Stereolithography of Semiconductor Silver and Acrylic-Based Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
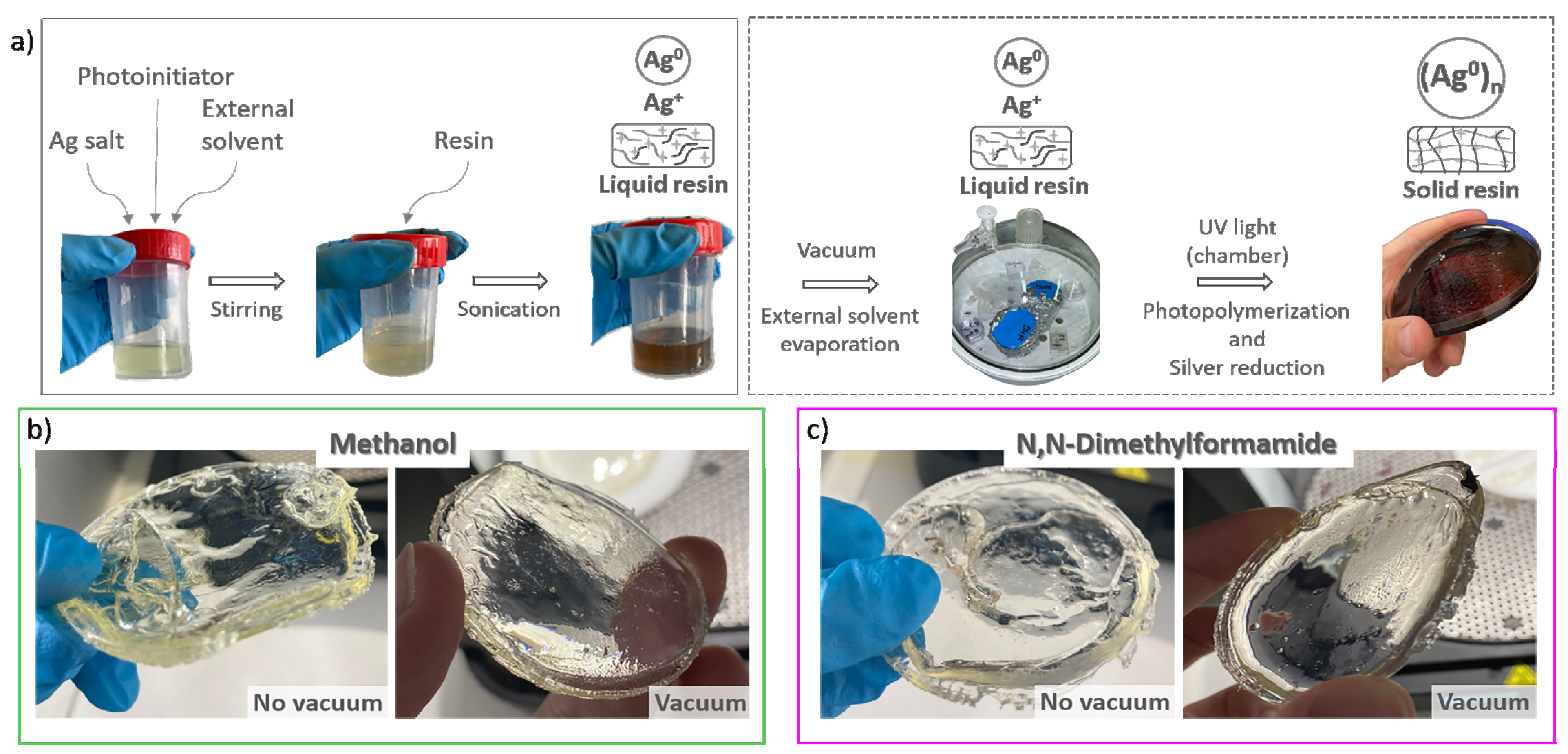
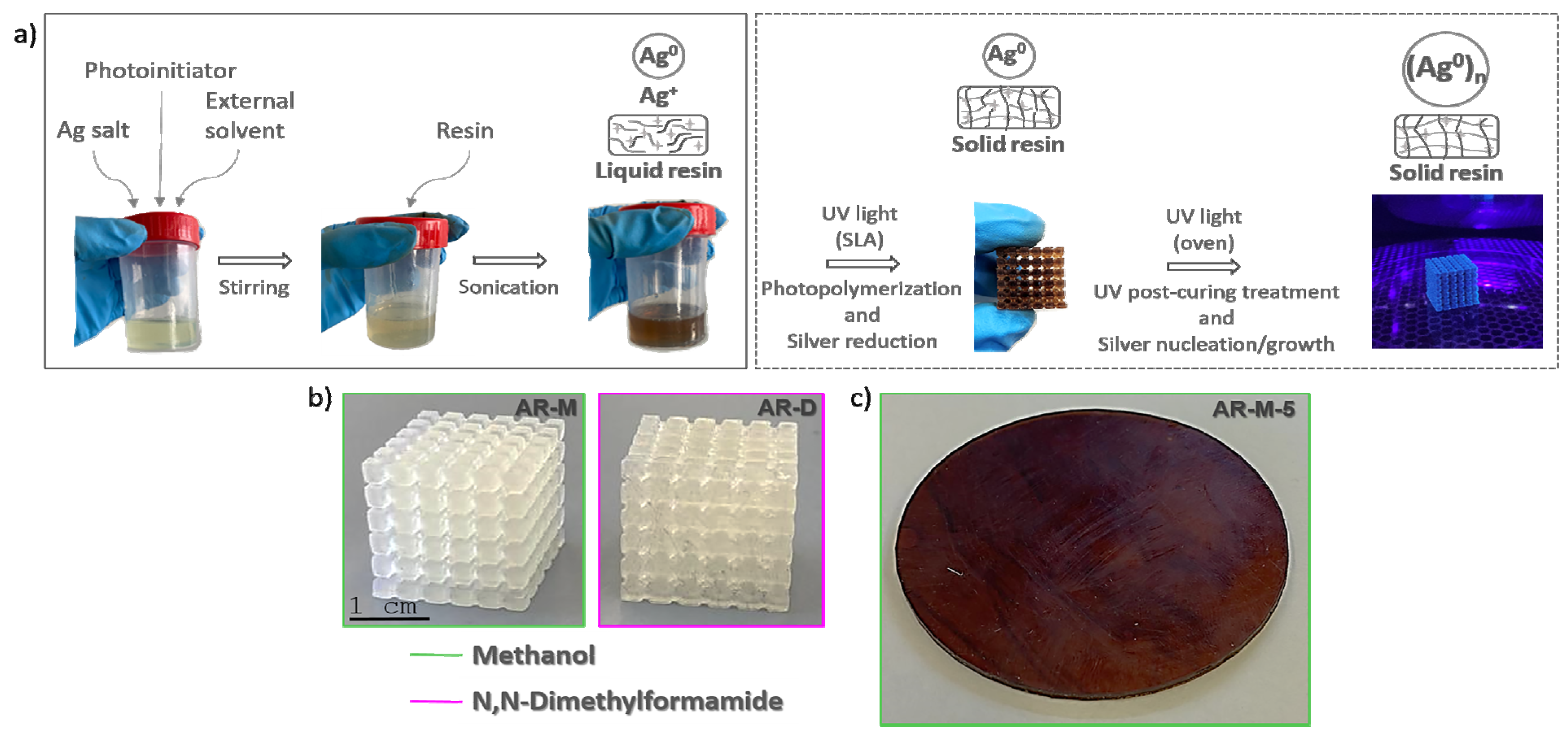
2.2. Sample Preparation
- -
- Using a UV chamber with a light source of 405 nm and a power of 1.25 mW/cm2 (FormCure, Formlabs, Somerville, MA, USA)) for 90 min. Previous to that, some solutions were left in vacuum during 24 h to evaporate the solvent.
- -
- Printing by SLA with Nobel 1.0 equipment, XYZprinting, Inc. (XYZprinting, New Taipei City, Taiwan)., using a 405 nm laser with an output power of 100 mW and a spot size that allows an XY resolution of 300 µm. All samples were printed with a layer height of 100 µm. Once printed, the samples were washed in IPA for several minutes. Post-processing of the samples was also performed inside a UV chamber with a light source of 405 nm and a power of 1.25 mW/cm2 (FormCure, Formlabs) for 60 min.
2.3. Characterization
3. Results
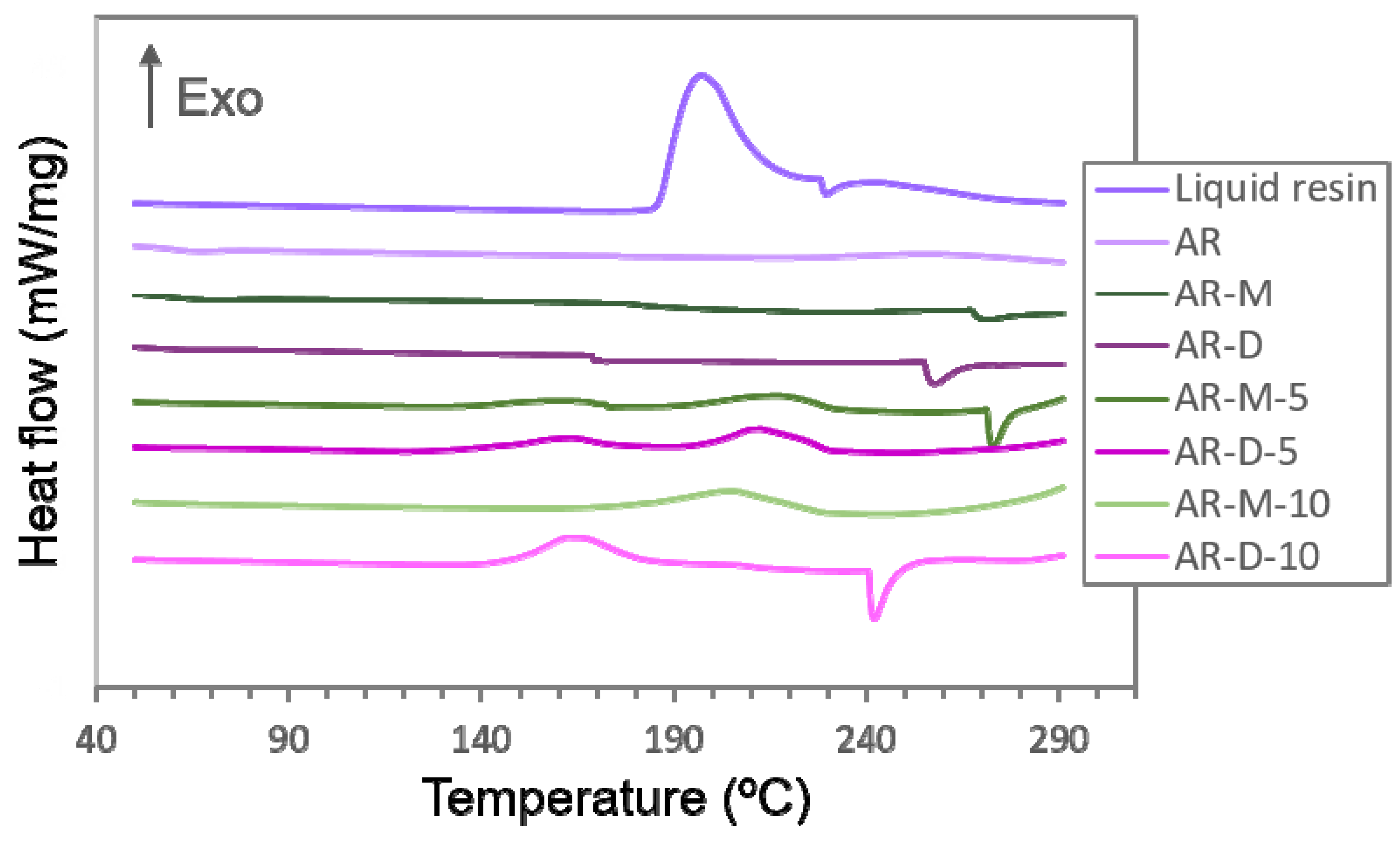
3.1. Fabrication of Nanocomposites: Photopolymerization in UV Chamber vs. SLA Printer
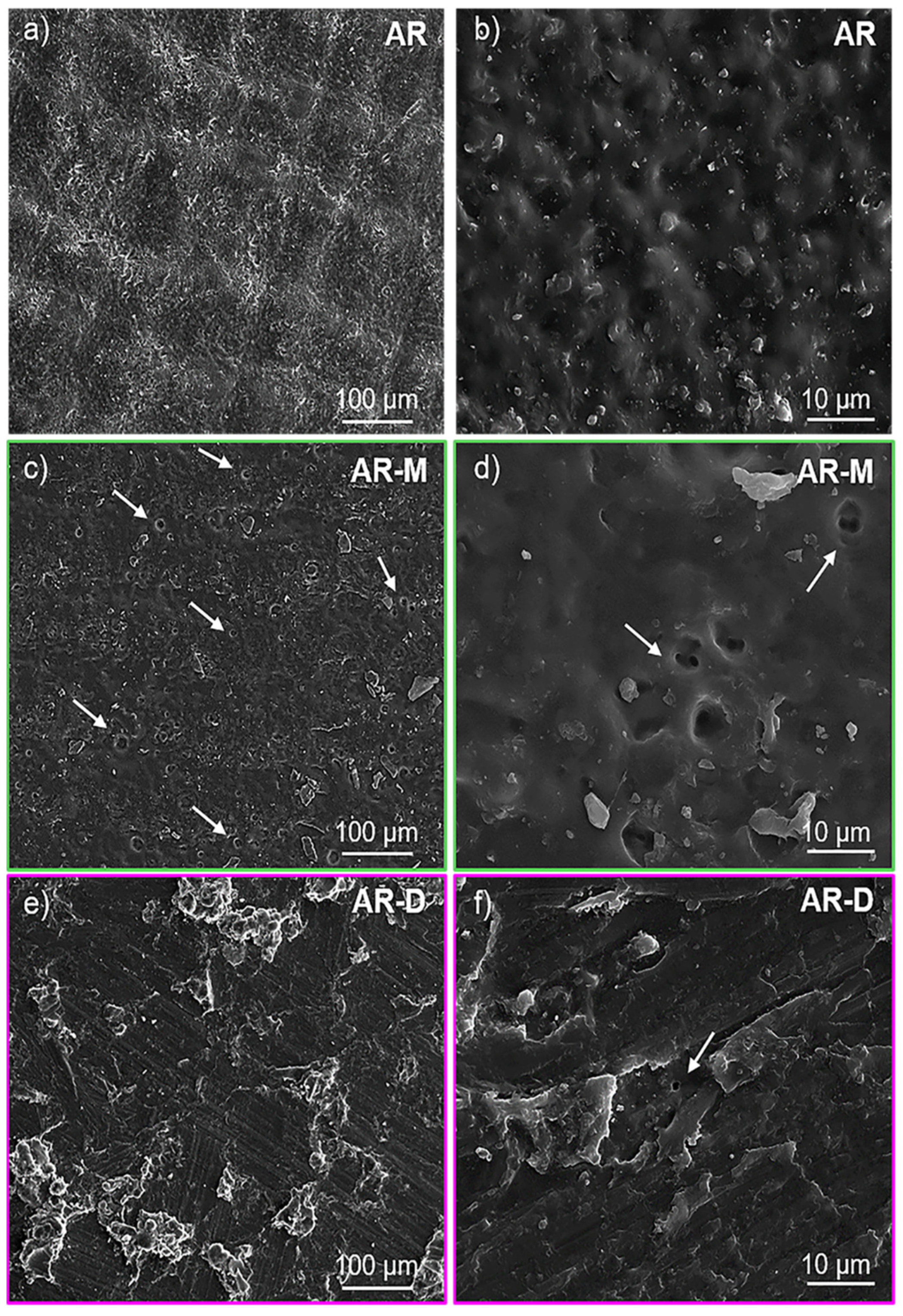
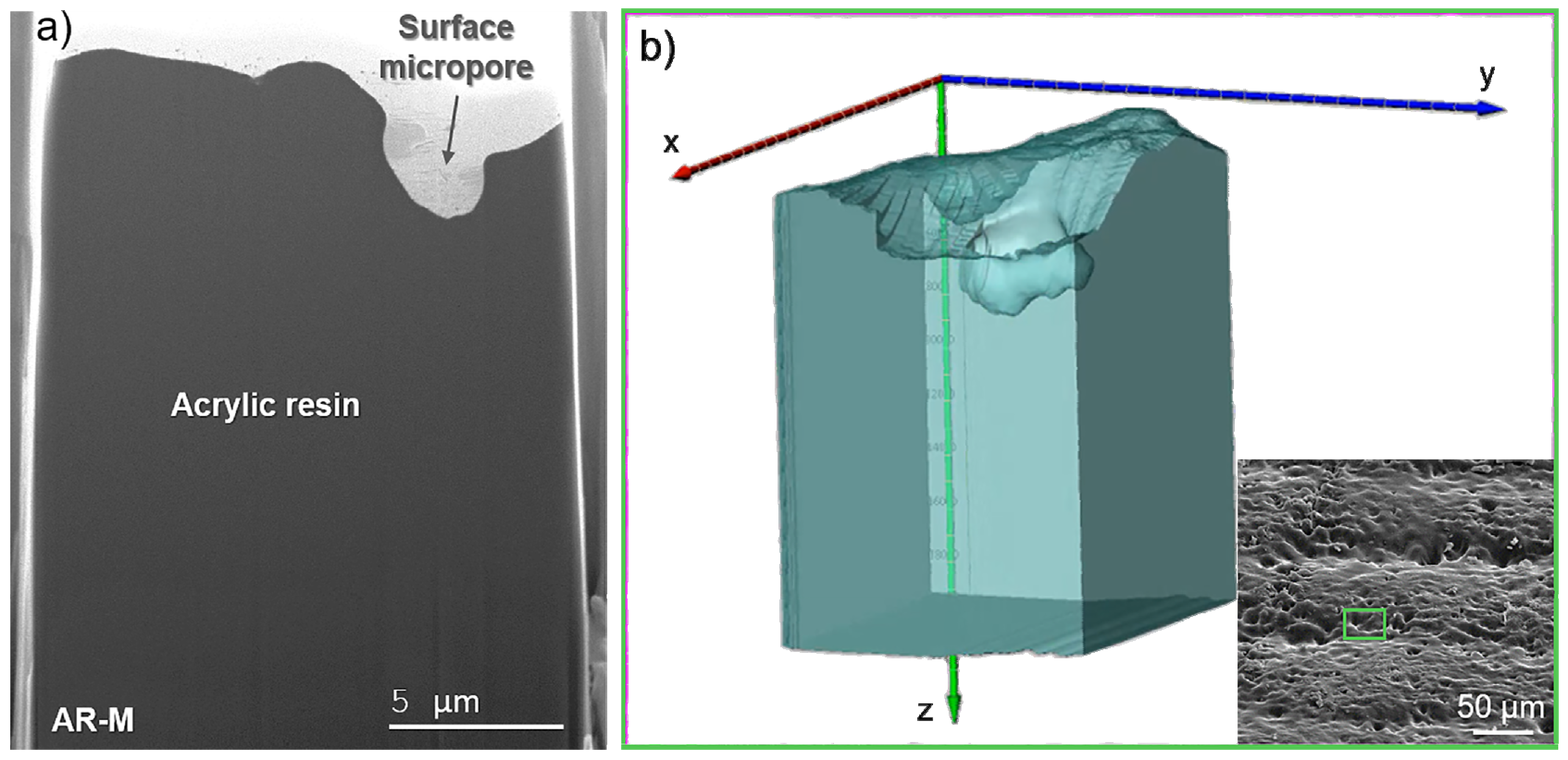
3.2. Morphological and Structural Characterization
3.3. Shore D Hardness Measurements and Analysis of the Degree of Cure
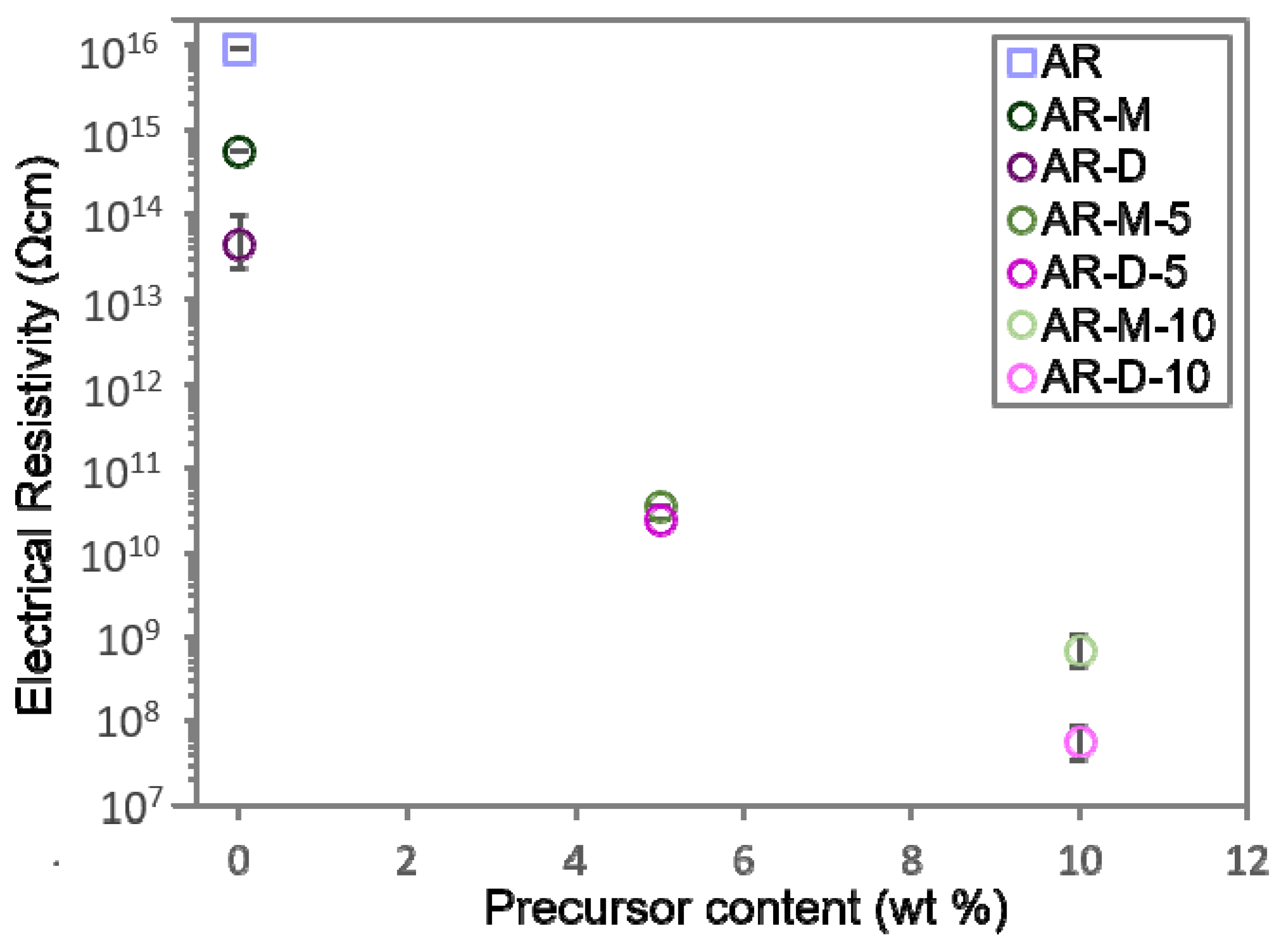
3.4. Electrical Properties of the Fabricated Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, X.; Yang, G.; Xu, Q.; Hou, W.; Zhu, J.J. Self-assembly of polyaniline/Au composites: From nanotubes to nanofibers. Macromol. Rapid Commun. 2006, 27, 31–36. [Google Scholar] [CrossRef]
- Williams, P.E.; Jones, S.T.; Walsh, Z.; Appel, E.A.; Abo-Hamed, E.K.; Scherman, O.A. Synthesis of conducting polymer-metal nanoparticle hybrids exploiting RAFT polymerization. ACS Macro Lett. 2015, 4, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Ju, H.; Kim, J. Effect of SrTiO3 Nanoparticles in Conductive Polymer on the Thermoelectric Performance for Efficient Thermoelectrics. Polymers 2020, 12, 777. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Saxena, P. Polymer Nanocomposites in Sensor Applications: A Review on Present Trends and Future Scope. Chin. J. Polym. Sci. 2021, 39, 665–691. [Google Scholar] [CrossRef]
- Idumah, C.I. Progress in polymer nanocomposites for bone regeneration and engineering. Polym. Polym. Compos. 2021, 29, 509–527. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, M.; Biesold, G.M.; Choi, W.; He, Y.; Li, Z.; Shen, D.; Lin, Z. Recent Advances in Synthesis, Properties, and Applications of Metal Halide Perovskite Nanocrystals/Polymer Nanocomposites. Adv. Mater. 2021, 33, 2005888. [Google Scholar] [CrossRef]
- Eid, A.I.; El kady, O.A.; Mohamed, L.Z.; Eessaa, A.K.; Esmail, S.A. Studying of physico-mechanical and electrical properties of polypropylene/ nano-copper composites for industrial applications. Egypt. J. Chem. 2019, 62, 1313–1320. [Google Scholar] [CrossRef]
- Huang, C.; Qian, X.; Yang, R. Thermal conductivity of polymers and polymer nanocomposites. Mater. Sci. Eng. R Rep. 2018, 132, 1–22. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Z.; Song, X.; Zhu, B.; Hsiai, T.; Wu, P.I.; Xiong, R.; Shi, J.; Chen, Y.; Zhou, Q.; et al. Three dimensional printing of high dielectric capacitor using projection based stereolithography method. Nano Energy 2016, 22, 414–421. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Negi, A.; He, J.; Hu, G.; Tian, S.; Liu, J. Synergistic effects of boron nitride (BN) nanosheets and silver (Ag) nanoparticles on thermal conductivity and electrical properties of epoxy nanocomposites. Polymers 2020, 12, 426. [Google Scholar] [CrossRef]
- Swathy, T.S.; Jinish Antony, M. Tangled silver nanoparticles embedded polythiophene-functionalized multiwalled carbon nanotube nanocomposites with remarkable electrical and thermal properties. Polymer 2020, 189, 122171. [Google Scholar] [CrossRef]
- Nasar, G.; Azhar Khan, M.; Nadeem, Q.; Amin, H.; Ahmad, N.; ur Rehman, J.; Khalil, U.; Saleem Khan, M. Silver-polymer nanocomposites: Structural, thermal and electromechanical elucidation for charge storage applications. Meas. J. Int. Meas. Confed. 2020, 156, 107615. [Google Scholar] [CrossRef]
- Starowicz, M.; Stypuła, B.; Banaś, J. Electrochemical synthesis of silver nanoparticles. Electrochem. Commun. 2006, 8, 227–230. [Google Scholar] [CrossRef]
- Lim, P.Y.; Liu, R.S.; She, P.L.; Hung, C.F.; Shih, H.C. Synthesis of Ag nanospheres particles in ethylene glycol by electrochemical-assisted polyol process. Chem. Phys. Lett. 2006, 420, 304–308. [Google Scholar] [CrossRef]
- Gawish, S.M.; Mosleh, S. Antimicrobial Polypropylene Loaded by Silver Nano Particles. Fibers Polym. 2020, 21, 19–23. [Google Scholar] [CrossRef]
- Chiolerio, A.; Roppolo, I.; Sangermano, M. Radical diffusion engineering: Tailored nanocomposite materials for piezoresistive inkjet printed strain measurement. RSC Adv. 2013, 3, 3446–3452. [Google Scholar] [CrossRef]
- Ghorbani, H.R.; Molaei, M. Antibacterial nanocomposite preparation of polypropylene-Silver using Corona discharge. Prog. Org. Coat. 2017, 112, 187–190. [Google Scholar] [CrossRef]
- Sangermano, M.; Yagci, Y.; Rizza, G. In situ synthesis of silver-epoxy nanocomposites by photoinduced electron transfer and cationic polymerization processes. Macromolecules 2007, 40, 8827–8829. [Google Scholar] [CrossRef]
- Sangermano, M.; Marchino, A.; Perruchas, S.; Gacoin, T.; Rizza, G. UV-cured nanostructured gold/acrylic coating. Macromol. Mater. Eng. 2008, 293, 964–968. [Google Scholar] [CrossRef]
- Fantino, E.; Chiappone, A.; Roppolo, I.; Manfredi, D.; Bongiovanni, R.; Pirri, C.F.; Calignano, F. 3D Printing of Conductive Complex Structures with in Situ Generation of Silver Nanoparticles. Adv. Mater. 2016, 28, 3712–3717. [Google Scholar] [CrossRef]
- Fantino, E.; Chiappone, A.; Calignano, F.; Fontana, M.; Pirri, F.; Roppolo, I. In situ thermal generation of silver nanoparticles in 3D printed polymeric structures. Materials 2016, 9, 589. [Google Scholar] [CrossRef] [PubMed]
- Roppolo, I.; Castellino, M.; Bejtka, K.; Rizza, G.; Perrone, D.; Coulon, P.E.; Chiappone, A.; Rajan, K.; Bocchini, S.; Ricciardi, C.; et al. Resistive Switching in Polymer Nanocomposites by Matrix-Controlled in Situ Nanoparticles Generation. J. Phys. Chem. C 2017, 121, 14285–14295. [Google Scholar] [CrossRef]
- González Flores, G.A.; Bertana, V.; Chiappone, A.; Roppolo, I.; Scaltrito, L.; Marasso, S.L.; Cocuzza, M.; Massaglia, G.; Quaglio, M.; Pirri, C.F.; et al. Single-Step 3D Printing of Silver-Patterned Polymeric Devices for Bacteria Proliferation Control. Macromol. Mater. Eng. 2022, 307, 2100596. [Google Scholar] [CrossRef]
- Sciancalepore, C.; Moroni, F.; Messori, M.; Bondioli, F. Acrylate-based silver nanocomposite by simultaneous polymerization–reduction approach via 3D stereolithography. Compos. Commun. 2017, 6, 11–16. [Google Scholar] [CrossRef]
- Valencia, L.M.; Herrera, M.; de la Mata, M.; de León, A.S.; Delgado, F.J.; Molina, S.I. Synthesis of Silver Nanocomposites for Stereolithography: In Situ Formation of Nanoparticles. Polymers 2022, 14, 1168. [Google Scholar] [CrossRef]
- Taormina, G.; Sciancalepore, C.; Bondioli, F.; Messori, M. Special resins for stereolithography: In situ generation of silver nanoparticles. Polymers 2018, 10, 212. [Google Scholar] [CrossRef]
- Pankaew, A.; Traiphol, N.; Traiphol, R. Synthesis of color-responsive polydiacetylene assemblies and polydiacetylene/zinc(II) ion/zinc oxide nanocomposites in water, toluene and mixed solvents: Toward large-scale production. Colloids Surf. A Physicochem. Eng. Asp. 2021, 617, 126431. [Google Scholar] [CrossRef]
- Arshad, M.U.; Raza, H.; Khan, M.B.; Hussain, A. Synthesis of 2D Molybdenum Disulfide (MoS2) for enhancement of mechanical and electrical properties of polystyrene (PS) polymer. Polym. Test. 2020, 90, 106646. [Google Scholar] [CrossRef]
- Ghorbani, F.; Tirgir, F.; Jafari, A. Construction and characterization of the novel polymer/Ag nanocomposite coated on glass bead as filter for inactivation of E. coli in water. Colloid Polym. Sci. 2021, 299, 1917–1932. [Google Scholar] [CrossRef]
- Bastani, S.; Mohseni, M. UV-Curable Nanocomposite Coatings and Materials; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 9780444633828. [Google Scholar]
- Andrzejewska, E. Free-Radical Photopolymerization of Multifunctional Monomers, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128178270. [Google Scholar]
- Hada, H.; Yonezawa, Y.; Yoshida, A.; Kurakake, A. Photoreduction of silver ion in aqueous and alcoholic solutions. J. Phys. Chem. 1976, 80, 2728–2731. [Google Scholar] [CrossRef]
- Król-Gracz, A.; Michalak, E.; Nowak, P.M.; Dyonizy, A. Photo-induced chemical reduction of silver bromide to silver nanoparticles. Cent. Eur. J. Chem. 2011, 9, 982–989. [Google Scholar] [CrossRef]
- Garra, P.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Photopolymerization processes of thick films and in shadow areas: A review for the access to composites. Polym. Chem. 2017, 8, 7088–7101. [Google Scholar] [CrossRef]
- Kim, L.U.; Kim, J.W.; Kim, C.K. Effects of molecular structure of the resins on the volumetric shrinkage and the mechanical strength of dental restorative composites. Biomacromolecules 2006, 7, 2680–2687. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.; He, Y.; Jiang, T.; Li, C.; Yang, W.; Nie, J. Polymerization shrinkage of (meth)acrylate determined by reflective laser beam scanning. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 923–928. [Google Scholar] [CrossRef]
- Ding, Y.; Xin, Y.; Zhang, Q.; Zou, Y. Acrylic resins with oxetane pendant groups for free radical and cationic dual-curing photoresists. Mater. Des. 2022, 213, 110370. [Google Scholar] [CrossRef]
- Ortiz, R.A.; Gomez, A.G.S.; Duarte, M.L.B.; Valdez, A.E.G. The effect of a dithiol spiroorthocarbonate on mechanical properties and shrinkage of a dental resin. Des. Monomers Polym. 2015, 18, 73–78. [Google Scholar] [CrossRef]
- Aktitiz, İ.; Varol, R.; Akkurt, N.; Saraç, M.F. In-situ synthesis of 3D printable mono- and Bi-metallic (Cu/Ag) nanoparticles embedded polymeric structures with enhanced electromechanical properties. Polym. Test. 2020, 90, 106724. [Google Scholar] [CrossRef]
- De León, A.S.; Molina, S.I. Influence of the degree of cure in the bulk properties of graphite nanoplatelets nanocomposites printed via stereolithography. Polymers 2020, 12, 1103. [Google Scholar] [CrossRef]
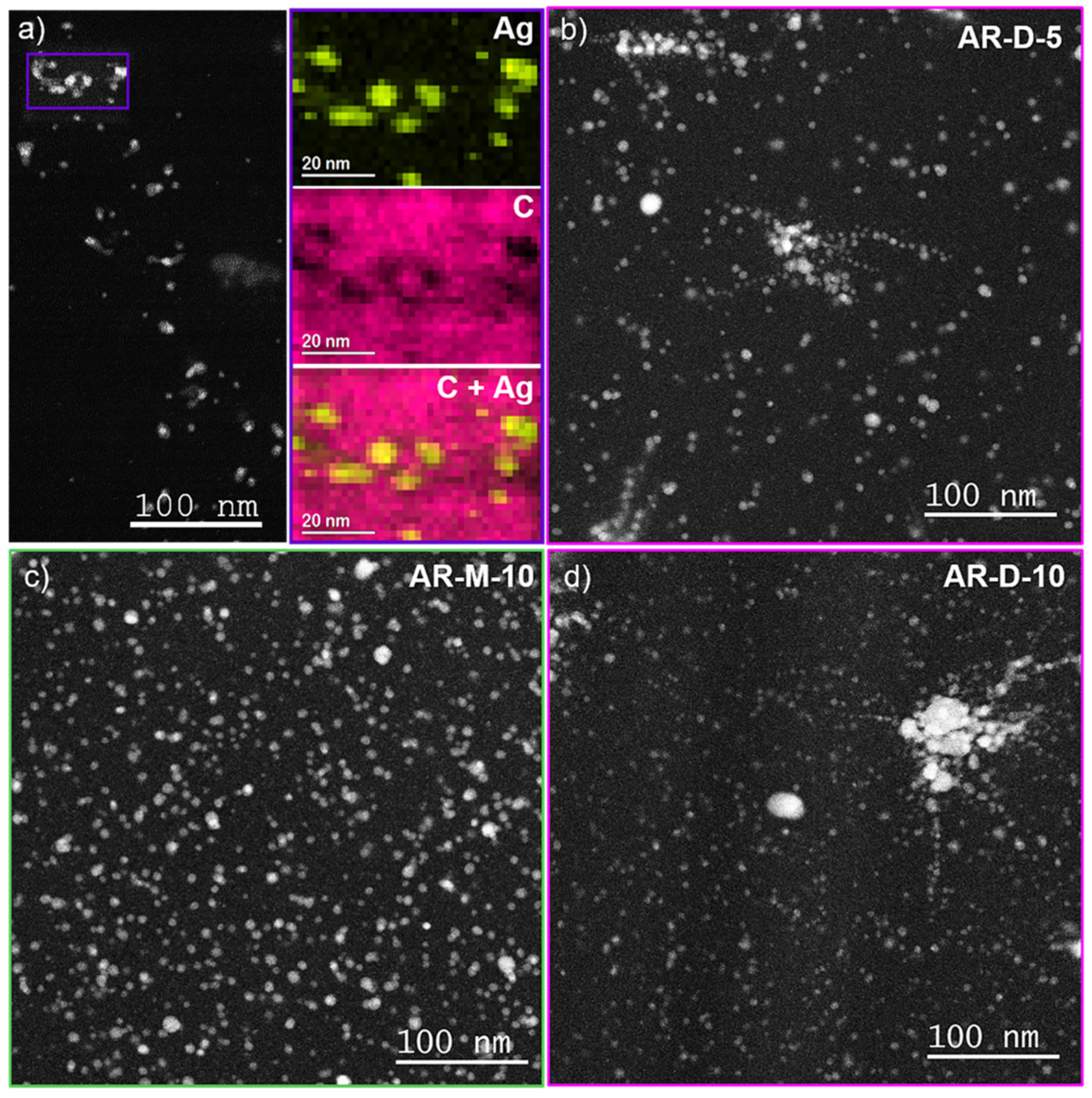
| Composite | External Solvent | AgClO4 Content (wt%) |
|---|---|---|
| AR ♦ | -- | -- |
| AR-M | Methanol | -- |
| AR-D | N,N-Dimethylformamide | -- |
| AR-M-5 | Methanol | 5 |
| AR-D-5 | N,N-Dimethylformamide | 5 |
| AR-M-10 | Methanol | 10 |
| AR-D-10 | N,N-Dimethylformamide | 10 |
| Composite | Shore D Hardness (HBD) | Standard Deviation |
|---|---|---|
| AR | 78.2 | ±2.4 |
| AR-M | 66.4 | ±2.6 |
| AR-D | 71.6 | ±3.1 |
| AR-M-5 | 38.0 | ±1.6 |
| AR-D-5 | 50.0 | ±2.5 |
| AR-M-10 | 30.2 | ±2.9 |
| AR-D-10 | 40.6 | ±3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia, L.M.; Herrera, M.; de la Mata, M.; Hernández-Saz, J.; Romero-Ocaña, I.; Delgado, F.J.; Benito, J.; Molina, S.I. Stereolithography of Semiconductor Silver and Acrylic-Based Nanocomposites. Polymers 2022, 14, 5238. https://doi.org/10.3390/polym14235238
Valencia LM, Herrera M, de la Mata M, Hernández-Saz J, Romero-Ocaña I, Delgado FJ, Benito J, Molina SI. Stereolithography of Semiconductor Silver and Acrylic-Based Nanocomposites. Polymers. 2022; 14(23):5238. https://doi.org/10.3390/polym14235238
Chicago/Turabian StyleValencia, Luisa M., Miriam Herrera, María de la Mata, Jesús Hernández-Saz, Ismael Romero-Ocaña, Francisco J. Delgado, Javier Benito, and Sergio I. Molina. 2022. "Stereolithography of Semiconductor Silver and Acrylic-Based Nanocomposites" Polymers 14, no. 23: 5238. https://doi.org/10.3390/polym14235238
APA StyleValencia, L. M., Herrera, M., de la Mata, M., Hernández-Saz, J., Romero-Ocaña, I., Delgado, F. J., Benito, J., & Molina, S. I. (2022). Stereolithography of Semiconductor Silver and Acrylic-Based Nanocomposites. Polymers, 14(23), 5238. https://doi.org/10.3390/polym14235238










