Recent Developments on Bioinspired Cellulose Containing Polymer Nanocomposite Cation and Anion Exchange Membranes for Fuel Cells (PEMFC and AFC)
Abstract
1. Introduction
- Low production cost
- Abundance in nature
- Facile manufacturing process without complicated steps
- Higher ion exchange capacity
- Excellent ion transport behavior
- Reasonable water uptake behavior
- Efficient mechanical stability
- Good thermal properties
- Higher chemical and electrochemical stability
- Insulating properties (to avoid the short circuit between cathode and anode)
- Lowest cross-over of oxygen and hydrogen
- Environmental friendly

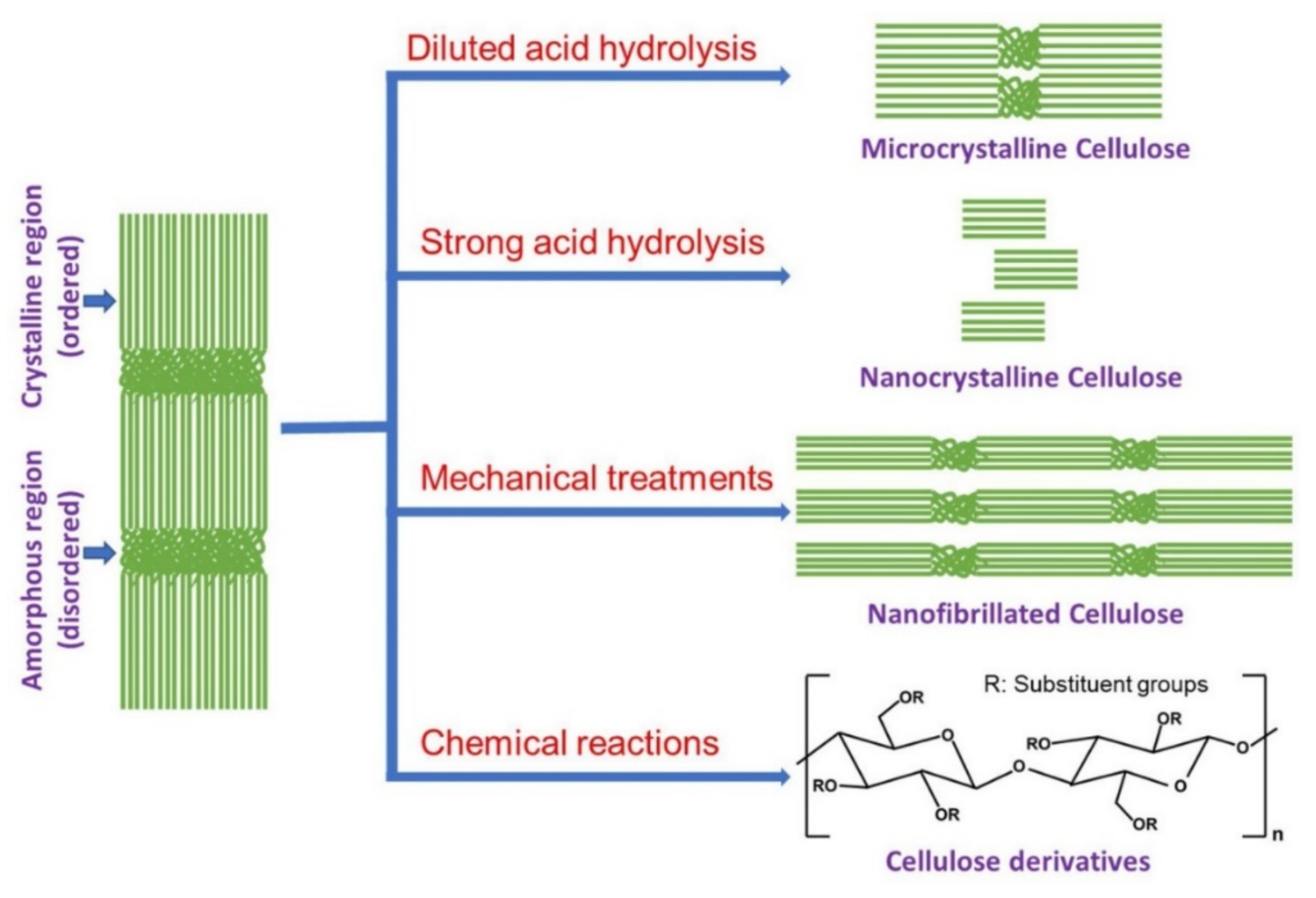
2. Developments of Different Cellulose Materials
3. Cellulose Containing Cation Exchange Membrane for PEMFC
3.1. Modification of Cellulose
3.2. Cellulose Derivatives with Nafion
3.3. CNC with Polyaryletherketone Family-Based Membranes
3.4. Different Kinds of Polymer Membrane Containing CNC Derivatives
3.5. Cellulose Derivatives Membrane with Inorganic Additives
4. Developments of Anion Exchange Membranes with Modified Cellulose for Alkaline Fuel Cell
4.1. Quaternized Cellulose Containing AEM Membrane
4.2. Impact of Sulfonated Cellulose and Cellulose Acetate in AEM Membrane
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hailemariam, A.; Erdiaw-Kwasie, M.O. Towards a circular economy: Implications for emission reduction and environmental sustainability. Bus. Strategy Environ. 2022. [Google Scholar] [CrossRef]
- Van Trinh, N.; Nguyen, X.L.; Kim, Y.; Yu, S. Characteristics of Water Transport of Membrane Electrolyte over Selected Temperature for Proton Exchange Membrane Fuel Cell. Polymers 2022, 14, 2972. [Google Scholar] [CrossRef]
- Lim, J.H.; Hou, J.; Lee, C.H. Dynamic Mechanical Fatigue Behavior of Polymer Electrolyte Membranes for Fuel Cell Electric Vehicles Using a Gas Pressure-Loaded Blister. Polymers 2021, 13, 4177. [Google Scholar] [CrossRef] [PubMed]
- Sadhasivam, T.; Palanisamy, G.; Roh, S.-H.; Kurkuri, M.D.; Kim, S.C.; Jung, H.-Y. Electro-analytical performance of bifunctional electrocatalyst materials in unitized regenerative fuel cell system. Int. J. Hydrogen Energy 2018, 43, 18169–18184. [Google Scholar] [CrossRef]
- Sharma, P.; Tinh, V.; Kim, D. Enhanced Ion Cluster Size of Sulfonated Poly (Arylene Ether Sulfone) for Proton Exchange Membrane Fuel Cell Application. Polymers 2021, 13, 1111. [Google Scholar] [CrossRef]
- Sharma, S.; Agarwal, S.; Jain, A. Significance of Hydrogen as Economic and Environmentally Friendly Fuel. Energies 2021, 14, 7389. [Google Scholar] [CrossRef]
- Escorihuela, J.; García-Bernabé, A.; Montero, Á.; Sahuquillo, Ó.; Giménez, E.; Compañ, V. Ionic Liquid Composite Polybenzimidazol Membranes for High Temperature PEMFC Applications. Polymers 2019, 11, 732. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, P.; Raizada, P.; Hussain, C.M. Impact of COVID-19 on greenhouse gases emissions: A critical review. Sci. Total Environ. 2022, 806, 150349. [Google Scholar] [CrossRef]
- Lee, S.; Nam, K.-H.; Seo, K.; Kim, G.; Han, H. Phase Inversion-Induced Porous Polybenzimidazole Fuel Cell Membranes: An Efficient Architecture for High-Temperature Water-Free Proton Transport. Polymers 2020, 12, 1604. [Google Scholar] [CrossRef]
- Zabat, L.H.; Sadaoui, N.A.; Abid, M.; Sekrafi, H. Threshold effects of renewable energy consumption by source in U.S. economy. Electr. Power Syst. Res. 2022, 213, 108669. [Google Scholar] [CrossRef]
- Bhukya, M.N.; Kumar, M.; Kant, A. Renewable Energy: Potential, Status, Targets and Challenges in Rajasthan. J. Phys. Conf. Ser. 2021, 1854, 012004. [Google Scholar] [CrossRef]
- Simari, C.; Prejanò, M.; Lufrano, E.; Sicilia, E.; Nicotera, I. Exploring the Structure–Performance Relationship of Sulfonated Polysulfone Proton Exchange Membrane by a Combined Computational and Experimental Approach. Polymers 2021, 13, 959. [Google Scholar] [CrossRef] [PubMed]
- Sadhasivam, T.; Kim, H.-T.; Jung, S.; Roh, S.-H.; Park, J.-H.; Jung, H.-Y. Dimensional effects of nanostructured Mg/MgH2 for hydrogen storage applications: A review. Renew. Sustain. Energy Rev. 2017, 72, 523–534. [Google Scholar] [CrossRef]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.-W. Hydrogen Storage in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 782–835. [Google Scholar] [CrossRef] [PubMed]
- Sadhasivam, T.; Hudson, M.S.L.; Pandey, S.K.; Bhatnagar, A.; Singh, M.K.; Gurunathan, K.; Srivastava, O. Effects of nano size mischmetal and its oxide on improving the hydrogen sorption behaviour of MgH2. Int. J. Hydrogen Energy 2013, 38, 7353–7362. [Google Scholar] [CrossRef]
- Thangarasu, S.; Oh, T.H. Impact of Polymers on Magnesium-Based Hydrogen Storage Systems. Polymers 2022, 14, 2608. [Google Scholar] [CrossRef]
- Dos Santos, K.G.; Eckert, C.T.; De Rossi, E.; Bariccatti, R.A.; Frigo, E.P.; Lindino, C.A.; Alves, H.J. Hydrogen production in the electrolysis of water in Brazil, a review. Renew. Sustain. Energy Rev. 2017, 68, 563–571. [Google Scholar] [CrossRef]
- Avargani, V.M.; Zendehboudi, S.; Saady, N.M.C.; Dusseault, M.B. A comprehensive review on hydrogen production and utilization in North America: Prospects and challenges. Energy Convers. Manag. 2022, 269, 115927. [Google Scholar] [CrossRef]
- Younas, M.; Shafique, S.; Hafeez, A.; Javed, F.; Rehman, F. An Overview of Hydrogen Production: Current Status, Potential, and Challenges. Fuel 2022, 316, 123317. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, X.; Meng, X. Recent advances on electrocatalytic and photocatalytic seawater splitting for hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 9087–9100. [Google Scholar] [CrossRef]
- Chakravarthi, S.; Meda, U.S.; Harti, A. Product Design of Hydrogen Sensing System. ECS Trans. 2022, 107, 3011–3025. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Thangarasu, S.; Palanisamy, G.; Im, Y.M.; Oh, T.H. An alternative platform of solid-state hydrides with polymers as composite/encapsulation for hydrogen storage applications: Effects in intermetallic and complex hydrides. Int. J. Hydrogen Energy 2022. [Google Scholar] [CrossRef]
- Millet, P. Hydrogen storage in hydride-forming materials. In Advances in Hydrogen Production, Storage and Distribution; Elsevier: Amsterdam, The Netherlands, 2014; pp. 368–409. [Google Scholar]
- Ding, X.; Chen, R.; Zhang, J.; Cao, W.; Su, Y.; Guo, J. Recent progress on enhancing the hydrogen storage properties of Mg-based materials via fabricating nanostructures: A critical review. J. Alloys Compd. 2021, 163137. [Google Scholar] [CrossRef]
- Singh, R.K.; Sadhasivam, T.; Sheeja, G.; Singh, P.; Srivastava, O. Effect of different sized CeO2 nano particles on decomposition and hydrogen absorption kinetics of magnesium hydride. Int. J. Hydrogen Energy 2013, 38, 6221–6225. [Google Scholar] [CrossRef]
- Boateng, E.; Chen, A. Recent advances in nanomaterial-based solid-state hydrogen storage. Mater. Today Adv. 2020, 6, 100022. [Google Scholar] [CrossRef]
- Rusman, N.; Dahari, M. A review on the current progress of metal hydrides material for solid-state hydrogen storage applications. Int. J. Hydrogen Energy 2016, 41, 12108–12126. [Google Scholar] [CrossRef]
- Camacho, M.D.L.N.; Jurburg, D.; Tanco, M. Hydrogen fuel cell heavy-duty trucks: Review of main research topics. Int. J. Hydrogen Energy 2022, 47, 29505–29525. [Google Scholar] [CrossRef]
- Duan, Z.; Zhang, L.; Feng, L.; Yu, S.; Jiang, Z.; Xu, X.; Hong, J. Research on Economic and Operating Characteristics of Hydrogen Fuel Cell Cars Based on Real Vehicle Tests. Energies 2021, 14, 7856. [Google Scholar] [CrossRef]
- Singla, M.K.; Nijhawan, P.; Oberoi, A.S. Hydrogen fuel and fuel cell technology for cleaner future: A review. Environ. Sci. Pollut. Res. 2021, 28, 15607–15626. [Google Scholar] [CrossRef]
- Granovskii, M.; Dincer, I.; A Rosen, M. Life cycle assessment of hydrogen fuel cell and gasoline vehicles. Int. J. Hydrogen Energy 2006, 31, 337–352. [Google Scholar] [CrossRef]
- Fan, L.; Tu, Z.; Chan, S.H. Recent development of hydrogen and fuel cell technologies: A review. Energy Rep. 2021, 7, 8421–8446. [Google Scholar] [CrossRef]
- Shen, C.; Xu, S.; Gao, Y. Analysis of Fuel Cell Stack Performance Attenuation and Individual Cell Voltage Uniformity Based on the Durability Cycle Condition. Polymers 2021, 13, 1199. [Google Scholar] [CrossRef] [PubMed]
- Sadhasivam, T.; Dhanabalan, K.; Roh, S.-H.; Kim, T.-H.; Park, K.-W.; Jung, S.; Kurkuri, M.D.; Jung, H.-Y. A comprehensive review on unitized regenerative fuel cells: Crucial challenges and developments. Int. J. Hydrogen Energy 2017, 42, 4415–4433. [Google Scholar] [CrossRef]
- Ghosh, S.; Das, S.; Mosquera, M.E.G. Conducting Polymer-Based Nanohybrids for Fuel Cell Application. Polymers 2020, 12, 2993. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, G.; Jung, H.-Y.; Sadhasivam, T.; Kurkuri, M.D.; Kim, S.C.; Roh, S.-H. A comprehensive review on microbial fuel cell technologies: Processes, utilization, and advanced developments in electrodes and membranes. J. Clean. Prod. 2019, 221, 598–621. [Google Scholar] [CrossRef]
- Mekhilef, S.; Saidur, R.; Safari, A. Comparative study of different fuel cell technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989. [Google Scholar] [CrossRef]
- Sazali, N.; Wan Salleh, W.N.; Jamaludin, A.S.; Mhd Razali, M.N. New Perspectives on Fuel Cell Technology: A Brief Review. Membranes 2020, 10, 99. [Google Scholar] [CrossRef]
- Sharaf, O.Z.; Orhan, M.F. An overview of fuel cell technology: Fundamentals and applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Jienkulsawad, P.; Chen, Y.-S.; Arpornwichanop, A. Modifying the Catalyst Layer Using Polyvinyl Alcohol for the Performance Improvement of Proton Exchange Membrane Fuel Cells under Low Humidity Operations. Polymers 2020, 12, 1865. [Google Scholar] [CrossRef]
- Kordesch, K.; Cifrain, M. A comparison between the alkaline fuel cell (AFC) and the polymer electrolyte membrane (PEM) fuel cell. In Handbook of Fuel Cells; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Choi, J.; Kyeong, M.; Kim, M.; Lee, S.-S.; Seo, B.; Park, H.; Park, H.-Y.; Henkensmeier, D.; Lee, S.; Kim, H.-J. Synthesis of Sulfonated Poly(Arylene Ether Sulfone)s Containing Aliphatic Moieties for Effective Membrane Electrode Assembly Fabrication by Low-Temperature Decal Transfer Methods. Polymers 2021, 13, 1713. [Google Scholar] [CrossRef]
- Dekel, D.R. Review of cell performance in anion exchange membrane fuel cells. J. Power Sources 2018, 375, 158–169. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Lee, K.H.; Chu, J.Y.; Ryu, S.K.; Kim, H.G.; Lee, J.-Y.; Lee, H.-K.; Yoo, D.J. Ameliorated Performance of Sulfonated Poly(Arylene Ether Sulfone) Block Copolymers with Increased Hydrophilic Oligomer Ratio in Proton-Exchange Membrane Fuel Cells Operating at 80% Relative Humidity. Polymers 2020, 12, 1871. [Google Scholar] [CrossRef]
- Martinez-Morlanes, M.J.; de la Torre-Gamarra, C.; Pérez-Prior, M.T.; Lara-Benito, S.; del Rio, C.; Várez, A.; Levenfeld, B. Sulfonated Polysulfone/TiO2(B) Nanowires Composite Membranes as Polymer Electrolytes in Fuel Cells. Polymers 2021, 13, 2030. [Google Scholar] [CrossRef]
- Son, B.; Park, J.; Kwon, O. Analysis of Ionic Domains on a Proton Exchange Membrane Using a Numerical Approximation Model Based on Electrostatic Force Microscopy. Polymers 2021, 13, 1258. [Google Scholar] [CrossRef]
- Ryu, S.; Kim, A.; Vinothkannan, M.; Lee, K.; Chu, J.; Yoo, D. Enhancing Physicochemical Properties and Single Cell Performance of Sulfonated Poly(arylene ether) (SPAE) Membrane by Incorporation of Phosphotungstic Acid and Graphene Oxide: A Potential Electrolyte for Proton Exchange Membrane Fuel Cells. Polymers 2021, 13, 2364. [Google Scholar] [CrossRef]
- Guo, Z.; Perez-Page, M.; Chen, J.; Ji, Z.; Holmes, S.M. Recent advances in phosphoric acid–based membranes for high–temperature proton exchange membrane fuel cells. J. Energy Chem. 2021, 63, 393–429. [Google Scholar] [CrossRef]
- Das, G.; Choi, J.-H.; Nguyen, P.K.T.; Kim, D.-J.; Yoon, Y.S. Anion Exchange Membranes for Fuel Cell Application: A Review. Polymers 2022, 14, 1197. [Google Scholar] [CrossRef]
- Kim, J.-D.; Matsushita, S.; Tamura, K. Crosslinked Sulfonated Polyphenylsulfone-Vinylon (CSPPSU-vinylon) Membranes for PEM Fuel Cells from SPPSU and Polyvinyl Alcohol (PVA). Polymers 2020, 12, 1354. [Google Scholar] [CrossRef]
- Alashkar, A.; Al-Othman, A.; Tawalbeh, M.; Qasim, M. A Critical Review on the Use of Ionic Liquids in Proton Exchange Membrane Fuel Cells. Membranes 2022, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Elwan, H.A.; Thimmappa, R.; Mamlouk, M.; Scott, K. Applications of poly ionic liquids in proton exchange membrane fuel cells: A review. J. Power Sources 2021, 510, 230371. [Google Scholar] [CrossRef]
- Ferriday, T.B.; Middleton, P.H. Alkaline fuel cell technology—A review. Int. J. Hydrogen Energy 2021, 46, 18489–18510. [Google Scholar] [CrossRef]
- Huang, J.; Yu, Z.; Tang, J.; Wang, P.; Tan, Q.; Wang, J.; Lei, X. A review on anion exchange membranes for fuel cells: Anion-exchange polyelectrolytes and synthesis strategies. Int. J. Hydrogen Energy 2022, 47, 27800–27820. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion exchange membranes for alkaline fuel cells: A review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Couture, G.; Alaaeddine, A.; Boschet, F.; Ameduri, B. Polymeric materials as anion-exchange membranes for alkaline fuel cells. Prog. Polym. Sci. 2011, 36, 1521–1557. [Google Scholar] [CrossRef]
- Shao, Y.; Yin, G.; Wang, Z.; Gao, Y. Proton exchange membrane fuel cell from low temperature to high temperature: Material challenges. J. Power Sources 2007, 167, 235–242. [Google Scholar] [CrossRef]
- Lufrano, E.; Simari, C.; Di Vona, M.; Nicotera, I.; Narducci, R. How the Morphology of Nafion-Based Membranes Affects Proton Transport. Polymers 2021, 13, 359. [Google Scholar] [CrossRef]
- Fernihough, O.; Cheshire, H.; Romano, J.-M.; Ibrahim, A.; El-Kharouf, A.; Du, S. Patterned Membranes for Proton Exchange Membrane Fuel Cells Working at Low Humidity. Polymers 2021, 13, 1976. [Google Scholar] [CrossRef]
- Seo, D.C.; Jeon, I.; Jeong, E.S.; Jho, J.Y. Mechanical Properties and Chemical Durability of Nafion/Sulfonated Graphene Oxide/Cerium Oxide Composite Membranes for Fuel-Cell Applications. Polymers 2020, 12, 1375. [Google Scholar] [CrossRef]
- Narducci, R.; Sgreccia, E.; Knauth, P.; Di Vona, M.L. Anion Exchange Membranes with 1D, 2D and 3D Fillers: A Review. Polymers 2021, 13, 3887. [Google Scholar] [CrossRef]
- Altaf, F.; Batool, R.; Gill, R.; Rehman, Z.U.; Majeed, H.; Ahmad, A.; Shafiq, M.; Dastan, D.; Abbas, G.; Jacob, K. Synthesis and electrochemical investigations of ABPBI grafted montmorillonite based polymer electrolyte membranes for PEMFC applications. Renew. Energy 2021, 164, 709–728. [Google Scholar] [CrossRef]
- Teixeira, F.C.; Teixeira, A.P.; Rangel, C. New proton conductive membranes of indazole- and condensed pyrazolebisphosphonic acid-Nafion membranes for PEMFC. Renew. Energy 2022, 196, 1187–1196. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, X.; Liu, M.; Liu, L.; Li, N. The effect of –NH− on quaternized polybenzimidazole anion exchange membranes for alkaline fuel cells. J. Membr. Sci. 2021, 626, 119178. [Google Scholar] [CrossRef]
- Liu, M.; Hu, X.; Hu, B.; Liu, L.; Li, N. Soluble poly(aryl piperidinium) with extended aromatic segments as anion exchange membranes for alkaline fuel cells and water electrolysis. J. Membr. Sci. 2022, 642, 119966. [Google Scholar] [CrossRef]
- Gómez, E.E.R.; Hernández, J.H.M.; Astaiza, J.E.D. Development of a Chitosan/PVA/TiO2 Nanocomposite for Application as a Solid Polymeric Electrolyte in Fuel Cells. Polymers 2020, 12, 1691. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S. Chitosan and alginate types of bio-membrane in fuel cell application: An overview. J. Power Sources 2015, 289, 71–80. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S. Sodium alginate/alumina composite biomembrane preparation and performance in DMFC application. Polym. Test. 2020, 81, 106183. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Muddasar, M.; Beaucamp, A.; Culebras, M.; Collins, M.N. Cellulose: Characteristics and applications for rechargeable batteries. Int. J. Biol. Macromol. 2022, 219, 788–803. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- He, X.; Lu, W.; Sun, C.; Khalesi, H.; Mata, A.; Andaleeb, R.; Fang, Y. Cellulose and cellulose derivatives: Different colloidal states and food-related applications. Carbohydr. Polym. 2021, 255, 117334. [Google Scholar] [CrossRef] [PubMed]
- Haldar, D.; Purkait, M.K. Micro and nanocrystalline cellulose derivatives of lignocellulosic biomass: A review on synthesis, applications and advancements. Carbohydr. Polym. 2020, 250, 116937. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Hussin, M.H.; Hui Chuin, C.T.; Sabar, S.; Fazita, M.R.N.; Taiwo, O.F.A.; Hassan, T.M.; Haafiz, M.K.M. Microcrystalline cellulose: Isolation, characterization and bio-composites application—A review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef]
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrard, B. Microcrystalline cellulose, a direct compression binder in a quality by design environment—A review. Int. J. Pharm. 2014, 473, 64–72. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Abd Mutalib, M.; Hir, Z.A.M.; Zain, M.M.; Mohamad, A.B.; Minggu, L.J.; Awang, N.A.; Salleh, W.N. An overview on cellulose-based material in tailoring bio-hybrid nanostructured photocatalysts for water treatment and renewable energy applications. Int. J. Biol. Macromol. 2017, 103, 1232–1256. [Google Scholar] [CrossRef]
- Islam, M.T.; Alam, M.M.; Patrucco, A.; Montarsolo, A.; Zoccola, M. Preparation of Nanocellulose: A Review. AATCC J. Res. 2014, 1, 17–23. [Google Scholar] [CrossRef]
- Torlopov, M.A.; Udoratina, E.V.; Martakov, I.S.; Sitnikov, P.A. Cellulose nanocrystals prepared in H3PW12O40-acetic acid system. Cellulose 2017, 24, 2153–2162. [Google Scholar] [CrossRef]
- De Souza Lima, M.M.; Borsali, R. Rodlike Cellulose Microcrystals: Structure, Properties, and Applications. Macromol. Rapid Commun. 2004, 25, 771–787. [Google Scholar] [CrossRef]
- Zhang, J.; Elder, T.J.; Pu, Y.; Ragauskas, A.J. Facile synthesis of spherical cellulose nanoparticles. Carbohydr. Polym. 2007, 69, 607–611. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, Z.; Zhou, J.; He, M.; Jiang, Q.; Li, S.; Ma, Y.; Liu, M.; Luo, S. Isolation and characterization of cellulose nanocrystals from pueraria root residue. Int. J. Biol. Macromol. 2019, 129, 1081–1089. [Google Scholar] [CrossRef]
- Pinto, L.O.; Bernardes, J.S.; Rezende, C.A. Low-energy preparation of cellulose nanofibers from sugarcane bagasse by modulating the surface charge density. Carbohydr. Polym. 2019, 218, 145–153. [Google Scholar] [CrossRef]
- Fischer, S.; Thümmler, K.; Volkert, B.; Hettrich, K.; Schmidt, I.; Fischer, K. Properties and applications of cellulose acetate. Macromol. Symp. 2008, 262, 89–96. [Google Scholar] [CrossRef]
- Sassi, J.-F.; Chanzy, H. Ultrastructural aspects of the acetylation of cellulose. Cellulose 1995, 2, 111–127. [Google Scholar] [CrossRef]
- Jogunola, O.; Eta, V.; Hedenström, M.; Sundman, O.; Salmi, T.; Mikkola, J.-P. Ionic liquid mediated technology for synthesis of cellulose acetates using different co-solvents. Carbohydr. Polym. 2016, 135, 341–348. [Google Scholar] [CrossRef]
- Fatima, A.; Yasir, S.; Khan, M.S.; Manan, S.; Ullah, M.W.; Ul-Islam, M. Plant extract-loaded bacterial cellulose composite membrane for potential biomedical applications. J. Bioresour. Bioprod. 2021, 6, 26–32. [Google Scholar] [CrossRef]
- Zou, Y.; Zhao, J.; Zhu, J.; Guo, X.; Chen, P.; Duan, G.; Liu, X.; Li, Y. A Mussel-Inspired Polydopamine-Filled Cellulose Aerogel for Solar-Enabled Water Remediation. ACS Appl. Mater. Interfaces 2021, 13, 7617–7624. [Google Scholar] [CrossRef]
- Deeksha, B.; Sadanand, V.; Hariram, N.; Rajulu, A.V. Preparation and properties of cellulose nanocomposite fabrics with in situ generated silver nanoparticles by bioreduction method. J. Bioresour. Bioprod. 2021, 6, 75–81. [Google Scholar] [CrossRef]
- De Medeiros, A.D.M.; Junior, C.J.G.D.S.; de Amorim, J.D.P.; Nascimento, H.A.D.; Converti, A.; Costa, A.F.D.S.; Sarubbo, L.A. Biocellulose for Treatment of Wastewaters Generated by Energy Consuming Industries: A Review. Energies 2021, 14, 5066. [Google Scholar] [CrossRef]
- Wang, X.; Yao, C.; Wang, F.; Li, Z. Cellulose-Based Nanomaterials for Energy Applications. Small 2017, 13, 1702240. [Google Scholar] [CrossRef]
- Li, S.; Huang, D.; Yang, J.; Zhang, B.; Zhang, X.; Yang, G.; Wang, M.; Shen, Y. Freestanding bacterial cellulose–polypyrrole nanofibres paper electrodes for advanced energy storage devices. Nano Energy 2014, 9, 309–317. [Google Scholar] [CrossRef]
- Vinodh, R.; Atchudan, R.; Kim, H.-J.; Yi, M. Recent Advancements in Polysulfone Based Membranes for Fuel Cell (PEMFCs, DMFCs and AMFCs) Applications: A Critical Review. Polymers 2022, 14, 300. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ko, H.; Nam, S.Y.; Kim, K. Study on Control of Polymeric Architecture of Sulfonated Hydrocarbon-Based Polymers for High-Performance Polymer Electrolyte Membranes in Fuel Cell Applications. Polymers 2021, 13, 3520. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Cruz, M.M.; Escorihuela, J.; Solorza-Feria, O.; Compañ, V. Proton Exchange Membrane Fuel Cells (PEMFCs): Advances and Challenges. Polymers 2021, 13, 3064. [Google Scholar] [CrossRef] [PubMed]
- Thangarasu, S.; Oh, T.H. Progress in poly(phenylene oxide) based cation exchange membranes for fuel cells and redox flow batteries applications. Int. J. Hydrogen Energy 2021, 46, 38381–38415. [Google Scholar] [CrossRef]
- Bayer, T.; Cunning, B.V.; Selyanchyn, R.; Nishihara, M.; Fujikawa, S.; Sasaki, K.; Lyth, S.M. High Temperature Proton Conduction in Nanocellulose Membranes: Paper Fuel Cells. Chem. Mater. 2016, 28, 4805–4814. [Google Scholar] [CrossRef]
- Jiang, G.-P.; Zhang, J.; Qiao, J.-L.; Jiang, Y.-M.; Zarrin, H.; Chen, Z.; Hong, F. Bacterial nanocellulose/Nafion composite membranes for low temperature polymer electrolyte fuel cells. J. Power Sources 2015, 273, 697–706. [Google Scholar] [CrossRef]
- Jiang, G.; Qiao, J.; Hong, F. Application of phosphoric acid and phytic acid-doped bacterial cellulose as novel proton-conducting membranes to PEMFC. Int. J. Hydrogen Energy 2012, 37, 9182–9192. [Google Scholar] [CrossRef]
- Gonçalves, R.F.; Lizundia, E.; Silva, M.M.; Costa, C.M.; Lanceros-Méndez, S. Mesoporous Cellulose Nanocrystal Membranes as Battery Separators for Environmentally Safer Lithium-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 3749–3761. [Google Scholar] [CrossRef]
- Huang, C.; Ji, H.; Yang, Y.; Guo, B.; Luo, L.; Meng, Z.; Fan, L.; Xu, J. TEMPO-oxidized bacterial cellulose nanofiber membranes as high-performance separators for lithium-ion batteries. Carbohydr. Polym. 2020, 230, 115570. [Google Scholar] [CrossRef]
- Palanisamy, G.; Sadhasivam, T.; Park, W.-S.; Bae, S.T.; Roh, S.-H.; Jung, H.-Y. Tuning the Ion Selectivity and Chemical Stability of a Biocellulose Membrane by PFSA Ionomer Reinforcement for Vanadium Redox Flow Battery Applications. ACS Sustain. Chem. Eng. 2020, 8, 2040–2051. [Google Scholar] [CrossRef]
- Islam, A.; Ong, H.L.; Villagracia, A.R.; Halim, K.A.A.; Ganganboina, A.B.; Doong, R.-A. Biomass–derived cellulose nanofibrils membrane from rice straw as sustainable separator for high performance supercapacitor. Ind. Crops Prod. 2021, 170, 113694. [Google Scholar] [CrossRef]
- Roh, S.-H.; Palanisamy, G.; Sadhasivam, T.; Jin, J.-E.; Shim, J.-Y.; Jung, H.-Y. Techno-Economical Feasibility of Biocellulose Membrane along with Polyethylene Film as a Separator for Lead-Acid Batteries. ACS Sustain. Chem. Eng. 2019, 7, 8789–8797. [Google Scholar] [CrossRef]
- Guccini, V.; Carlson, A.; Yu, S.; Lindbergh, G.; Lindström, R.W.; Salazar-Alvarez, G. Highly proton conductive membranes based on carboxylated cellulose nanofibres and their performance in proton exchange membrane fuel cells. J. Mater. Chem. A 2019, 7, 25032–25039. [Google Scholar] [CrossRef]
- Sharma, S.K.; Sharma, P.R.; Wang, L.; Pagel, M.; Borges, W.; Johnson, K.I.; Raut, A.; Gu, K.; Bae, C.; Rafailovich, M.; et al. Nitro-oxidized carboxylated cellulose nanofiber based nanopapers and their PEM fuel cell performance. Sustain. Energy Fuels 2022, 6, 3669–3680. [Google Scholar] [CrossRef]
- Bayer, T.; Cunning, B.V.; Šmíd, B.; Selyanchyn, R.; Fujikawa, S.; Sasaki, K.; Lyth, S.M. Spray deposition of sulfonated cellulose nanofibers as electrolyte membranes in fuel cells. Cellulose 2021, 28, 1355–1367. [Google Scholar] [CrossRef]
- Jankowska, I.; Ławniczak, P.; Pogorzelec-Glaser, K.; Łapiński, A.; Pankiewicz, R.; Tritt-Goc, J. Cellulose microfibers surface treated with imidazole as new proton conductors. Mater. Chem. Phys. 2020, 239, 122056. [Google Scholar] [CrossRef]
- Lindner, Ł.; Bielejewski, M.; Markiewicz, E.; Łapiński, A.; Pankiewicz, R.; Tritt-Goc, J. Synthesis and characterization of triazole based nanocrystalline cellulose solid proton conductors. Eur. Polym. J. 2021, 161, 110825. [Google Scholar] [CrossRef]
- Selyanchyn, O.; Bayer, T.; Klotz, D.; Selyanchyn, R.; Sasaki, K.; Lyth, S.M. Cellulose Nanocrystals Crosslinked with Sulfosuccinic Acid as Sustainable Proton Exchange Membranes for Electrochemical Energy Applications. Membranes 2022, 12, 658. [Google Scholar] [CrossRef]
- Selim, A.; Szijjártó, G.P.; Románszki, L.; Tompos, A. Development of WO3–Nafion Based Membranes for Enabling Higher Water Retention at Low Humidity and Enhancing PEMFC Performance at Intermediate Temperature Operation. Polymers 2022, 14, 2492. [Google Scholar] [CrossRef]
- Mazzapioda, L.; Panero, S.; Navarra, M.A. Polymer Electrolyte Membranes Based on Nafion and a Superacidic Inorganic Additive for Fuel Cell Applications. Polymers 2019, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- Hambardzumyan, A.; Vayer, M.; Foulon, L.; Pernes, M.; Devers, T.; Bigarré, J.; Aguié-Béghin, V. Nafion membranes reinforced by cellulose nanocrystals for fuel cell applications: Aspect ratio and heat treatment effects on physical properties. J. Mater. Sci. 2022, 57, 4684–4703. [Google Scholar] [CrossRef]
- An, V.N.; Phong, L.P.N.; Van Nhi, N.; Van, T.T.T.; Nhan, H.T.C.; Van Hieu, L. Effect of imidazole-doped nanocrystalline cellulose on the characterization of Nafion films of fuel cells. J. Chem. Technol. Biotechnol. 2021, 96, 3114–3121. [Google Scholar] [CrossRef]
- Vu, A.N.; Tran, A.M.; Nguyen, L.H.; Van Nguyen, H.; Le, N.P.P.; Van Le, H. Fabrication of ternary composite based on imidazole, nafion, and cellulose nanocrystals for fuel cell application. Sci. Technol. Dev. J. Nat. Sci. 2022, 6, 1991–2003. [Google Scholar]
- Ni, C.; Wei, Y.; Zhao, Q.; Liu, B.; Sun, Z.; Gu, Y.; Zhang, M.; Hu, W. Novel proton exchange membranes based on structure-optimized poly(ether ether ketone ketone)s and nanocrystalline cellulose. Appl. Surf. Sci. 2018, 434, 163–175. [Google Scholar] [CrossRef]
- Ni, C.; Wang, H.; Zhao, Q.; Liu, B.; Sun, Z.; Zhang, M.; Hu, W.; Liang, L. Crosslinking effect in nanocrystalline cellulose reinforced sulfonated poly(aryl ether ketone) proton exchange membranes. Solid State Ionics 2018, 323, 5–15. [Google Scholar] [CrossRef]
- Pereira, F.; Vallé, K.; Belleville, P.; Morin, A.; Lambert, S.; Sanchez, C. Advanced Mesostructured Hybrid Silica−Nafion Membranes for High-Performance PEM Fuel Cell. Chem. Mater. 2008, 20, 1710–1718. [Google Scholar] [CrossRef]
- Yao, Y.; Ji, L.; Lin, Z.; Li, Y.; Alcoutlabi, M.; Hamouda, H.; Zhang, X. Sulfonated Polystyrene Fiber Network-Induced Hybrid Proton Exchange Membranes. ACS Appl. Mater. Interfaces 2011, 3, 3732–3737. [Google Scholar] [CrossRef]
- Zhao, Q.; Wei, Y.; Ni, C.; Wang, L.; Liu, B.; Liu, J.; Zhang, M.; Men, Y.; Sun, Z.; Xie, H.; et al. Effect of aminated nanocrystal cellulose on proton conductivity and dimensional stability of proton exchange membranes. Appl. Surf. Sci. 2019, 466, 691–702. [Google Scholar] [CrossRef]
- Vilela, C.; Martins, A.P.C.; Sousa, N.; Silvestre, A.J.D.; Figueiredo, F.M.L.; Freire, C.S.R. Poly(bis[2-(methacryloyloxy)ethyl] phosphate)/Bacterial Cellulose Nanocomposites: Preparation, Characterization and Application as Polymer Electrolyte Membranes. Appl. Sci. 2018, 8, 1145. [Google Scholar] [CrossRef]
- Mesbah, F.; El Gayar, D.; Farag, H.; Tamer, T.M.; Omer, A.M.; Mohy-Eldin, M.S.; Khalifa, R.E. Development of highly ionic conductive cellulose acetate-g-poly (2-acrylamido-2-methylpropane sulfonic acid-co-methyl methacrylate) graft copolymer membranes. J. Saudi Chem. Soc. 2021, 25, 101318. [Google Scholar] [CrossRef]
- Nohara, T.; Arita, T.; Tabata, K.; Saito, T.; Shimada, R.; Nakazaki, H.; Suzuki, Y.; Sato, R.; Masuhara, A. Novel Filler-Filled-Type Polymer Electrolyte Membrane for PEFC Employing Poly(vinylphosphonic acid)-b-polystyrene-Coated Cellulose Nanocrystals as a Filler. ACS Appl. Mater. Interfaces 2022, 14, 8353–8360. [Google Scholar] [CrossRef]
- Etuk, S.S.; Lawan, I.; Zhou, W.; Jiang, Y.; Zhang, Q.; Wei, X.; Zhang, M.; Fernando, G.F.; Yuan, Z. Synthesis and characterization of triazole based sulfonated nanocrystalline cellulose proton conductor. Cellulose 2020, 27, 3197–3209. [Google Scholar] [CrossRef]
- Rogalsky, S.; Bardeau, J.-F.; Makhno, S.; Babkina, N.; Tarasyuk, O.; Cherniavska, T.; Orlovska, I.; Kozyrovska, N.; Brovko, O. New proton conducting membrane based on bacterial cellulose/polyaniline nanocomposite film impregnated with guanidinium-based ionic liquid. Polymer 2018, 142, 183–195. [Google Scholar] [CrossRef]
- Naumi, F.; Natanael, C.L.; Rahayu, I.; Indrarti, L.; Hendrana, S. Polymer Electrolyte Membrane Fuel Cell based on Sulfonated Polystyrene and Phosphoric Acid with Biocellulose as a Matrix. Res. J. Chem. Environ. 2018, 22, 289–293. [Google Scholar]
- Madih, K.; El-Shazly, A.; Elkady, M.; Aziz, A.N.; Youssef, M.E.; Khalifa, R.E. A facile synthesis of cellulose acetate reinforced graphene oxide nanosheets as proton exchange membranes for fuel cell applications. J. Saudi Chem. Soc. 2022, 26, 101435. [Google Scholar] [CrossRef]
- Wang, L.; Zuo, X.; Raut, A.; Isseroff, R.; Xue, Y.; Zhou, Y.; Sandhu, B.; Schein, T.; Zeliznyak, T.; Sharma, P.; et al. Operation of proton exchange membrane (PEM) fuel cells using natural cellulose fiber membranes. Sustain. Energy Fuels 2019, 3, 2725–2732. [Google Scholar] [CrossRef]
- Vilela, C.; Morais, J.D.; Silva, A.C.Q.; Muñoz-Gil, D.; Figueiredo, F.M.L.; Silvestre, A.J.D.; Freire, C.S.R. Flexible Nanocellulose/Lignosulfonates Ion-Conducting Separators for Polymer Electrolyte Fuel Cells. Nanomaterials 2020, 10, 1713. [Google Scholar] [CrossRef]
- Vilela, C.; Silva, A.C.; Domingues, E.; Gonçalves, G.; Martins, M.A.; Figueiredo, F.M.L.; Santos, S.A.O.; Freire, C.S.R. Conductive polysaccharides-based proton-exchange membranes for fuel cell applications: The case of bacterial cellulose and fucoidan. Carbohydr. Polym. 2020, 230, 115604. [Google Scholar] [CrossRef]
- Bano, S.; Negi, Y.S.; Illathvalappil, R.; Kurungot, S.; Ramya, K. Studies on nano composites of SPEEK/ethylene glycol/cellulose nanocrystals as promising proton exchange membranes. Electrochimica Acta 2019, 293, 260–272. [Google Scholar] [CrossRef]
- Li, L.; Liu, L.; Qing, Y.; Zhang, Z.; Yan, N.; Wu, Y.; Tian, C. Stretchable alkaline poly(acrylic acid) electrolyte with high ionic conductivity enhanced by cellulose nanofibrils. Electrochimica Acta 2018, 270, 302–309. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Kim, A.R.; Kumar, G.G.; Yoo, D.J. Sulfonated graphene oxide/Nafion composite membranes for high temperature and low humidity proton exchange membrane fuel cells. RSC Adv. 2018, 8, 7494–7508. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.; Kamarudin, S.K.; Timmiati, S.N.; Masdar, M. New composite membrane poly (vinyl alcohol)/graphene oxide for direct ethanol–proton exchange membrane fuel cell. J. Appl. Polym. Sci. 2019, 136, 46928. [Google Scholar] [CrossRef]
- Lee, H.; Han, J.; Kim, K.; Kim, J.; Kim, E.; Shin, H.; Lee, J.-C. Highly sulfonated polymer-grafted graphene oxide composite membranes for proton exchange membrane fuel cells. J. Ind. Eng. Chem. 2019, 74, 223–232. [Google Scholar] [CrossRef]
- Gorgieva, S.; Osmić, A.; Hribernik, S.; Božič, M.; Svete, J.; Hacker, V.; Wolf, S.; Genorio, B. Efficient chitosan/nitrogen-doped reduced graphene oxide composite membranes for direct alkaline ethanol fuel cells. Int. J. Mol. Sci. 2021, 22, 1740. [Google Scholar] [CrossRef]
- Thong, P.T.; Sadhasivam, T.; Lim, H.; Jin, C.-S.; Ryi, S.-K.; Park, W.; Kim, H.T.; Roh, S.-H.; Jung, H.-Y. High Oxidizing Stability and Ion Selectivity of Hybrid Polymer Electrolyte Membrane for Improving Electrochemical Performance in Vanadium Redox Flow Battery. J. Electrochem. Soc. 2018, 165, A2321–A2329. [Google Scholar] [CrossRef]
- Hren, M.; Božič, M.; Fakin, D.; Kleinschek, K.S.; Gorgieva, S. Alkaline membrane fuel cells: Anion exchange membranes and fuels. Sustain. Energy Fuels 2021, 5, 604–637. [Google Scholar] [CrossRef]
- Zhou, T.; Shao, R.; Chen, S.; He, X.; Qiao, J.; Zhang, J. A review of radiation-grafted polymer electrolyte membranes for alkaline polymer electrolyte membrane fuel cells. J. Power Sources 2015, 293, 946–975. [Google Scholar] [CrossRef]
- Park, E.J.; Kim, Y.S. Quaternized aryl ether-free polyaromatics for alkaline membrane fuel cells: Synthesis, properties, and performance—A topical review. J. Mater. Chem. A 2018, 6, 15456–15477. [Google Scholar] [CrossRef]
- Lin, X.; Varcoe, J.R.; Poynton, S.D.; Liang, X.; Ong, A.L.; Ran, J.; Li, Y.; Xu, T. Alkaline polymer electrolytes containing pendant dimethylimidazolium groups for alkaline membrane fuel cells. J. Mater. Chem. A 2013, 1, 7262–7269. [Google Scholar] [CrossRef]
- Yanagi, H.; Fukuta, K. Anion Exchange Membrane and Ionomer for Alkaline Membrane Fuel Cells (AMFCs). ECS Trans. 2008, 16, 257–262. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Qiao, J.; Baker, R.; Zhang, J. Alkaline polymer electrolyte membranes for fuel cell applications. Chem. Soc. Rev. 2013, 42, 5768–5787. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, J.; Li, S.; Zhang, S. Synthesis of multi-block poly(arylene ether sulfone) copolymer membrane with pendant quaternary ammonium groups for alkaline fuel cell. J. Power Sources 2011, 196, 4445–4450. [Google Scholar] [CrossRef]
- Tuan, C.M.; Kim, D. Anion-exchange membranes based on poly(arylene ether ketone) with pendant quaternary ammonium groups for alkaline fuel cell application. J. Membr. Sci. 2016, 511, 143–150. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Zhang, S. Novel Hydroxide-Conducting Polyelectrolyte Composed of an Poly(arylene ether sulfone) Containing Pendant Quaternary Guanidinium Groups for Alkaline Fuel Cell Applications. Macromolecules 2010, 43, 3890–3896. [Google Scholar] [CrossRef]
- Dong, X.; Xue, B.; Qian, H.; Zheng, J.; Li, S.; Zhang, S. Novel quaternary ammonium microblock poly (p-phenylene-co-aryl ether ketone)s as anion exchange membranes for alkaline fuel cells. J. Power Sources 2017, 342, 605–615. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, X.; Jin, C. Development of a high-performance anion exchange membrane using poly(isatin biphenylene) with flexible heterocyclic quaternary ammonium cations for alkaline fuel cells. J. Mater. Chem. A 2019, 7, 6883–6893. [Google Scholar] [CrossRef]
- Kumari, M.; Douglin, J.C.; Dekel, D.R. Crosslinked quaternary phosphonium-functionalized poly(ether ether ketone) polymer-based anion-exchange membranes. J. Membr. Sci. 2021, 626, 119167. [Google Scholar] [CrossRef]
- Lee, K.H.; Chu, J.Y.; Kim, A.R.; Yoo, D.J. Fabrication of high-alkaline stable quaternized poly (arylene ether ketone)/graphene oxide derivative including zwitterion for alkaline fuel cells. ACS Sustain. Chem. Eng. 2021, 9, 8824–8834. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Son, T.Y.; Im, K.S.; Chae, J.E.; Kim, H.J.; Kim, T.H.; Nam, S.Y. Anion Exchange Composite Membranes Composed of Quaternary Ammonium-Functionalized Poly (2, 6-dimethyl-1, 4-phenylene oxide) and Silica for Fuel Cell Application. ACS Omega 2021, 6, 10168–10179. [Google Scholar] [CrossRef]
- Chen, J.; Guan, M.; Li, K.; Tang, S. High-performance COF-based composite anion exchange membrane sandwiched by GO layers for alkaline H2/O2 fuel cell application. J. Ind. Eng. Chem. 2021, 104, 136–145. [Google Scholar] [CrossRef]
- Samsudin, A.M.; Bodner, M.; Hacker, V. A Brief Review of Poly(Vinyl Alcohol)-Based Anion Exchange Membranes for Alkaline Fuel Cells. Polymers 2022, 14, 3565. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, H.G.; Dhanasekaran, P.; Nagaraju, N.; Neeshma, M.; Dass, B.M.; Dhavale, V.M.; Unni, S.M.; Bhat, S.D. Anion Exchange Membranes for Alkaline Polymer Electrolyte Fuel Cells—A Concise Review. Materials 2022, 15, 5601. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Park, B.J.; Yoon, H.H. A bionanocomposite based on 1,4-diazabicyclo-[2.2.2]-octane cellulose nanofiber cross-linked-quaternary polysulfone as an anion conducting membrane. J. Mater. Chem. A 2016, 4, 15554–15564. [Google Scholar] [CrossRef]
- Schmitt, F.; Granet, R.; Sarrazin, C.; Mackenzie, G.; Krausz, P. Synthesis of anion exchange membranes from cellulose: Crosslinking with diiodobutane. Carbohydr. Polym. 2011, 86, 362–366. [Google Scholar] [CrossRef]
- Lu, Y.; Armentrout, A.A.; Li, J.; Tekinalp, H.L.; Nanda, J.; Ozcan, S. A cellulose nanocrystal-based composite electrolyte with superior dimensional stability for alkaline fuel cell membranes. J. Mater. Chem. A 2015, 3, 13350–13356. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, J.; Qiao, J.; Liu, L.; Jiang, G.; Zhang, J.; Liu, Y. High durable poly (vinyl alcohol)/Quaterized hydroxyethylcellulose ethoxylate anion exchange membranes for direct methanol alkaline fuel cells. J. Power Sources 2013, 227, 291–299. [Google Scholar] [CrossRef]
- Roh, S.-H.; Lim, M.-H.; Sadhasivam, T.; Jung, H.-Y. Investigation on physico-chemical and electrochemical performance of poly(phenylene oxide)-based anion exchange membrane for vanadium redox flow battery systems. Electrochim. Acta 2019, 325, 134944. [Google Scholar] [CrossRef]
- Willdorf-Cohen, S.; Mondal, A.N.; Dekel, D.R.; Diesendruck, C.E. Chemical stability of poly (phenylene oxide)-based ionomers in an anion exchange-membrane fuel cell environment. J. Mater. Chem. A 2018, 6, 22234–22239. [Google Scholar] [CrossRef]
- Xu, T.; Wu, D.; Wu, L. Poly (2,6-dimethyl-1, 4-phenylene oxide) (PPO)—A versatile starting polymer for proton conductive membranes (PCMs). Prog. Polym. Sci. 2008, 33, 894–915. [Google Scholar] [CrossRef]
- Sadhasivam, T.; Kim, H.-T.; Park, W.-S.; Lim, H.; Ryi, S.-K.; Roh, S.-H.; Jung, H.-Y. Low permeable composite membrane based on sulfonated poly(phenylene oxide) (sPPO) and silica for vanadium redox flow battery. Int. J. Hydrogen Energy 2017, 42, 19035–19043. [Google Scholar] [CrossRef]
- Cho, C.G.; Kim, S.H.; Park, Y.C.; Kim, H.; Park, J.-W. Fuel cell membranes based on blends of PPO with poly(styrene-b-vinylbenzylphosphonic acid) copolymers. J. Membr. Sci. 2008, 308, 96–106. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; Ru, Y.; Hu, M.; Din, L.; Xu, T. Anion exchange membranes (AEMs) based on poly(2,6-dimethyl-1,4-phenylene oxide) (PPO) and its derivatives. Polym. Chem. 2015, 6, 5809–5826. [Google Scholar] [CrossRef]
- Msomi, P.F.; Nonjola, P.; Ndungu, P.G.; Ramonjta, J. Quaternized poly(2.6 dimethyl-1.4 phenylene oxide)/polysulfone blend composite membrane doped with ZnO-nanoparticles for alkaline fuel cells. J. Appl. Polym. Sci. 2018, 135, 45959. [Google Scholar] [CrossRef]
- Gopi, K.H.; Peera, S.G.; Bhat, S.; Sridhar, P.; Pitchumani, S. Preparation and characterization of quaternary ammonium func-tionalized poly(2,6-dimethyl-1,4-phenylene oxide) as anion exchange membrane for alkaline polymer electrolyte fuel cells. Int. J. Hydrogen Energy 2014, 39, 2659–2668. [Google Scholar] [CrossRef]
- Chen, N.; Liu, Y.; Long, C.; Li, R.; Wang, F.; Zhu, H. Enhanced performance of ionic-liquid-coated silica/quaternized poly(2,6-dimethyl-1,4-phenylene oxide) composite membrane for anion exchange membrane fuel cells. Electrochim. Acta 2017, 258, 124–133. [Google Scholar] [CrossRef]
- Sheng, W.; Zhou, X.; Wu, L.; Shen, Y.; Huang, Y.; Liu, L.; Dai, S.; Li, N. Quaternized poly(2,6-dimethyl-1,4-phenylene oxide) anion exchange membranes with pendant sterically-protected imidazoliums for alkaline fuel cells. J. Membr. Sci. 2020, 601, 117881. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z.; Bai, L.; Shao, C.; Chen, R.; Zhao, P.; Chu, X.; Li, N. Quaternized poly (2, 6-dimethyl-1, 4-phenylene oxide) anion exchange membranes based on isomeric benzyltrimethylammonium cations for alkaline fuel cells. J. Membr. Sci. 2020, 606, 118133. [Google Scholar] [CrossRef]
- Letsau, T.T.; Govender, P.P.; Msomi, P.F. Imidazolium-Quaternized Poly(2,6-Dimethyl-1,4-Phenylene Oxide)/Zeolitic Imidazole Framework-8 Composite Membrane as Polymer Electrolyte for Fuel-Cell Application. Polymers 2022, 14, 595. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, S.; Wan, R.; Yang, Y.; He, R. Functionalized graphene oxide cross-linked poly(2,6-dimethyl-1,4-phenylene ox-ide)-based anion exchange membranes with superior ionic conductivity. J. Power Sources 2022, 517, 230720. [Google Scholar] [CrossRef]
- Lee, K.H.; Chu, J.Y.; Kim, A.R.; Yoo, D.J. Simultaneous improvement of anion conductivity and cell durability through the formation of dense ion clusters of F-doped graphitic carbon nitride/quaternized poly(phenylene oxide) composite membrane. J. Membr. Sci. 2022, 650, 120384. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.; Sui, Z.; Shui, T.; Wang, S. A novel anion exchange membrane based on silicone/polyphenylene oxide with excellent ionic conductivity for AEMFC. Polym. Adv. Technol. 2022. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, J.; Liao, Y.; Li, C.; Wei, Z. Enhanced Conductivity of Anion-Exchange Membrane by Incorporation of Quaternized Cellulose Nanocrystal. ACS Appl. Mater. Interfaces 2018, 10, 23774–23782. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Park, B.J.; Kim, J.; Kang, D.; Yoon, H.H. Quaternized cellulose and graphene oxide crosslinked polyphenylene oxide based anion exchange membrane. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
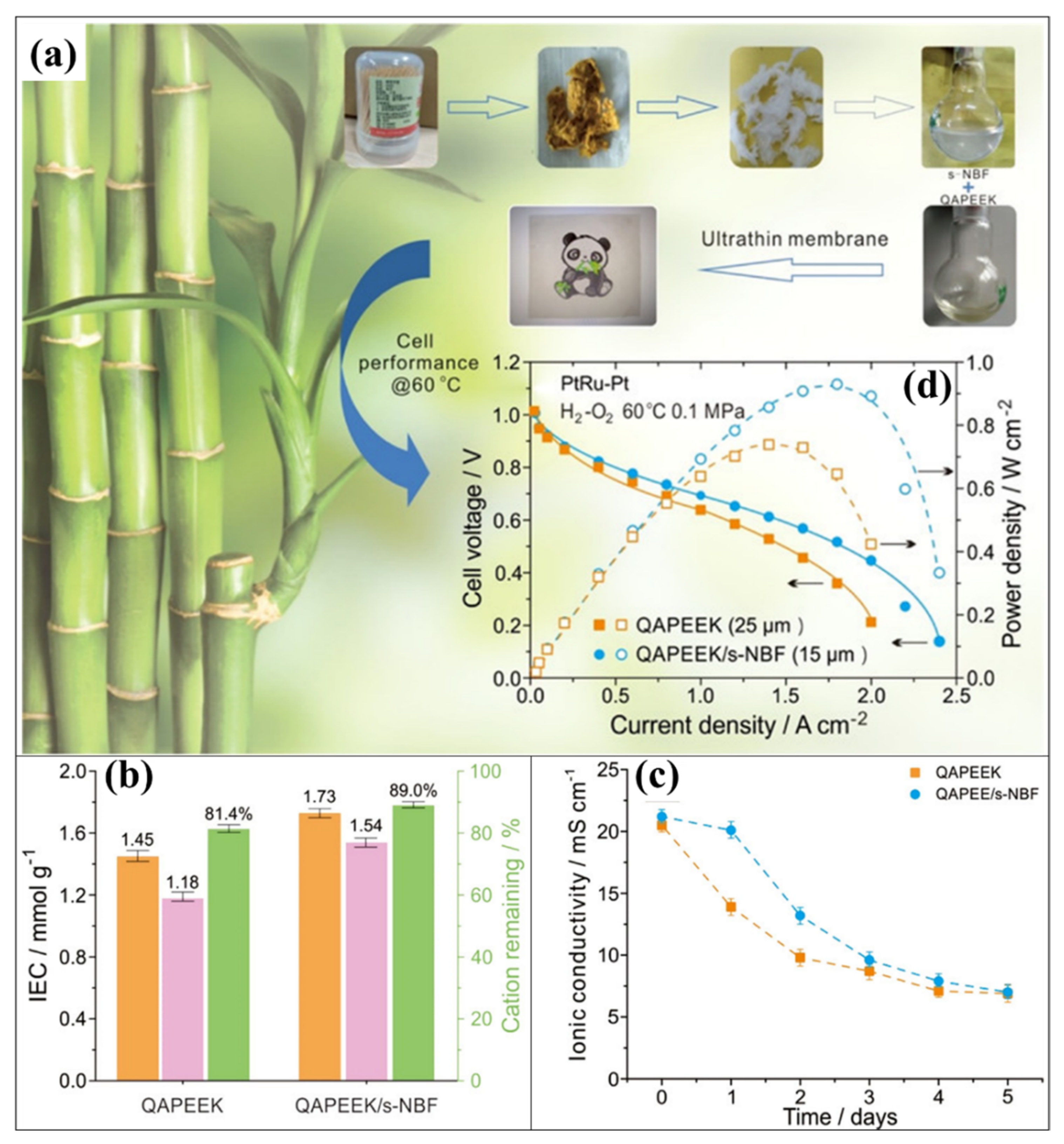
- Peng, Y.; Wang, Y.; Wei, X.; Zhou, J.; Peng, H.; Xiao, L.; Lu, J.; Zhuang, L. Sulfonated Nanobamboo Fiber-Reinforced Quaternary Ammonia Poly(ether ether ketone) Membranes for Alkaline Polymer Electrolyte Fuel Cells. ACS Appl. Mater. Interfaces 2018, 10, 33581–33588. [Google Scholar] [CrossRef]
- Samaniego, A.J.; Arabelo, A.K.; Sarker, M.; Mojica, F.; Madrid, F.J.; Chuang, P.-Y.A.; Ocon, J.; Espiritu, R. Fabrication of cellulose acetate-based radiation grafted anion exchange membranes for fuel cell application. J. Appl. Polym. Sci. 2021, 138, 49947. [Google Scholar] [CrossRef]


| Cellulose/Cellulose Derivatives | Polymers/Additives | IEC (meq/g) | Water Uptake (WU) and Swelling Ratio (SR) | Proton Conductivity (IC) | Fuel Cell Test (mW cm−2) | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| T (°C) | WU (%) | SR (%) | T (°C) | IC (S/cm) | |||||
| - | Nafion 112 | 0.91 | 38 | RT | 9.0 ± 0.5 × 10−3 | [119] | |||
| 100 | 9.9 × 10−2 | [120] | |||||||
| - | Nafion 117 | 0.92 | 40 | 16.5 | 9 | 80 | 95 × 10−3 | 140.8 (at 60 °C and 80% RH) | [46] |
| CNF | - | 0.016 | - | - | - | - | - | - | [108] |
| Sulfonated CNF | - | 0.900 | 26 ± 3 | 14 ± 5 | 120 | 2 × 10−3 | 156 (at 80 °C; 95% RH; 0.1 MPa) | ||
| - | Nafion | 0.92 | - | 26 ± 2 | 16 ± 2 | 120 | ~0.1 | - | |
| Nitro-oxidized carboxy CNF: carboxylate | - | - | - | - | - | 80 | 10.4 × 10−3 | - | [107] |
| Nitro-oxidized carboxy CNF: carboxylic acid | - | - | - | - | - | 80 | 14.6 × 10−3 | 19.1 (at 80 °C; 21 psi) | |
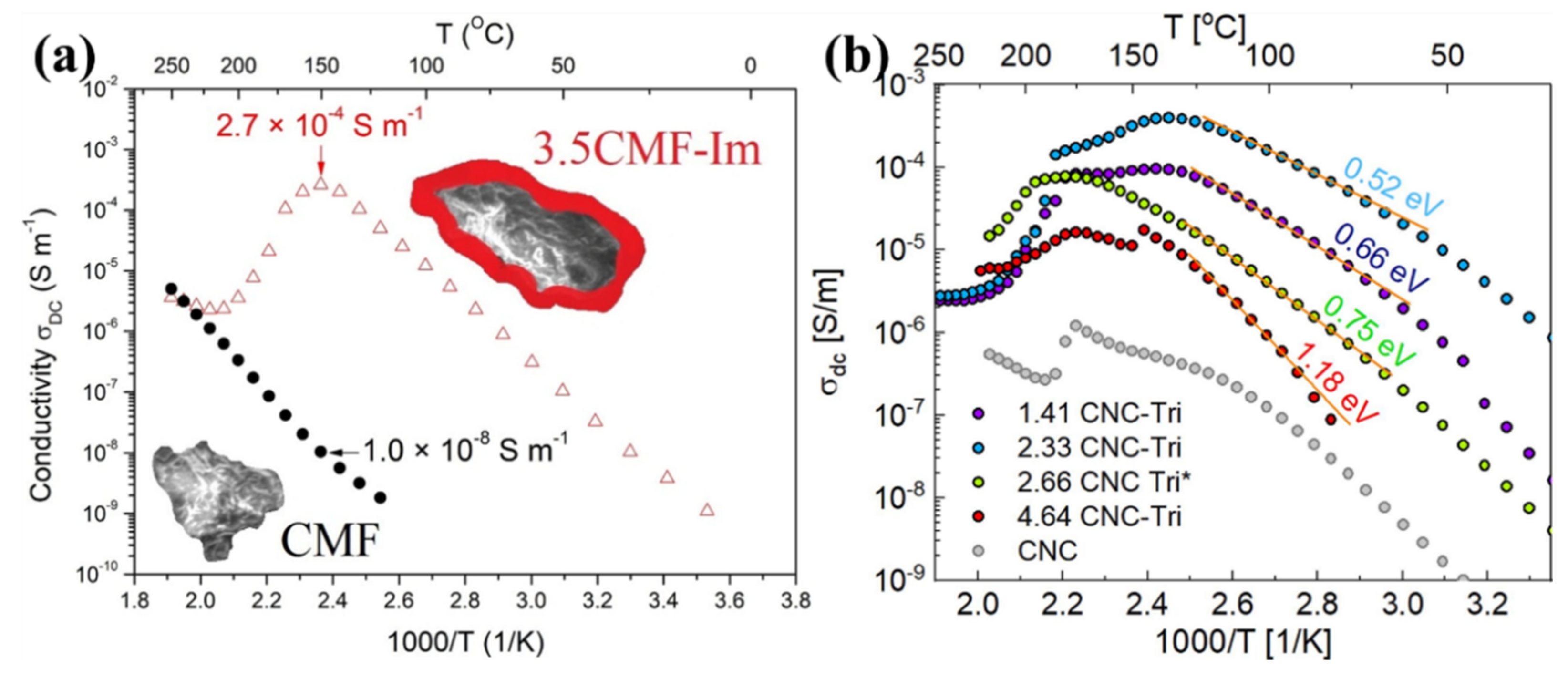
| CMF | - | - | - | - | - | 150 | 1.0 × 10−8 | - | [109] |
| CMF | imidazole molecules | - | - | - | - | 150 | 2.7 × 10−4 | - | |
| CNC | 1H-1,2,3 triazole | - | - | - | - | 160 | 4.0 × 10−4 @ anhydrous conditions | - | [110] |
| CNC | - | - | - | - | - | 20 @ ~96% RH | 0.4 × 10−3 | - | [111] |
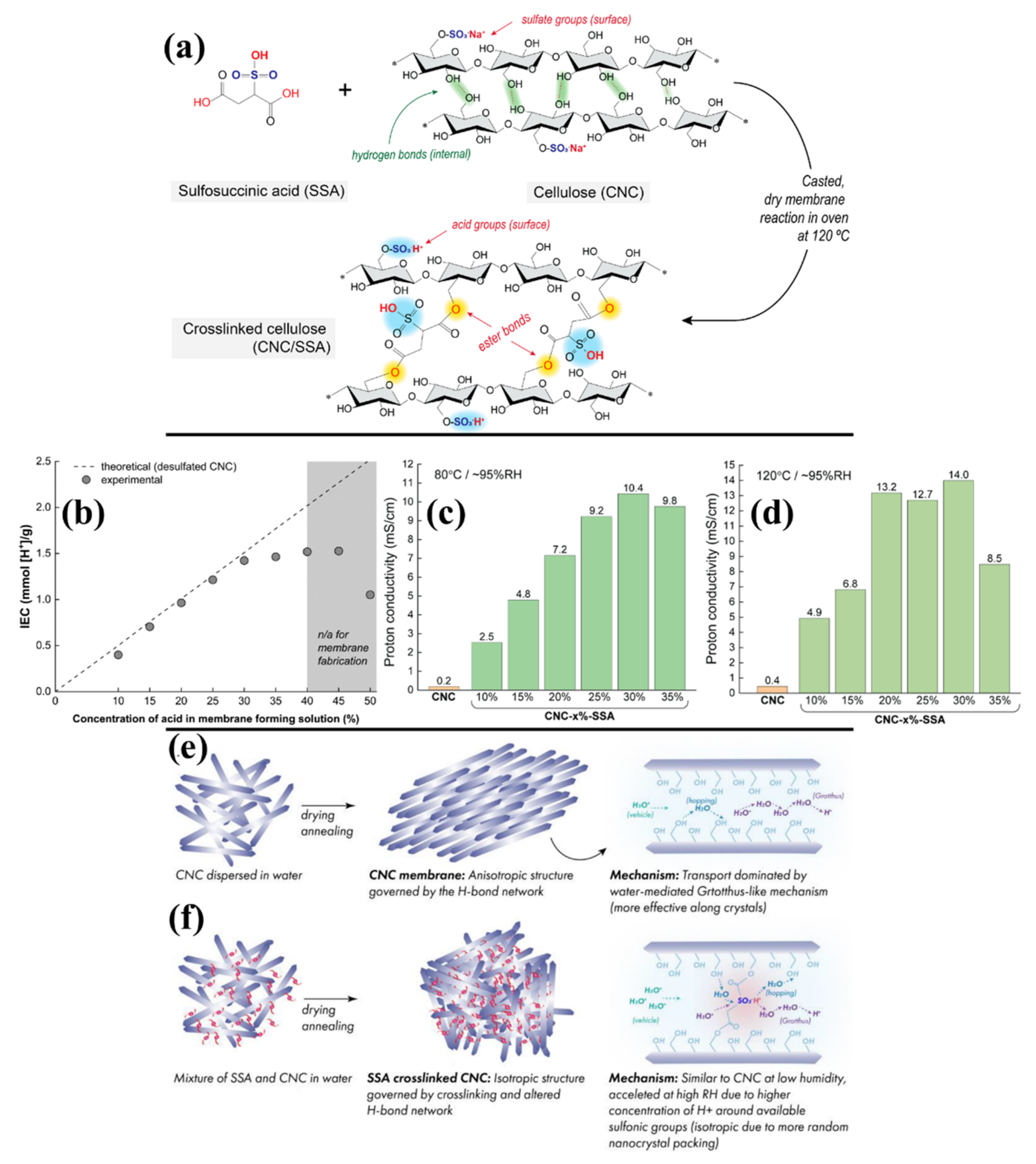
| CNC | Sulfosuccinic Acid (SSA)−10% | 0.399 | - | - | - | 4.8 × 10−3 | - | ||
| CNC | SSA–15% | 0.705 | - | - | - | 7.5 × 10−3 | - | ||
| CNC | SSA–20% | 0.964 | - | - | - | 11.6 × 10−3 | - | ||
| CNC | SSA–25% | 1.214 | - | - | - | 12.7 × 10−3 | - | ||
| CNC | SSA–30% | 1.423 | - | - | - | 14.0 × 10−3 | - | ||
| CNC | SSA–35% | 1.464 | - | - | - | 10.1 × 10−3 | - | ||
| CNC—Ramie | - | - | - | 21 | - | - | - | - | [114] |
| CNC—Tunicate | - | - | - | 21 | - | - | - | - | |
| CNC–Ramie (5%) | Nafion | - | 29.5 | - | - | - | - | ||
| CNC–Tunicate (5%) | Nafion | 1.07 ± 0.01 | - | 28 | - | - | 100 × 10−3 | - | |
| - | Nafion | 1.07 ± 0.01 | - | - | - | - | 90 × 10−3 | - | |
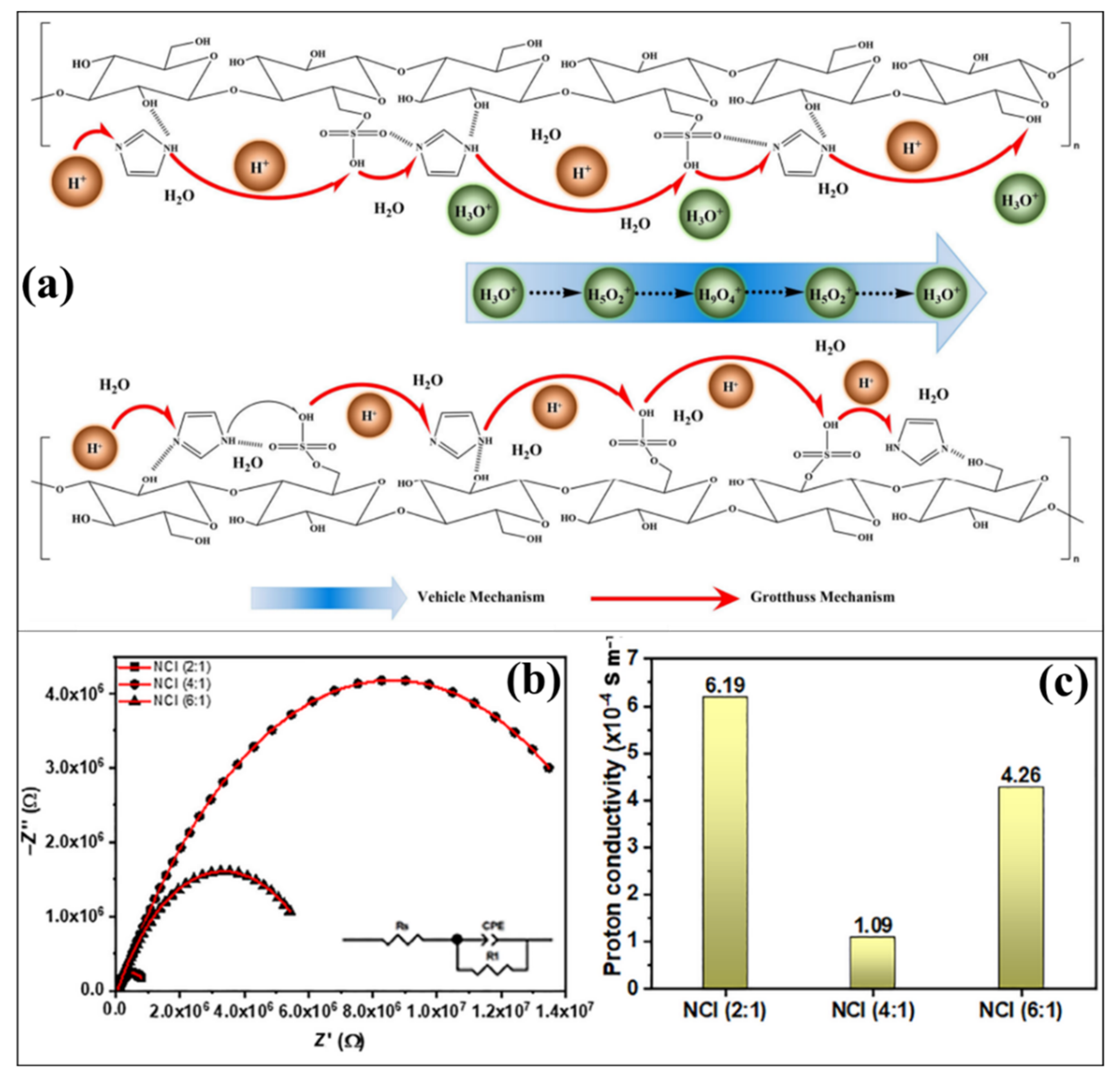
| CNC | - | - | - | - | - | 25 | 0.19 × 10−4 | - | [115] |
| Im doped CNC | - | - | - | - | - | 1.20 × 10−4 | - | ||
| Im doped CNC | Nafion (2:1) | - | - | - | - | 6.19 × 10−4 | - | ||
| Im doped CNC | Nafion (4:1) | - | - | - | - | 1.09 × 10−4 | - | ||
| Im doped CNC | Nafion (6:1) | - | - | - | - | 4.26 × 10−4 | - | ||
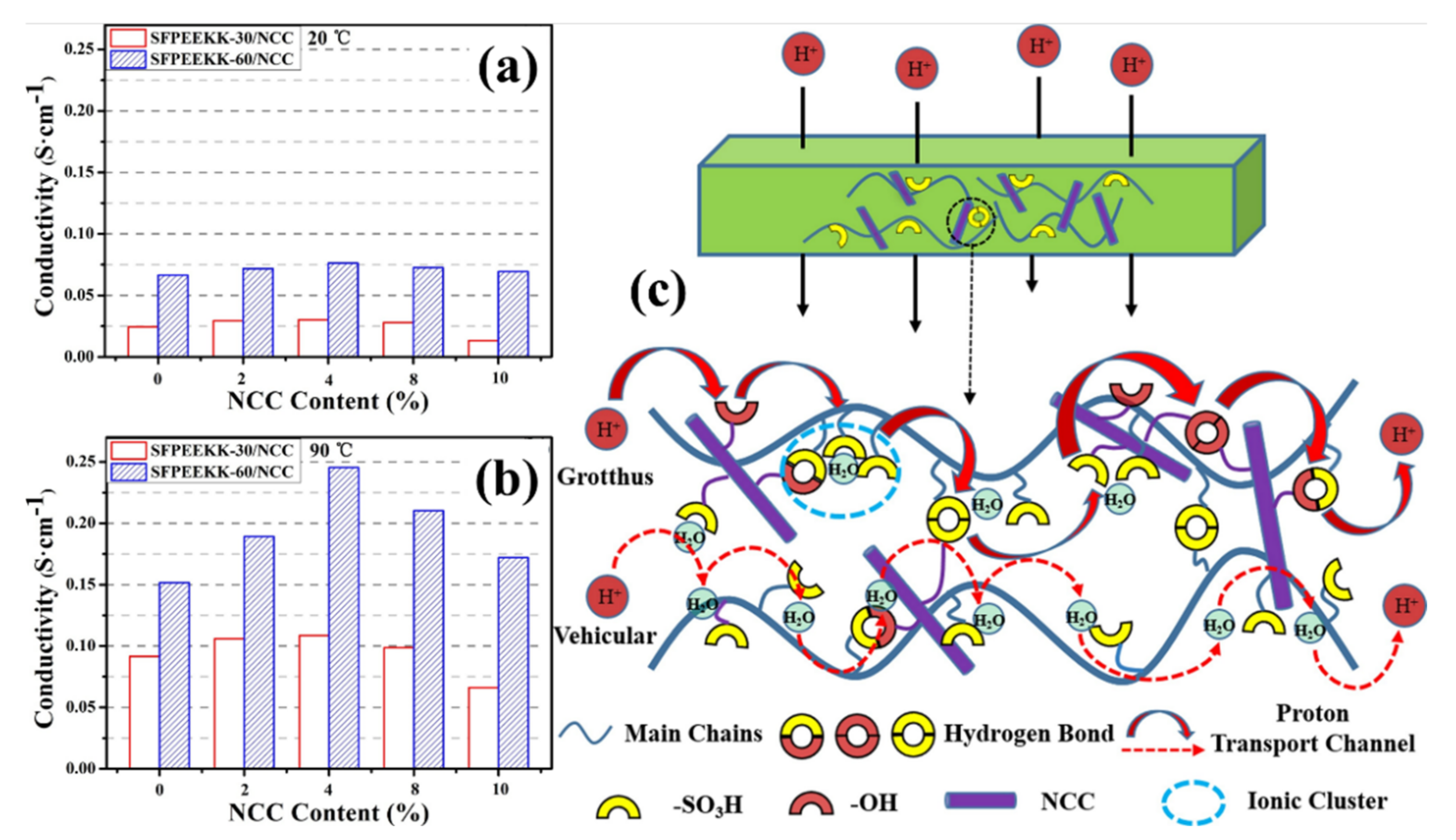
| - | SFPEEKK-30 | 0.92 | 20 | 7.81 | 2.11 | 20 | 0.024 | - | [117] |
| 90 | 17.69 | 7.33 | 90 | 0.092 | - | ||||
| CNC-2% | SFPEEKK-30 | 0.93 | 20 | 13.59 | 2.81 | 20 | 0.029 | - | |
| 90 | 25.34 | 11.45 | 90 | 0.106 | - | ||||
| CNC-4% | SFPEEKK-30 | 0.98 | 20 | 16.00 | 4.16 | 20 | 0.030 | - | |
| 90 | 26.98 | 14.97 | 90 | 0.109 | - | ||||
| CNC-8% | SFPEEKK-30 | 0.96 | 20 | 11.80 | 3.30 | 20 | 0.028 | - | |
| 90 | 21.33 | 12.77 | 90 | 0.099 | - | ||||
| CNC-10% | SFPEEKK-30 | 0.85 | 20 | 9.24 | 2.39 | 20 | 0.013 | - | |
| 90 | 20.18 | 9.21 | 90 | 0.066 | - | ||||
| - | SFPEEKK-60 | 1.72 | 20 | 33.95 | 10.13 | 20 | 0.066 | - | |
| 90 | 92.86 | 24.43 | 90 | 0.152 | - | ||||
| CNC-2% | SFPEEKK-60 | 1.91 | 20 | 35.06 | 10.28 | 20 | 0.072 | - | |
| 90 | 104.15 | 26.34 | 90 | 0.189 | - | ||||
| CNC-4% | SFPEEKK-60 | 2.03 | 20 | 36.29 | 11.42 | 20 | 0.076 | - | |
| 90 | 115.73 | 32.6 | 90 | 0.245 | - | ||||
| CNC-8% | SFPEEKK-60 | 1.98 | 20 | 35.08 | 10.68 | 20 | 0.073 | - | |
| 90 | 107.24 | 27.43 | 90 | 0.210 | - | ||||
| CNC-10% | SFPEEKK-60 | 1.84 | 20 | 32.58 | 9.57 | 20 | 0.069 | - | |
| 90 | 79.34 | 21.27 | 90 | 0.172 | - | ||||
| - | SPAEK-COOH-10 | 2.013 | 80 | 53.95 | 16.84 | 80 | 0.162 | - | [118] |
| - | SPAEK-COOH-30 | 2.04 | 80 | - | - | 80 | 0.201 | - | |
| CNC-5 composite | SPAEK-COOH-10 | 2.37 | 80 | 58.47 | 19.65 | 80 | 0.177 | - | |
| CNC-8 composite | SPAEK-COOH-10 | 2.31 | 80 | 55.54 | 18.79 | 80 | 0.171 | - | |
| CNC-2 cross-linking | SPAEK-COOH-10 | 2.15 | 80 | 48.95 | 14.17 | 80 | 0.138 | - | |
| CNC-5 cross-linking | SPAEK-COOH-10 | 2.16 | 80 | 50.67 | 14.20 | 80 | 0.152 | - | |
| CNC-8 cross-linking | SPAEK-COOH-10 | 2.23 | 80 | 51.58 | 14.54 | 80 | 0.160 | - | |
| CNC-10 cross-linking | SPAEK-COOH-10 | 2.19 | 80 | 48.02 | 16.16 | 80 | 0.142 | - | |
| - | Ph-SPEEKK | - | 80 | 46.7 ± 2 | 14.6 ± 1.6 | 80 | 0.078 | 104 | [121] |
| sCNC-5 | Ph-SPEEKK | - | 80 | 49.9 ± 4 | 19.4 ± 1.4 | 80 | 0.102 | - | |
| Am1-sCNC-2 | Ph-SPEEKK | - | 80 | 48.1 ± 3 | 11.1 ± 1.9 | 80 | 0.120 | - | |
| Am1-sCNC-5 | Ph-SPEEKK | - | 80 | 54.7 ± 4 | 11.9 ± 2.0 | 80 | 0.127 | - | |
| Am1-sCNC-8 | Ph-SPEEKK | - | 80 | 52.8 ± 4 | 12.3 ± 2.3 | 80 | 0.107 | - | |
| Am3-sCNC-2 | Ph-SPEEKK | - | 80 | 58.4 ± 4 | 11.3 ± 1.0 | 80 | 0.126 | - | |
| Am3-sCNC-5 | Ph-SPEEKK | - | 80 | 58.3 ± 3 | 13.8 ± 1.8 | 80 | 0.133 | 227 (at 75 °C) | |
| Am3-sCNC-8 | Ph-SPEEKK | - | 80 | 61.6 ± 3 | 12.4 ± 1.4 | 80 | 0.090 | - | |
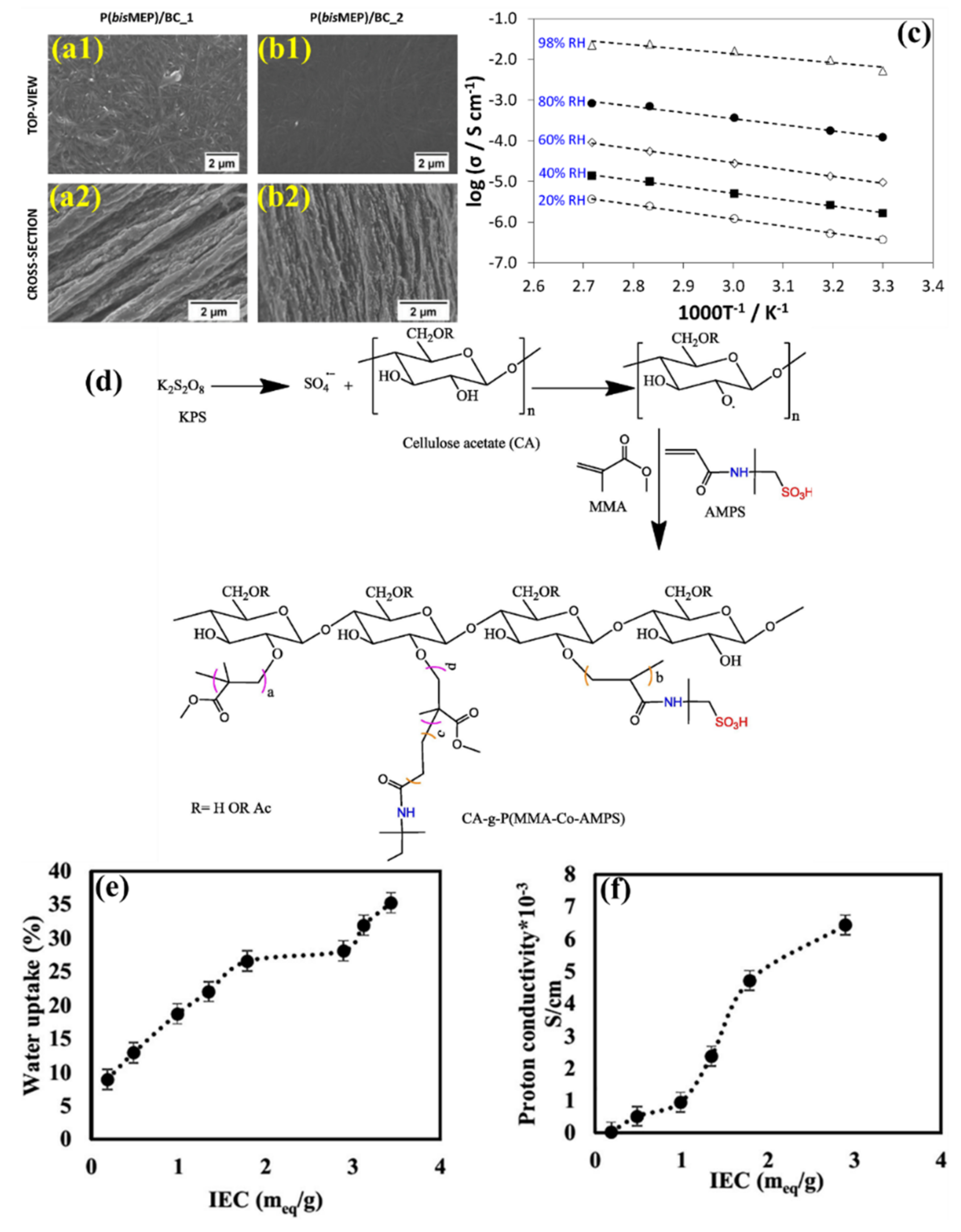
| BC | - | – | RT | 121 ± 11 | - | - | - | - | [122] |
| - | P(bisMEP) | 3.5 ± 0.02 | - | - | - | - | - | - | |
| BC_1 | P(bisMEP) | 1.1 ± 0.1 | 79 ± 6 | - | 94 | 22.4 @98% RH | - | ||
| BC_2 | P(bisMEP) | 3.0 ± 0.05 | 155 ± 8 | - | 80 | 27.2 @98% RH | - | ||
| CA | - | - | 0.035 × 10−3 | - | [123] | ||||
| CA | p(AMPAS-co-MMA) | 3.4 | 27 | - | - | 6.44 × 10−3 | - | ||
| - | Nafion | - | - | - | - | 60 @ 95% RH | 1.6 × 10−1 | - | [124] |
| CNC | PVPA-b-PS | - | - | - | - | 3.8 × 10−2 | - | ||
| CNC | PVPA-b-PS/PC | - | - | - | - | 1.8 × 10−2 | - | ||
| CNC:PVA (1:1) | - | [125] | |||||||
| CNC | PVA–2 mmol 1,2,4-triazole | - | - | - | - | 120 | 0.0986 × 10−3 | - | |
| - | - | - | - | 140 | 0.0918 × 10−3 | - | |||
| CNC | PVA–3 mmol 1,2,4-triazole | - | - | - | - | 120 | 3.1 × 10−3 | - | |
| - | - | - | - | 140 | 2.0 × 10−3 | - | |||
| CNC | PVA–4 mmol 1,2,4-triazole | - | - | - | - | 120 | 4.54 × 10−3 | - | |
| - | - | - | - | 140 | 2.82 × 10−3 | - | |||
| CNC | PVA–5 mmol 1,2,4-triazole | - | - | - | - | 120 | 13 × 10−3 | - | |
| - | - | - | - | 140 | 8.4 × 10−3 | - | |||
| - | BG-BF4 | - | - | - | - | 25 | 2.1 × 10−2 | - | [126] |
| - | - | - | - | 180 | 1.8 × 10−1 | - | |||
| BC | BG–BF4 (60%) | - | - | - | - | 25 | 3.5 × 10−5 | - | |
| - | - | - | - | 180 | 1.9 × 10−4 | - | |||
| BC | BG–BF4 (80%) | - | - | - | - | 25 | 1.2 × 10−4 | - | |
| - | - | - | - | 180 | 4.5 × 10−4 | - | |||
| BC | BG–BF4 (95%) | - | - | - | - | 25 | 1.6 × 10−3 | - | |
| - | - | - | - | 180 | 5.2 × 10−2 | - | |||
| BC | PAN—BG-BF4 (80%) | - | - | - | - | 25 | 1.5 × 10−4 | - | |
| - | - | - | - | 180 | 4 × 10−3 | - | |||
| BC | PAN—BG-BF4 (95%) | - | - | - | - | 25 | 1.2 × 10−2 | - | |
| - | - | - | - | 180 | 1 × 10−1 | - | |||
| - | Nafion | - | - | - | - | - | 19.04 × 10−3 | - | [127] |
| BC | sPS | - | - | - | - | - | 7.17 × 10−3 | - | |
| BC | sPS+(0.04%) H3PO4 | - | - | - | - | - | 2.02 × 10−3 | - | |
| BC | sPS + (0.2%) H3PO4 | - | - | - | - | - | 3.12 × 10−3 | - | |
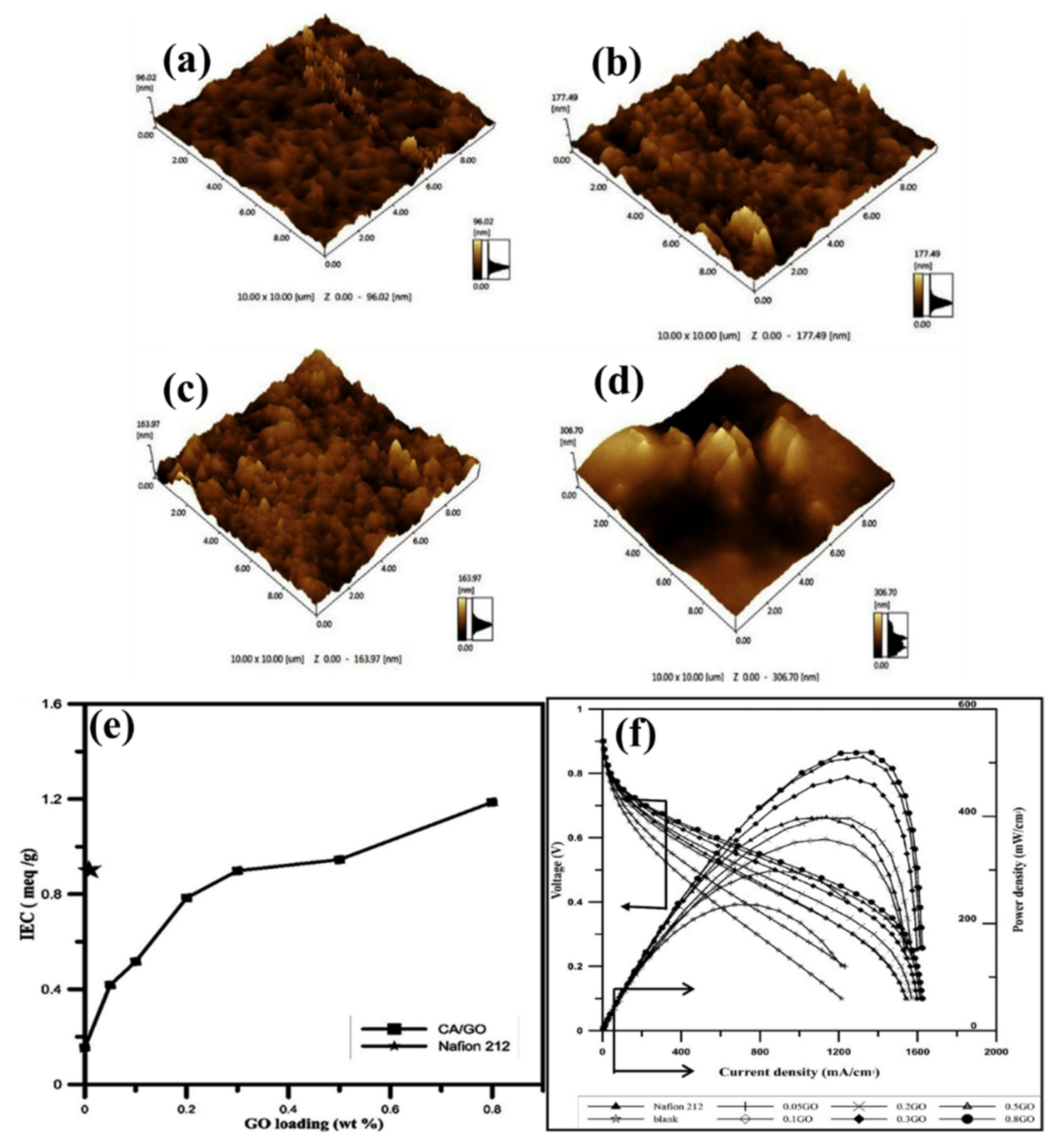
| - | Nafion 212 | 0.913 | - | - | - | - | 6.94 × 10−3 | 401 (at 60 °C) | [128] |
| CA | - | 0.15 | 8.96 | - | - | 1.21 × 10−3 | 235 (at 60 °C) | ||
| CA | GO–0.05 wt.% | 0.4 | 11.17 | - | - | 1.97 × 10−3 | - | ||
| CA | GO–0.1 wt.% | 0.5 | 11.48 | - | - | 3.79 × 10−3 | - | ||
| CA | GO–0.2 wt.% | 0.8 | 18.97 | - | - | 6.92 × 10−3 | - | ||
| CA | GO–0.3 wt.% | 0.913 | 20.77 | - | - | 9.26 × 10−3 | - | ||
| CA | GO–0.5 wt.% | 0.95 | 22.99 | - | - | 13.41 × 10−3 | - | ||
| CA | GO–0.8 wt.% | 1.18 | 24.06 | - | - | 15.5 × 10−3 | 519 (at 60 °C) | ||
| - | Nafion | 1.0 | - | - | - | - | - | - | [129] |
| Cellulose fiber membrane | Nafion | 0.15 | - | - | - | 30 | 0.007 | 23 (at 80 °C) | |
| - | - | - | 90 | 0.015 | |||||
| Cellulose fiber membrane | Resorcinol bis(diphenyl phosphate) | 0.04 | - | - | - | 30 | 0.003 | 10 (at 60 °C) | |
| - | - | - | 90 | 0.010 | |||||
| Bacterial nanocellulose (BNC) | - | - | RT | 21.8 ± 2.1 | - | - | - | - | [130] |
| - | Lignosulfonates (LS) | - | 214 ± 5.7 | - | - | - | - | ||
| BNC (2:1) | LS | - | 55.6 ± 2.4 | - | - | - | - | ||
| BNC (4:3) | LS | - | 78.0 ± 3.7 | - | 30 | 7.3 × 10−3 | - | ||
| - | - | - | 80 | 1.9 × 10−2 | - | ||||
| BNC | - | - | RT | 22 ± 2 | - | 94 @ 80 % RH | 3.7 × 10−7 | - | [131] |
| - | Fucoidan | - | 68 ± 3 | - | - | - | |||
| BNC—57 wt.% | Fucoidan-43 wt.% | - | 45 ± 3 | - | 1.3 × 10−4 | - | |||
| BNC—67 wt.% | Fucoidan-33 wt.% | - | 32 ± 2 | - | 7.7 × 10−5 | - | |||
| Cellulose/Cellulose Derivatives | Polymers/Additives | IEC (meq g−1) | Water Uptake (WU) and Swelling Ratio(SR) | Ion Conductivity | Tensile Strength (MPa) | Fuel Cell Test Results (mW cm−2) | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| (°C) | WU (%) | SR (%) | (°C) | (IC) (mS cm−1) | ||||||
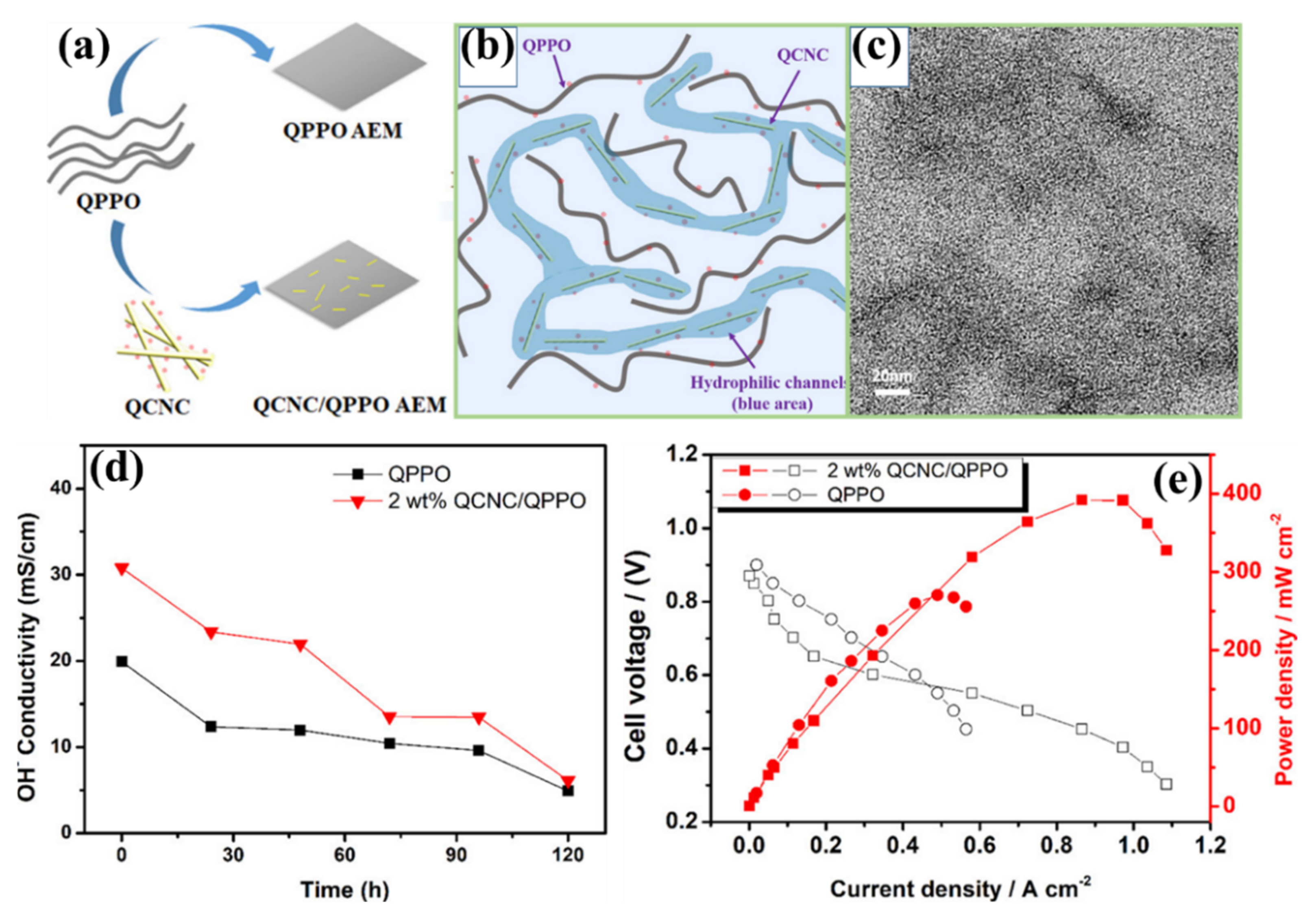
| QPPO | 1.00 | - | 15.1 ± 1.1 | 2.2 ± 0.1 | 20 | 16.7 ± 0.2 | 28.3 ± 0.9 | 270 (at 60 °C) | [175] | |
| QCNC-0.5 wt.% | QPPO | 1.01 | - | 18.0 ± 2.2 | 2.4 ± 0.4 | 20 | 19.3 ± 0.6 | 28.9 ± 2.5 | - | |
| QCNC-1 wt.% | QPPO | 1.06 | - | 17.7 ± 1.4 | 2.2 ± 0.2 | 20 | 21.3 ± 0.6 | 28.6 ± 0.6 | - | |
| QCNC-2 wt.% | QPPO | 1.05 | - | 16.9 ± 0.4 | 2.6 ± 0.3 | 20 | 28.0 ± 0.1 | 30.9 ± 0.5 | 392 (at 60 °C) | |
| - | - | - | 80 | 60 | ||||||
| QCNC-3 wt.% | QPPO | 1.00 | - | 16.8 ± 0.2 | 2.4 ± 0.0 | 20 | 20.5 ± 0.3 | 22.8 ± 2.8 | - | |
| QCNC-4 wt.% | QPPO | 1.04 | - | 17.6 ± 0.8 | 2.3 ± 0.4 | 20 | 13.9 ± 0.7 | 20.2 ± 2.7 | - | |
| QPPO | 0.85 | - | 25.34 | 12.07 | RT | 20.20 | - | - | [176] | |
| QCF-1% | QPPO | 1.12 | - | 40.52 | 11.56 | RT | 31.93 | - | - | |
| QCF-0.5% | QPPO−QGO1% | 2.35 | - | 78.87 | 11.49 | RT | 79.71 | - | - | |
| 80 | 157.32 | - | - | |||||||
| QCF-1% | QPPO−QGO1% | 2.64 | - | 88.97 | 17.21 | RT | 114.64 | - | - | |
| 80 | 215.66 | - | - | |||||||
| QCF-2% | QPPO−QGO0.5% | 2.09 | - | 86.19 | 22.88 | RT | 60.23 | - | - | |
| QCF-3% | QPPO−QGO0.5% | 1.82 | - | 88.13 | 25.55 | RT | 68.92 | - | - | |
| QAPEEK (1:0) | 1.70 | 30 | - | 20.0 | 30 | 20.7 | - | 760 (at 60 °C) | [177] | |
| 80 | - | 27.5 | 80 | 110 | - | |||||
| SCNF | QAPEEK (12:1) | 1.66 | 30 | - | 17.5 | 30 | 22.0 | - | - | |
| 80 | - | 22.5 | 80 | 103 | - | - | ||||
| SCNF | QAPEEK (8:1) | 1.76 | 30 | - | 7.5 | 30 | 21.4 | - | 930 (at 60 °C) | |
| 80 | - | 7.5 | 80 | 119 | - | |||||
| SCNF | QAPEEK (6:1) | 1.59 | 30 | - | 5.0 | 30 | 11.11 | - | - | |
| 80 | - | 5.0 | 80 | 56.1 | - | - | ||||
| SCNF | QAPEEK (4.8:1) | 1.51 | 30 | - | 2.5 | 30 | 7.6 | - | - | |
| 80 | - | 2.5 | 80 | 31.5 | - | - | ||||
| cellulose acetate | vinylbenzyl chloride-grafted (CA-g-VBC) | - | - | - | - | - | 16.3 | - | - | [178] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thangarasu, S.; Oh, T.-H. Recent Developments on Bioinspired Cellulose Containing Polymer Nanocomposite Cation and Anion Exchange Membranes for Fuel Cells (PEMFC and AFC). Polymers 2022, 14, 5248. https://doi.org/10.3390/polym14235248
Thangarasu S, Oh T-H. Recent Developments on Bioinspired Cellulose Containing Polymer Nanocomposite Cation and Anion Exchange Membranes for Fuel Cells (PEMFC and AFC). Polymers. 2022; 14(23):5248. https://doi.org/10.3390/polym14235248
Chicago/Turabian StyleThangarasu, Sadhasivam, and Tae-Hwan Oh. 2022. "Recent Developments on Bioinspired Cellulose Containing Polymer Nanocomposite Cation and Anion Exchange Membranes for Fuel Cells (PEMFC and AFC)" Polymers 14, no. 23: 5248. https://doi.org/10.3390/polym14235248
APA StyleThangarasu, S., & Oh, T.-H. (2022). Recent Developments on Bioinspired Cellulose Containing Polymer Nanocomposite Cation and Anion Exchange Membranes for Fuel Cells (PEMFC and AFC). Polymers, 14(23), 5248. https://doi.org/10.3390/polym14235248







