar] [CrossRef]
- de Souza, F.M.; Choi, J.; Bhoyate, S.; Kahol, P.K.; Gupta, R.K. Expendable Graphite as an Efficient Flame-Retardant for Novel Partial Bio-Based Rigid Polyurethane Foams. C-J. Carbon Res. 2020, 6, 27. [Google Scholar] [CrossRef]
- Benaniba, M.T.; Belhaneche-Bensemra, N.; Gelbard, G. Epoxidation of sunflower oil with peroxoacetic acid in presence of ion exchange resin by various processes. Energy Educ. Sci. Technol. 2008, 21, 71–82. [Google Scholar]
- Guo, A.; Oil, A.; Society, C.; Examiner, P.; Niland, P.D. Process for the Preparation of Vegetable Ol-Based Polyols and Electroninsulating Casting Compounds Created from Vegetable Oil-Based Polyols. U.S. Patent No. 6,107,433, 22 August 2000. [Google Scholar]
- Szycher, M. Szycher’s Handbook of Polyurethanes, 1st ed.; CRC Press: New York, NY, USA, 1999; Available online: https://books.google.com/books?hl=pt-BR&lr=&id=g93wwhc0gr0C&oi=fnd&pg=PA1&dq=Szycher’s+handbook+of+polyurethanes&ots=zz-t5c3gHi&sig=9fPvVzh4M4Ti_wRRsc9MrTXE6MU#v=onepage&q=Szycher’shandbookofpolyurethanes&f=false (accessed on 18 October 2022).
- Liang, P.; Wang, H.; Chen, C.; Ge, F.; Liu, D.; Li, S.; Han, B.; Xiong, X.; Zhao, S. The Use of Fourier Transform Infrared Spectroscopy for Quantifcation of adulteration in Virgin Walnut Oil. J. Spectrosc. 2013, 1, 64–69. [Google Scholar]
- Salim, R.M.; Asik, J.; Sarjadi, M.S. Chemical functional groups of extractives, cellulose and lignin extracted from native Leucaena leucocephala bark. Wood Sci. Technol. 2021, 55, 295–313. [Google Scholar] [CrossRef]
- Arniza, M.Z.; Hoong, S.S.; Idris, Z.; Yeong, S.K.; Hassan, H.A.; Din, A.K.; Choo, Y.M. Synthesis of transesterified palm olein-based Polyol and rigid polyurethanes from this polyol, JAOCS. J. Am. Oil Chem. Soc. 2015, 92, 243–255. [Google Scholar] [CrossRef]
- Gupta, R.K.; Ionescu, M.; Wan, X.; Radojcic, D.; Petroviƈ, Z.S. Synthesis of a Novel Limonene Based Mannich Polyol for Rigid Polyurethane Foams. J. Polym. Environ. 2015, 23, 261–268. [Google Scholar] [CrossRef]
- Lee, S.T.; Ramesh, N.S. Polymeric Foams: Mechanisms and Materials [Book Review], 1st ed.; CRC Press: Boca Raton, FL, USA, 2004; Volume 1, p. 360. [Google Scholar]
- Savas, L.A.; Deniz, T.K.; Tayfun, U.; Dogan, M. Effect of microcapsulated red phosphorus on flame retardant, thermal and mechanical properties of thermoplastic polyurethane composites filled with huntite&hydromagnesite mineral. Polym. Degrad. Stab. 2017, 135, 121–129. [Google Scholar]
- Leszczyńska, A.; Njuguna, J.; Pielichowski, K.; Banerjee, J.R. Polymer/montmorillonite nanocomposites with improved thermal properties: Part I. Factors influencing thermal stability and mechanisms of thermal stability improvement. Thermochim. Acta. 2007, 453, 75–96. [Google Scholar] [CrossRef]
- Sardari, A.; Alvani, A.A.S.; Ghaffarian, S.R. Castor oil-derived water-based polyurethane coatings: Structure manipulation for property enhancement. Prog. Org. Coatings. 2019, 133, 198–205. [Google Scholar] [CrossRef]
- de Souza, F.M.; Kahol, P.K.; Gupta, R.K. Introduction to Polyurethane Chemistry. In Polyurethane Chemistry: Renewable Polyols and Isocyanates; American Chemical Society: Washington, DC, USA, 2021; pp. 1–24. [Google Scholar]
- Czupryński, B.; Paciorek-Sadowska, J.; Liszkowska, J. Properties of rigid polyurethane-polyisocyanurate foams modified with the selected fillers. J. Appl. Polym. Sci. 2010, 115, 2460–2469. [Google Scholar] [CrossRef]
- Agrawal, A.; Kaur, R.; Walia, R.S. Investigation on flammability of rigid polyurethane foam-mineral fillers composite. Fire Mater. 2019, 43, 917–927. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Chen, J.; Mishra, S.R.; Gupta, R.K. Highly flame-retardant polyurethane foam based on reactive phosphorus polyol and limonene-based polyol. J. Appl. Polym. Sci. 2018, 135, 16–19. [Google Scholar] [CrossRef]
- Ramanujam, S.; Zequine, C.; Bhoyate, S.; Neria, B.; Kahol, P.; Gupta, R. Novel Biobased Polyol Using Corn Oil for Highly Flame-Retardant Polyurethane Foams. C 2019, 5, 13. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Hu, L.; Zhou, Y. Synthesis of rigid polyurethane foams with castor oil-based flame retardant polyols. Ind. Crops Prod. 2014, 52, 380–388. [Google Scholar] [CrossRef]
- Lorenzetti, A.; Modesti, M.; Besco, S.; Hrelja, D.; Donadi, S. Influence of phosphorus valency on thermal behaviour of flame retarded polyurethane foams. Polym. Degrad. Stab. 2011, 96, 1455–1461. [Google Scholar] [CrossRef]
- Feng, F.; Qian, L. The flame retardant behaviors and synergistic effect of expandable graphite and dimethyl methylphosphonate in rigid polyurethane foams. Polym. Compos. 2014, 35, 301–309. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Zavargo, Z.; Flyn, J.H.; Macknight, W.J. Thermal degradation of segmented polyurethanes. J. Appl. Polym. Sci. 1994, 51, 1087–1095. [Google Scholar] [CrossRef]
- Modesti, M.; Lorenzetti, A.; Simioni, F.; Camino, G. Expandable graphite as an intumescent flame retardant in polyisocyanurate–polyurethane foams. Polym. Degrad. Stab. 2002, 77, 195–202. [Google Scholar] [CrossRef]
- Modesti, M.; Lorenzetti, A. Halogen-free flame retardants for polymeric foams. Polym. Degrad. Stab. 2002, 78, 167–173. [Google Scholar] [CrossRef]
- Camino, G.; Duquesne, S.; Delobel, R.; Eling, B.; Lindsay, C.; Roels, T. Mechanism of Expandable Graphite Fire Retardant Action in Polyurethanes. ACS Symp. Ser. 2001, 797, 90–109. [Google Scholar]
- Camino, G.; Costa, L.; di Cortemiglia, M.P.L. Overview of fire retardant mechanisms. Polym. Degrad. Stab. 1991, 33, 131–154. [Google Scholar] [CrossRef]
- de Souza, F.M.; Gupta, R.K.; Kahol, P.K. Recent Development on Flame Retardants for Polyurethanes. In Polyurethane Chemistry: Renewable Polyols and Isocyanates; American Chemical Society: Washington, DC, USA, 2021; pp. 187–223. [Google Scholar]
- Yang, L.; Shroll, R.M.; Zhang, J.; Lourderaj, U.; Hase, W.L. Theoretical investigation of mechanisms for the gas-phase unimolecular decomposition of DMMP. J. Phys. Chem. A. 2009, 113, 13762–13771. [Google Scholar] [CrossRef] [PubMed]
- Weil, E.D.; Levchik, S.V. Phosphorus Flame Retardants. Kirk-Othmer Encycl. Chem. Technol. 2000, 2017, 1–34. [Google Scholar]
- Gu, L.; Luo, Y. Flame retardancy and thermal decomposition of phosphorus-containing waterborne polyurethanes modified by halogen-free flame retardants. Ind. Eng. Chem. Res. 2015, 54, 2431–2438. [Google Scholar] [CrossRef]
- Wang, W.; He, K.; Dong, Q.; Zhu, N.; Fan, Y.; Wang, F.; Xia, Y.; Li, H.; Wang, J.; Yuan, Z.; et al. Synergistic effect of aluminum hydroxide and expandable graphite on the flame retardancy of polyisocyanurate–polyurethane foams. J. Appl. Polym. Sci. 2014, 131, 4. [Google Scholar] [CrossRef]
- Acuña, P.; Li, Z.; Santiago-Calvo, M.; Villafañe, F.; Rodríguez-Perez, M.á.; Wang, D.Y. Influence of the characteristics of expandable graphite on the morphology, thermal properties, fire behaviour and compression performance of a rigid polyurethane foam. Polymers 2019, 11, 168. [Google Scholar] [CrossRef]

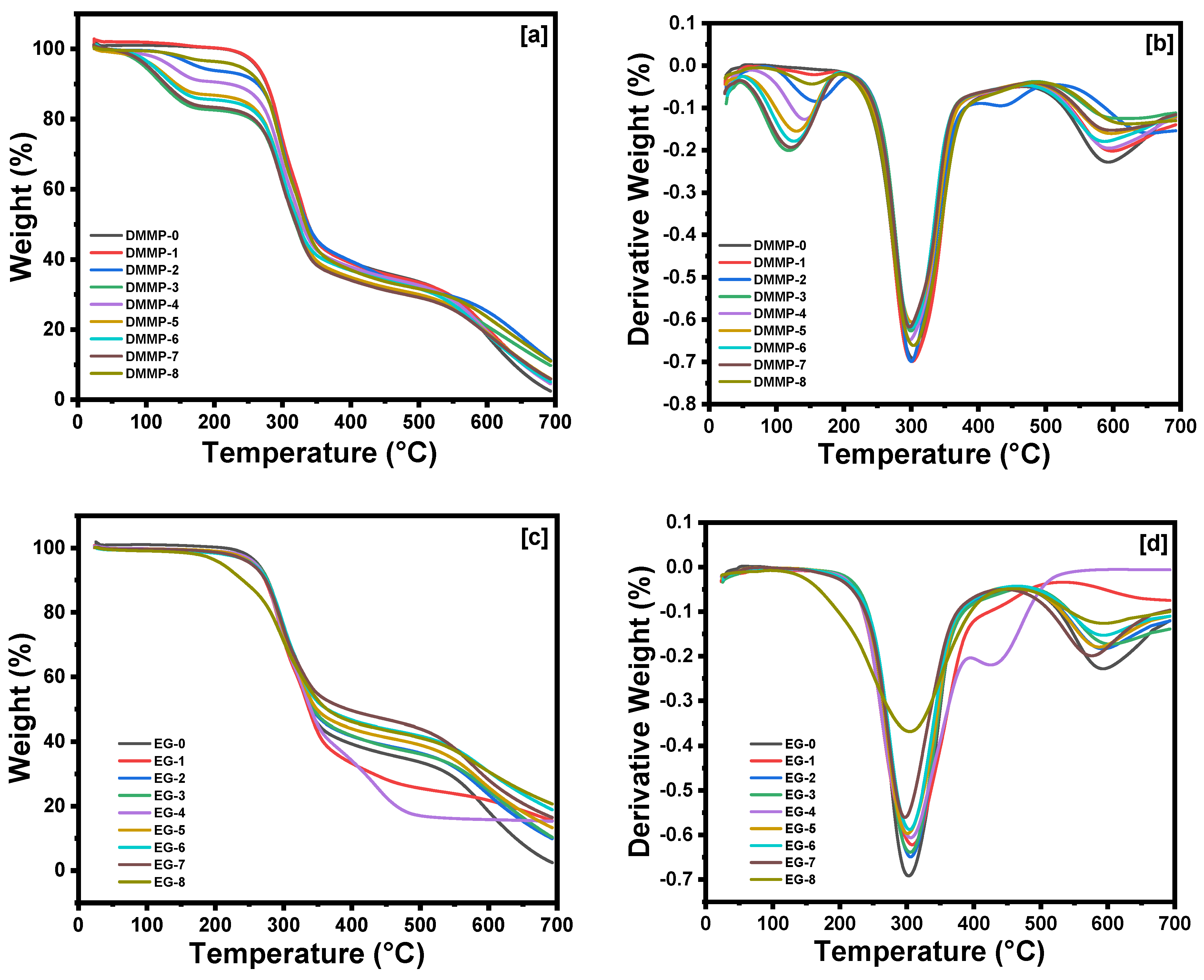
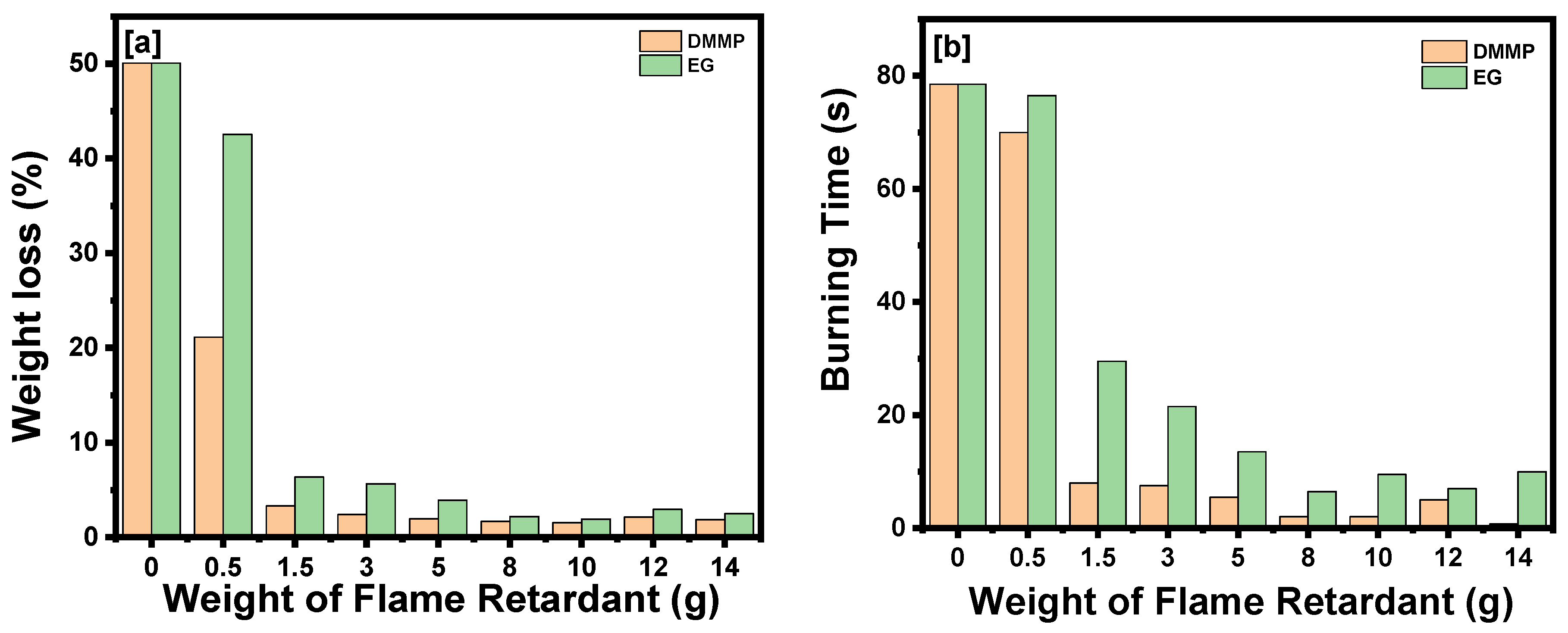

| Sample Code | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|---|
| DMMP or EG (g) | 0 | 0.5 | 1.5 | 3 | 5 | 8 | 10 | 12 | 14 |
| wt.% | 0 | 0.97 | 2.87 | 5.57 | 8.96 | 13.61 | 16.44 | 19.1 | 21.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asare, M.A.; Kote, P.; Chaudhary, S.; de Souza, F.M.; Gupta, R.K. Sunflower Oil as a Renewable Resource for Polyurethane Foams: Effects of Flame-Retardants. Polymers 2022, 14, 5282. https://doi.org/10.3390/polym14235282
Asare MA, Kote P, Chaudhary S, de Souza FM, Gupta RK. Sunflower Oil as a Renewable Resource for Polyurethane Foams: Effects of Flame-Retardants. Polymers. 2022; 14(23):5282. https://doi.org/10.3390/polym14235282
Chicago/Turabian StyleAsare, Magdalene A., Prashant Kote, Sahilkumar Chaudhary, Felipe M. de Souza, and Ram K. Gupta. 2022. "Sunflower Oil as a Renewable Resource for Polyurethane Foams: Effects of Flame-Retardants" Polymers 14, no. 23: 5282. https://doi.org/10.3390/polym14235282
APA StyleAsare, M. A., Kote, P., Chaudhary, S., de Souza, F. M., & Gupta, R. K. (2022). Sunflower Oil as a Renewable Resource for Polyurethane Foams: Effects of Flame-Retardants. Polymers, 14(23), 5282. https://doi.org/10.3390/polym14235282






