Dope Dyeing of Regenerated Cellulose Fibres with Leucoindigo as Base for Circularity of Denim
Abstract
1. Introduction
- -
- Cotton fibres are short staple fibres with a fibre length up to 50 mm. The direct fibre recycling would be favourable from the point of a life cycle analysis (LCA) [7]. However, the short fibre length and the high twist in the yarn prevent a simple re-opening of a cotton textile into individual cotton fibres with a final fibre length of 20 mm and higher. Shorter fibre lengths limit yarn production to high yarn counts with comparable low mechanical strength [8].
- -
- The complex chemical structure of cellulose prevents recycling via depolymerisation and polymerisation [9].
- The viscose process is a robust technology. Information is required if and how indigo dyeing could be integrated into the production of regenerated cellulose fibres to serve as feedstock for denim products.
- During dope formation, the cellulose matrix dissolves and the indigo dye is released into the spin dope. Information is required if agglomeration of the molecular disperse indigo blue leads to coagulates which would prevent fibre spinning. Intensive bleeding of dye into the acidic coagulation bath also would prevent future technical implementation.
- For the denim market, dark blue fibres are needed. Regenerated fibres from denim textiles are light blue as dye loss may be expected during the use phase of garments. Could the colour depth of the regenerated fibres be increased at the stage of fibre production by addition of indigo dye to produce dark blue fibres, which then makes the environmentally unfavourable warp dyeing process obsolete?
2. Experimental
2.1. Chemicals and Materials
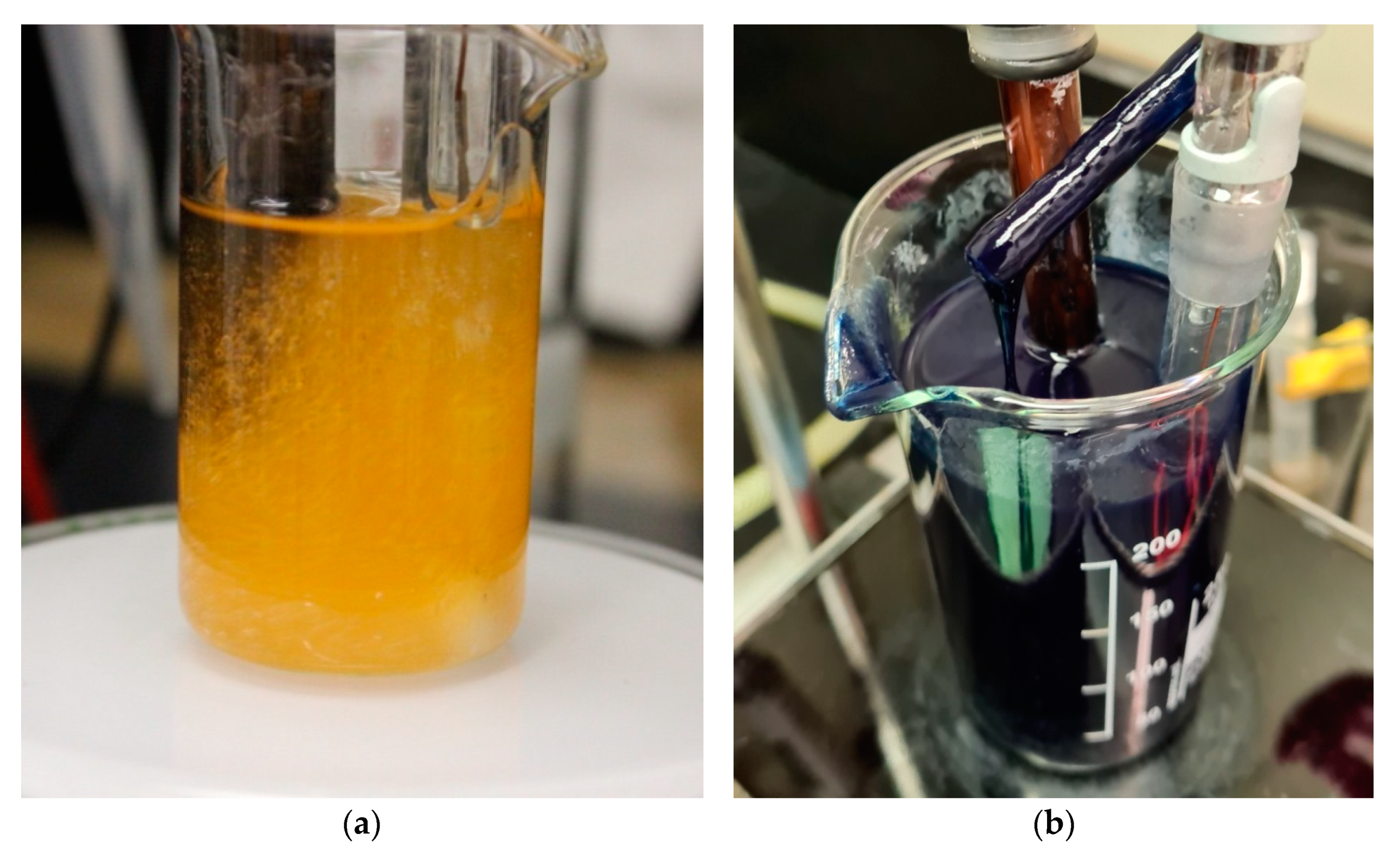
2.2. Preparation of Indigo Dyed Viscose
2.3. Cellulose Dissolution and Regeneration
2.3.1. Continuous Monitoring of the Redox Potential
2.3.2. Variations of the Regeneration Bath
- (a)
- 10 wt% H2SO4
- (b)
- 10 wt% H2SO4 + 10 wt% Na2SO4
- (c)
- 10 wt% H2SO4 + 10 wt% Na2SO4 + 5 wt% ZnSO4
- (d)
- 10 wt% H2SO4 + 10 wt% Na2SO4 + 5 wt% ZnSO4 + 2 wt% glucose
2.3.3. Variations of Carbon Disulphide Amounts Added in the Xanthation Step
2.3.4. Regeneration from Mixture of Dyed and Undyed Cellulose Fibres
2.3.5. Addition of Pre-Reduced Indigo in the Viscose Process
2.4. Analysis of Indigo Content
2.5. Colour Measurement and Determination of K/S Value
2.6. Determination of Indigo Particle Size
3. Results and Discussion
3.1. Indigo Dyeing of Regenerated Cellulose Fibres
3.2. Compatibility of Indigo Dye with the Viscose Process
3.3. Dissolution and Regeneration of Indigo Dyed CV
3.4. Addition of Indigo to the Spin Dope
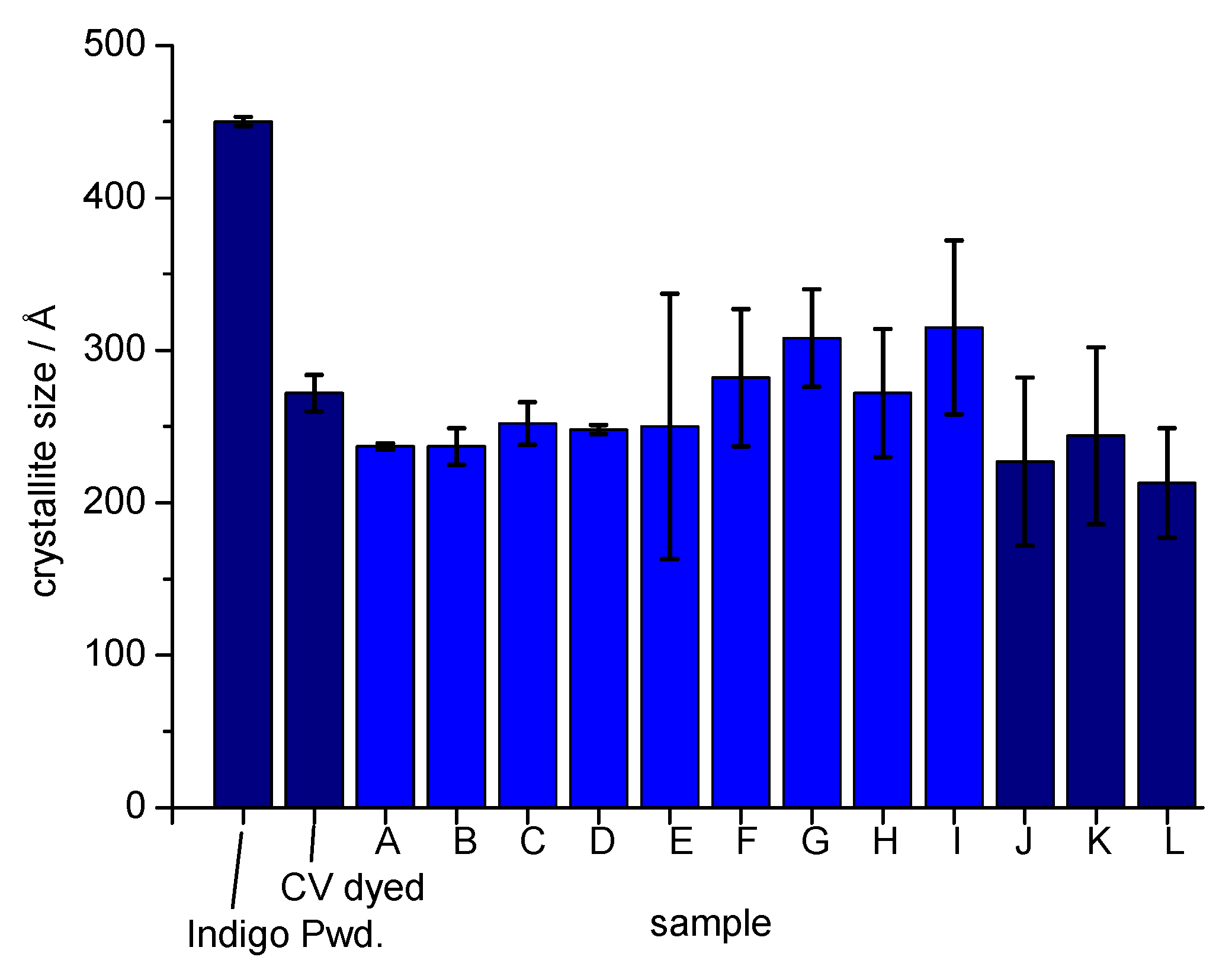
3.5. Determination of Indigo Crystallite Size
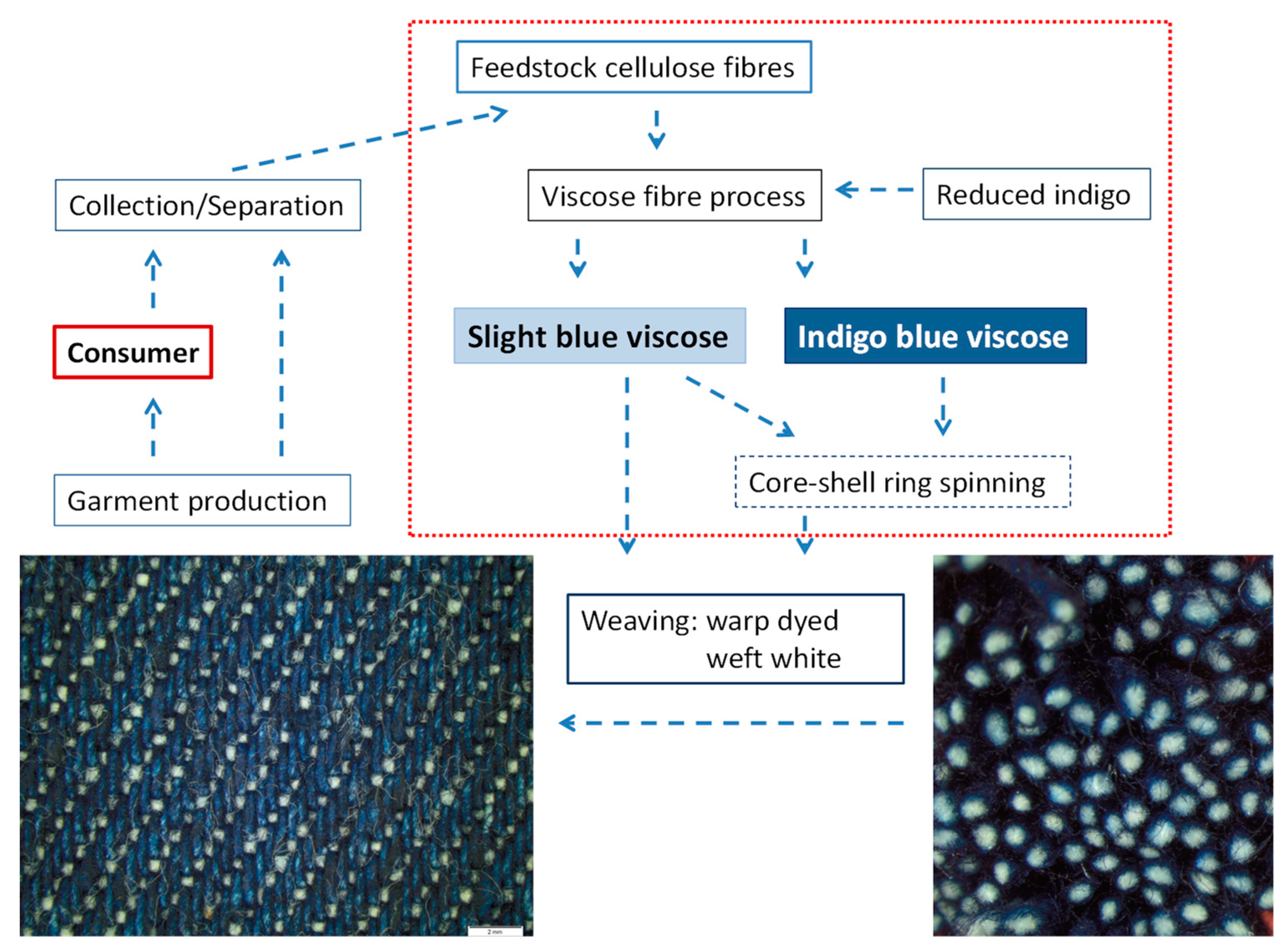
4. Process Balances—Model Calculations
4.1. Impact of Circularity in Denim on Cotton Consumption
4.2. Combination of Indigo Dyeing and Viscose Fibre Technology
4.3. Savings through Omission of Yarn Dyeing
5. Conclusions
- Extension of the concept to other techniques of regenerated cellulose fibre production e.g., use of ionic liquids.
- Investigation of the behaviour of sulphur dyes during fibre regeneration as these dyes often are used for topping or bottoming of indigo dyed yarn.
- Evaluation of techniques to separate other minor fibre components e.g., elastane fibres, polyester sewing threads during fibre regeneration. A technique to separate elastane from other fibres already is in experimental evaluation.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Commission. EU Strategy for Sustainable and Circular Textiles; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Duhoux, T.; Maes, E.; Hirschnitz-Garbers, M.; Peeters, K.; Asscherickx, L.; Christis, M.; Stubbe, B.; Colignon, P.; Hinzmann, M.; Sachdeva, A. Study on the Technical, Regulatory, Economic and Environmental Effectiveness of Textile Fibres Recycling; Final Report to the European Commission, Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs; European Commission: Brussels, Belgium, 2021. [Google Scholar] [CrossRef]
- Nørup, N.; Pihl, K.; Damgaard, A.; Scheutz, C. Evaluation of a European textile sorting centre: Material flow analysis and life cycle inventory. Resour. Conserv. Recycl. 2019, 143, 310–319. [Google Scholar] [CrossRef]
- De Oliveira Neto, G.C.; Correia, J.M.F.; Silva, P.C.; de Oliveira Sanches, A.G.; Lucato, W.C. Cleaner Production in the textile industry and its relationship to sustainable development goals. J. Clean. Prod. 2019, 228, 1514–1525. [Google Scholar] [CrossRef]
- Yousef, S.; Tatariants, M.; Tichonovas, M.; Sarwar, Z.; Jonuškienė, I.; Kliucininkas, L. A new strategy for using textile waste as a sustainable source of recovered cotton. Resour. Conserv. Recycl. 2019, 145, 359–369. [Google Scholar] [CrossRef]
- Action, P. The Deadly Chemicals in Cotton; Environmental Justice Foundation in Collaboration with Pesticide Action Network UK: London, UK, 2007. [Google Scholar]
- Esteve-Turrillas, F.; de la Guardia, M. Environmental impact of Recover cotton in textile industry. Resour. Conserv. Recycl. 2017, 116, 107–115. [Google Scholar] [CrossRef]
- Aronsson, J.; Persson, A. Tearing of post-consumer cotton T-shirts and jeans of varying degree of wear. J. Eng. Fibers Fabr. 2020, 15, 1–9. [Google Scholar] [CrossRef]
- Klemm, D.; Philipp, B.; Heinze, T.; Heinze, U.; Wagenknecht, W. Comprehensive Cellulose Chemistry; Volume I: Fundamentals and Analytical Methods; Wiley-VCH Verlag: Weinheim, Germany, 1998. [Google Scholar]
- Manian, A.P.; Pham, T.; Bechtold, T. Regenerated cellulosic fibers. In Handbook of Properties of Textile and Technical Fibres, 2nd ed.; Bunsell, A.R., Ed.; The Textile Institute Book Series; Woodhead Publishing: Cambridge, UK, 2018; pp. 329–343. [Google Scholar] [CrossRef]
- Munasinghe, P.; Druckman, A.; Dissanayake, D. A systematic review of the life cycle inventory of clothing. J. Clean. Prod. 2021, 320, 128852. [Google Scholar] [CrossRef]
- Shen, L. Bio-Based and Recycled Polymers for Cleaner Production An Assessment of Plastics and Fibres; Utrecht University: Utrecht, The Netherlands, 2011. [Google Scholar]
- Haslinger, S.; Hummel, M.; Anghelescu-Hakala, A.; Määttänen, M.; Sixta, H. Upcycling of cotton polyester blended textile waste to new man-made cellulose fibers. Waste Manag. 2019, 97, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Han, W.; Xu, Z.; Zhang, J.; Kong, F.; Zhang, J.; Zhang, X.; Jia, F. Complete recycling and valorization of waste textiles for value-added transparent films via an ionic liquid. J. Environ. Chem. Eng. 2021, 9, 106182. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Liu, T.; Liu, T.; Zhang, J.; Liu, H. Eco-friendly post-consumer cotton waste recycling for regenerated cellulose fibers. Carbohydr. Polym. 2019, 206, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhang, L. Rapid Dissolution of Cellulose in LiOH/Urea and NaOH/Urea Aqueous Solutions. Macromol. Biosci. 2005, 5, 539–548. [Google Scholar] [CrossRef] [PubMed]
- El Seoud, O.A.; Kostag, M.; Jedvert, K.; Malek, N.I. Cellulose Regeneration and Chemical Recycling: Closing the “Cellulose Gap” Using Environmentally Benign Solvents. Macromol. Mater. Eng. 2020, 305, 1900832. [Google Scholar] [CrossRef]
- Makarov, I.S.; Golova, L.K.; Smyslov, A.G.; Vinogradov, M.I.; Palchikova, E.E.; Legkov, S.A. Flax Noils as a Source of Cellulose for the Production of Lyocell Fibers. Fibers 2022, 10, 45. [Google Scholar] [CrossRef]
- Bechtold, T.; Pham, T. Textile Chemistry; De Gruyter: Berlin, Germany; Boston, MA, USA, 2019. [Google Scholar]
- Akı, S.U.; Candan, C.; Nergis, B.; Önder, N.S. Understanding Denim Recycling: A Quantitative Study with Lifecycle Assessment Methodology. In Waste in Textile and Leather Sectors; Körlü, A., Ed.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Paul, R.; Blackburn, R.S.; Bechtold, T. Indigo and Indigo Colorants. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag: Weinheim, Germany, 2021; pp. 1–16. [Google Scholar] [CrossRef]
- Contin, B.; Kohan, L.; Duarte, L.O.; Fernandes, P.R.B.; Ruchel-Soares, R.; Siqueira, M.U.; Baruque-Ramos, J. Brazilian Cotton Jeans Recycling: Characterization of Shredded Pre-consumer Waste. Mater. Circ. Econ. 2022, 4, 1–10. [Google Scholar] [CrossRef]
- Manian, A.P.; Ruef, H.; Bechtold, T. Mass Coloration of Regenerated Cellulosics—A Review. Lenzing. Ber. 2006, 85, 87–90. [Google Scholar]
- Haslinger, S.; Wang, Y.; Rissanen, M.; Lossa, M.B.; Tanttu, M.; Ilen, E.; Määttänen, M.; Harlin, A.; Hummel, M.; Sixta, H. Recycling of vat and reactive dyed textile waste to new colored man-made cellulose fibers. Green Chem. 2019, 21, 5598–5610. [Google Scholar] [CrossRef]
- Regenerated Cellulosic Fibres: Viscose, Modal, Lyocell, Cupro, (Tri-)Acetate. Available online: https://win-win.info/index.php/sustainable-concepts/regenerated-cellulosic-fibres/ (accessed on 12 February 2022).
- Opperskalski, S.; Ridler, S.J.; Siew, S.; Tan, E.; Barsley, L.; Denes, H.; Gillespie, A.; Gosai, A.; Heaton, A.; Hyde, T.; et al. Preferred Fiber and Materials—Market Report 2021. Text. Exch. 2021, 1–118. [Google Scholar]
- Zheng, J.; Wang, Y.; Zhang, Q.; Wang, D.; Wang, S.; Jiao, M. Study on structure and properties of natural indigo spun-dyed viscose fiber. e-Polymers 2021, 21, 327–335. [Google Scholar] [CrossRef]
- Abu-Rous, M.; Öztürk, H.B.; Kininmonth, M.A.; Sandhofer, A.; Schuster, K.C. Spundyed Cellulosic Fibre. WO2021224112A1, 5 April 2020. [Google Scholar]
- Manian, A.P.; Paul, B.; Lanter, H.; Bechtold, T.; Pham, T. Cellulose Fibre Degradation in Cellulose/Steel Hybrid Geotextiles under Outdoor Weathering Conditions. Polymers 2022, 14, 4179. [Google Scholar] [CrossRef]
- BASF. BASF Ratgeber Cellulosefasern: Schlichten, Vorbehandeln, Färben (B 375 d/B 77), B 375 d; BASF AG: Ludwigshafen, Germany, 1977. [Google Scholar]
- Krässig, H.; Schurz, J.; Steadman, R.; Schliefer, K.; Albrecht, W.; Mohring, M.; Schlosser, H. Cellulose. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Alexandre, S.C.D. Pre-Treatment of Pre-and Post-Consumer Viscose Textile Waste for the Purpose of Upcycling to High Quality Textile Fibres via Ioncell ® Process; Tecnico Lisboa: Lisbon, Portugal, 2021. [Google Scholar]
- Scherrer, P. Of the Size and Internal Structure of Colloidal Particles by Means of Rontgen Rays. Nachr. Ges. Wiss. Gott. 1918, 2, 96–100. [Google Scholar]
- Langford, J.I.; Wilson, A.J.C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Bechtold, T.; Turcanu, A.; Geissler, S.; Ganglberger, E. Process balance and product quality in the production of natural indigo from Polygonum tinctorium Ait. applying low-technology methods. Bioresour. Technol. 2002, 81, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Abdelileh, M.; Manian, A.P.; Rhomberg, D.; Ben Ticha, M.; Meksi, N.; Aguiló-Aguayo, N.; Bechtold, T. Calcium-iron-D-gluconate complexes for the indirect cathodic reduction of indigo in denim dyeing: A greener alternative to non-regenerable chemicals. J. Clean. Prod. 2020, 266, 121753. [Google Scholar] [CrossRef]
- Bechtold, T.; Berktold, F.; Turcanu, A. The redox behaviour of CI Sulphur Black 1—A’basis for improved understanding of sulphur dyeing. Color. Technol. 2000, 116, 215–221. [Google Scholar] [CrossRef]
- Wolf, A. A new crystalline phase of indigo. Die Naturwissenschaften 1980, 67, 453. [Google Scholar] [CrossRef]
- Kettner, F.; Hüter, L.; Schäfer, J.; Röder, K.; Purgahn, U.; Krautscheid, H. Selective crystallization of indigo B by a modified sublimation method and its redetermined structure. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, o2867. [Google Scholar] [CrossRef]
- Ütebay, B.; Çelik, P.; Çay, A. Effects of cotton textile waste properties on recycled fibre quality. J. Clean. Prod. 2019, 222, 29–35. [Google Scholar] [CrossRef]
- Bálint, A. Business Unit Textile Chemicals. Denim Book—From Cotton to Fashion; Clariant International Ltd.: Singapore; Muttenz, Switzerland, 2012. [Google Scholar]
- Esin, S.; Osman, B. Fatigue behaviour of core-spun yarns containing filament by means of cyclic dynamic loading. IOP Conf. Series: Mater. Sci. Eng. 2017, 254, 082012. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, X.; Ding, X. Carbon and water footprints assessment of cotton jeans using the method based on modularity: A full life cycle perspective. J. Clean. Prod. 2022, 332, 130042. [Google Scholar] [CrossRef]
- Saikhao, L.; Setthayanond, J.; Karpkird, T.; Bechtold, T.; Suwanruji, P. Green reducing agents for indigo dyeing on cotton fabrics. J. Clean. Prod. 2018, 197, 106–113. [Google Scholar] [CrossRef]
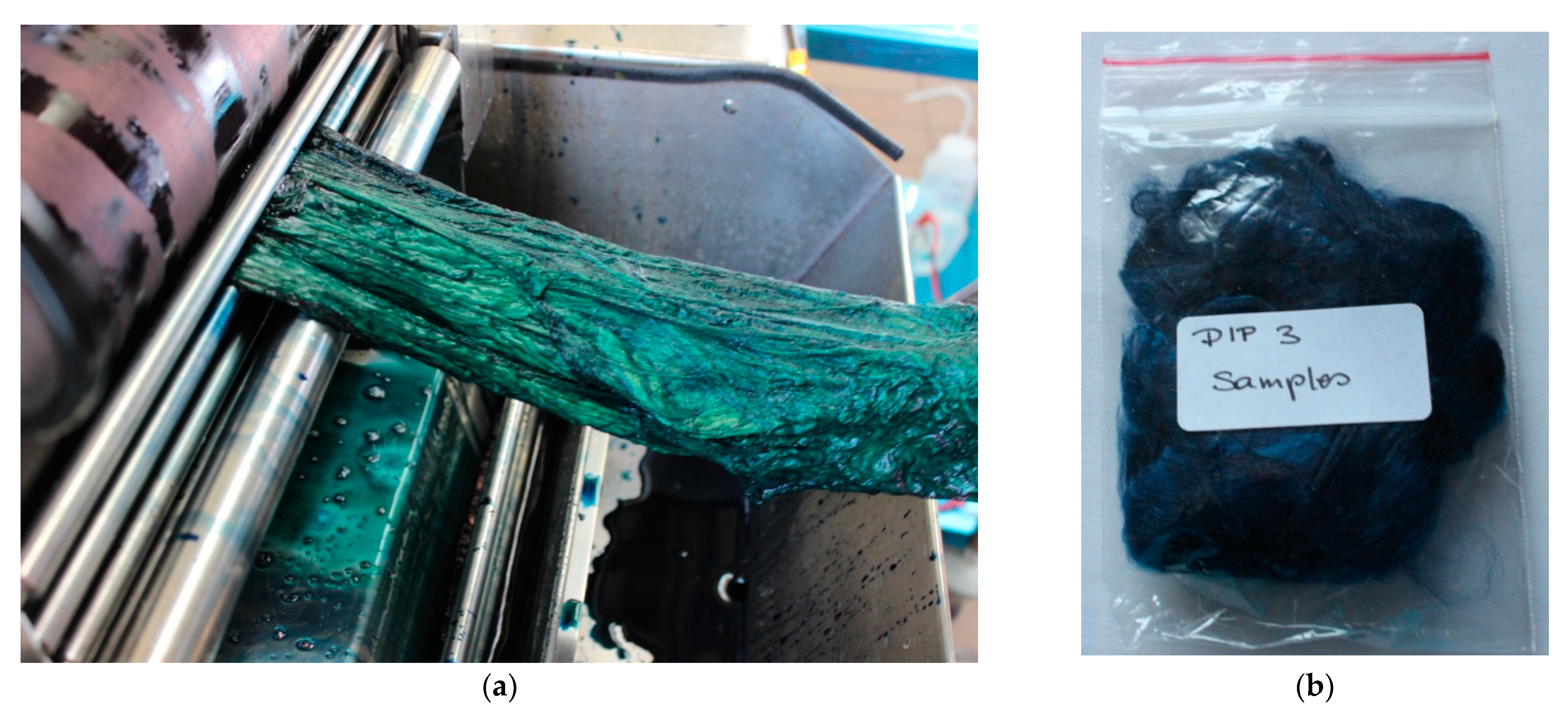

 ) undyed CV1, (
) undyed CV1, ( ) 3 dip dyed CV1.
) 3 dip dyed CV1.
 ) undyed CV1, (
) undyed CV1, ( ) 3 dip dyed CV1.
) 3 dip dyed CV1.



| Sample | CIELab Coordinates | K/S | ||
|---|---|---|---|---|
| L* | a* | b* | ||
| CV1 1 dip | 38.41 ± 16.27 | −2.70 ± 1.69 | −19.41 ± 7.04 | 11.09 ± 7.85 |
| CV1 2 dips | 21.47 ± 7.52 | 0.19 ± 0.70 | −19.82 ± 7.00 | 28.93 ± 10.22 |
| CV1 3 dips | 18.10 ± 7.09 | 0.52 ± 1.00 | −15.08 ± 5.87 | 31.02 ± 11.39 |
| Co a | 41.82 ± 1.28 | −1.89 ± 0.21 | −22.08 ± 0.62 | 4.15 ± 0.21 |
| Co a | 30.56 ± 0.38 | 0.40 ± 0.14 | −21.62 ± 0.04 | 8.1 ± 0.00 |
| Co b | 20.00 ± 0.20 | 1.23 ± 1.80 | −13.71 ± 0.23 | |
| Co (Indigo pwd.) c | 39.1 | −3.7 | −24.6 | 11.01 |
| (Indigo sol.) c | 29.6 | −1.4 | −23.1 | 18.87 |
| Undyed CV1 | 87.84 ± 0.69 | −0.12 ± 0.07 | 0.74 ± 0.29 | 0.06 ± 0.01 |
| Experiment | Variation | CIELab Coordinates | K/S | Indigo Content | ||
|---|---|---|---|---|---|---|
| wt% | L* | a* | b* | g kg−1 | ||
| A | 45 CS2 | 14.54 ± 0.10 | 0.51 ± 0.09 | −9.31 ± 0.21 | 34.22 ± 0.25 a | 39.84 ± 1.53 |
| B | 60 CS2 | 14.43 ± 0.30 | 0.94 ± 0.11 | −8.31 ± 0.16 | 31.94 ± 1.30 a | 37.92 ± 0.68 |
| C | 75 CS2 | 18.71 ± 0.42 | 0.66 ± 0.07 | −14.13 ± 0.04 | 27.24 ± 1.19 a | 31.65 ± 0.80 |
| D | 25 dyed CV1, 75 undyed CV2, 30 CS2 | 29.91 ± 0.25 | −1.65 ± 0.03 | −15.97 ± 0.08 | 12.31 ± 0.28 b | 10.78 ± 1.35 |
| E | 25 dyed CV1, 75 undyed CV2, 75 CS2 | 34.31 ± 0.64 | −2.59 ± 0.09 | −16.06 ± 0.25 | 9.08 ± 0.41 b | 11.91 ± 0.53 |
| F | 10 H2SO4 | 15.25 ± 0.33 | 0.31 ± 0.13 | −9.41 ± 0.25 | 33.05 ± 1.25 a | 37.54 ± 0.43 |
| G | 10 H2SO4, 10 Na2SO4 | 14.54 ± 0.67 | 0.39 ± 0.20 | −9.15 ± 0.33 | 35.02 ± 1.56 a | 41.30 ± 0.82 |
| H | 10 H2SO4, 10 Na2SO4, 5 ZnSO4 | 16.32 ± 1.67 | 0.31 ± 0.19 | −9.87 ± 0.93 | 29.83 ± 4.08 a | 43.02 ± 0.54 |
| I | 10 H2SO4, 10 Na2SO4, 5 ZnSO4 2 glucose | 14.36 ± 1.01 | 0.39 ± 0.15 | −8.27 ± 0.31 | 34.49 ± 3.66 a | 39.54 ± 0.40 |
| Feedstock | CV1 3 dips | 18.10 ± 7.09 | 0.52 ± 1.00 | −15.08 ± 5.87 | 31.02 ± 11.39 b | 30.11 ± 7.78 |
| Experiment | Indigo Added | CIELab Coordinates | K/S | Indigo Content | ||
|---|---|---|---|---|---|---|
| % | L* | a* | b* | g kg−1 | ||
| J | 0.81 ± 0.05 | 18.22 ± 0.41 | −1.18 ± 0.32 | −8.66 ± 0.81 | 28.08 ± 1.52 | 13.01 ± 0.89 |
| K | 2.28 ± 0.16 | 14.30 ± 0.84 | −0.38 ± 0.28 | −5.77 ± 0.45 | 32.47 ± 4.33 | 29.61 ± 2.04 |
| L | 4.29 ± 0.16 | 13.76 ± 1.49 | 0.76 ± 0.38 | −4.98 ± 1.80 | 28.67 ± 4.11 | 50.12 ± 2.18 |
| Reference dyeings | ||||||
| M1 | 2.87 | 19.38 ± 0.27 | 3.21 ± 0.42 | −17.80 ± 0.82 | 23.52 ± 0.36 | |
| M2 | 3.27 | 17.86 ± 0.36 | 2.62 ± 0.05 | −15.69 ± 0.45 | 24.14 ± 0.46 | |
| N1 c | - | 20.00 ± 0.20 | 1.23 ± 1.80 | −13.71 ± 0.23 | ||
| N2 d | (indigo pwd.) | 39.1 | −3.7 | −24.6 | 11.01 | |
| N3 d | (indigo sol.) | 29.6 | −1.4 | −23.1 | 18.87 | |
| Technical Dyeing Range | Global Production | |||
|---|---|---|---|---|
| Period | 1 Day | Year | Year | |
| Production | tons | 15 | 3750 | 1,500,000 |
| Water | m3 | 75 | 18,750 | 7,500,000 |
| Na2S2O4 | tons | 0.050–0.126 | 12.5–31.5 | 5000–12,600 |
| NaOH | tons | 0.050–0.126 | 12.5–31.5 | 5000–12,600 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manian, A.P.; Müller, S.; Braun, D.E.; Pham, T.; Bechtold, T. Dope Dyeing of Regenerated Cellulose Fibres with Leucoindigo as Base for Circularity of Denim. Polymers 2022, 14, 5280. https://doi.org/10.3390/polym14235280
Manian AP, Müller S, Braun DE, Pham T, Bechtold T. Dope Dyeing of Regenerated Cellulose Fibres with Leucoindigo as Base for Circularity of Denim. Polymers. 2022; 14(23):5280. https://doi.org/10.3390/polym14235280
Chicago/Turabian StyleManian, Avinash P., Sophia Müller, Doris E. Braun, Tung Pham, and Thomas Bechtold. 2022. "Dope Dyeing of Regenerated Cellulose Fibres with Leucoindigo as Base for Circularity of Denim" Polymers 14, no. 23: 5280. https://doi.org/10.3390/polym14235280
APA StyleManian, A. P., Müller, S., Braun, D. E., Pham, T., & Bechtold, T. (2022). Dope Dyeing of Regenerated Cellulose Fibres with Leucoindigo as Base for Circularity of Denim. Polymers, 14(23), 5280. https://doi.org/10.3390/polym14235280






