Effect of Processing Time of Steam-Explosion for the Extraction of Cellulose Fibers from Phoenix canariensis Palm Leaves as Potential Renewable Feedstock for Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Extraction of Cellulose Fibers from Palm Leaves Waste
2.3. Experimental Techniques
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
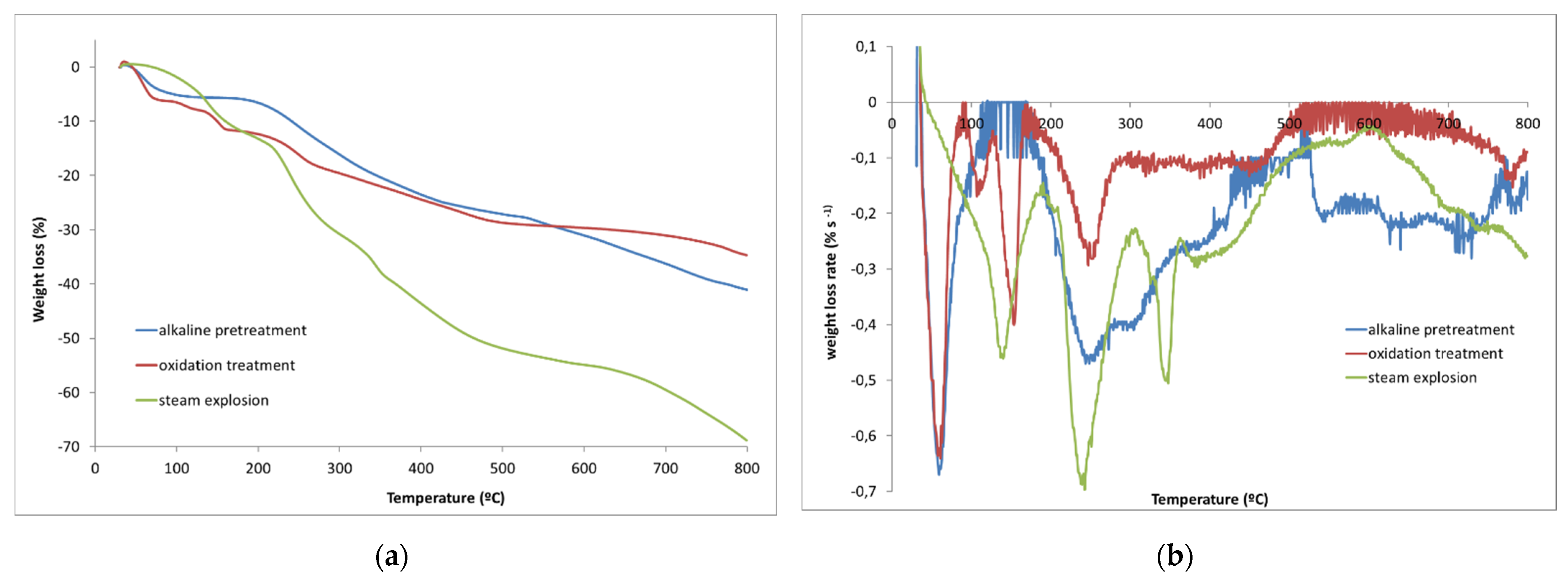
2.3.2. Thermogravimetric Analysis (TGA)
2.3.3. X-ray Diffraction (XRD)
2.3.4. Elemental Analysis (EA)
2.3.5. Scanning Electron Microscopy (SEM)
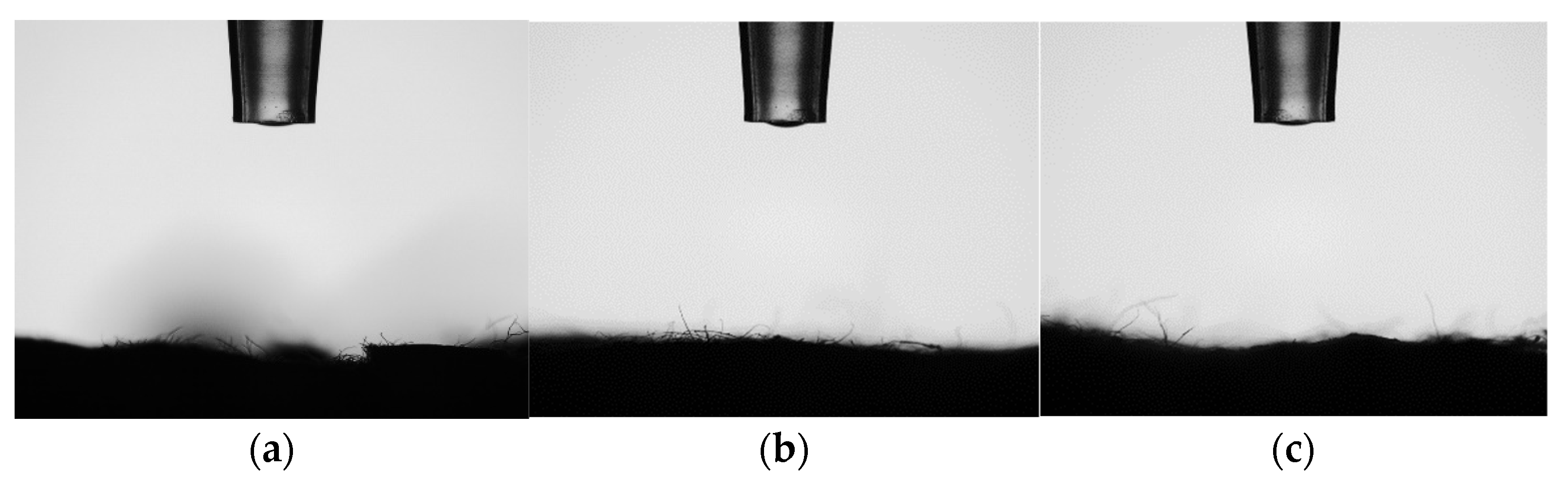
2.3.6. Water Contact Angles (WCA) Measurements
2.3.7. Determination of the Static Absorption of Water
3. Results and Discussion
3.1. Characterization of Cellulose Fibers
3.2. Characterization of the Generated Waste Solutions at Each Extraction Stage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asada, C.; Sholahuddin; Nakamura, Y. Biorefinery System of Lignocellulosic Biomass Using Steam Explosion. In Cellulose Science and Derivatives; Sand, A., Banga, S., Eds.; IntechOpen: London, UK, 2021; ISBN 978-1-83969-578-0. [Google Scholar]
- Beluns, S.; Gaidukovs, S.; Platnieks, O.; Barkane, A.; Gaidukova, G.; Grase, L.; Nabels-Sneiders, M.; Kovalovs, A.; Thakur, V.K. Clean Manufacturing of Cellulose Nanopapers by Incorporating Lignin and Xylan as Sustainable Additives. Carbohydr. Polym. Technol. Appl. 2022, 3, 100207. [Google Scholar] [CrossRef]
- Chopra, L. Manikanika Extraction of Cellulosic Fibers from the Natural Resources: A Short Review. Mater. Today Proc. 2022, 48, 1265–1270. [Google Scholar] [CrossRef]
- Arun, R.; Shruthy, R.; Preetha, R.; Sreejit, V. Biodegradable Nano Composite Reinforced with Cellulose Nano Fiber from Coconut Industry Waste for Replacing Synthetic Plastic Food Packaging. Chemosphere 2022, 291, 132786. [Google Scholar] [CrossRef] [PubMed]
- Mendes, I.S.F.; Prates, A.; Evtuguin, D.V. Production of Rayon Fibres from Cellulosic Pulps: State of the Art and Current Developments. Carbohydr. Polym. 2021, 273, 118466. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, J.; Thrän, D.; Bezama, A. The Circularity of Potential Bio-Textile Production Routes: Comparing Life Cycle Impacts of Bio-Based Materials Used within the Manufacturing of Selected Leather Substitutes. J. Clean. Prod. 2021, 287, 125470. [Google Scholar] [CrossRef]
- Sun, D.; Lv, Z.-W.; Rao, J.; Tian, R.; Sun, S.-N.; Peng, F. Effects of Hydrothermal Pretreatment on the Dissolution and Structural Evolution of Hemicelluloses and Lignin: A Review. Carbohydr. Polym. 2022, 281, 119050. [Google Scholar] [CrossRef]
- Yogalakshmi, K.N.; Sivashanmugam, P.; Kavitha, S.; Kannah, Y.; Varjani, S.; AdishKumar, S.; Kumar, G. Lignocellulosic Biomass-Based Pyrolysis: A Comprehensive Review. Chemosphere 2022, 286, 131824. [Google Scholar] [CrossRef]
- Nahak, B.K.; Preetam, S.; Sharma, D.; Shukla, S.K.; Syväjärvi, M.; Toncu, D.-C.; Tiwari, A. Advancements in Net-Zero Pertinency of Lignocellulosic Biomass for Climate Neutral Energy Production. Renew. Sustain. Energy Rev. 2022, 161, 112393. [Google Scholar] [CrossRef]
- Wang, Y.; Akbarzadeh, A.; Chong, L.; Du, J.; Tahir, N.; Awasthi, M.K. Catalytic Pyrolysis of Lignocellulosic Biomass for Bio-Oil Production: A Review. Chemosphere 2022, 297, 134181. [Google Scholar] [CrossRef]
- Shanks, R.A. Chemistry and Structure of Cellulosic Fibres as Reinforcements in Natural Fibre Composites. In Natural Fibre Composites; Elsevier: Philadelphia, PA, USA, 2014; pp. 66–83. ISBN 978-0-85709-524-4. [Google Scholar]
- Fidale, L.C.; Ruiz, N.; Heinze, T.; Seoud, O.A.E. Cellulose Swelling by Aprotic and Protic Solvents: What Are the Similarities and Differences? Macromol. Chem. Phys. 2008, 209, 1240–1254. [Google Scholar] [CrossRef]
- Bandgar, P.S.; Jain, S.; Panwar, N.L. A Comprehensive Review on Optimization of Anaerobic Digestion Technologies for Lignocellulosic Biomass Available in India. Biomass Bioenergy 2022, 161, 106479. [Google Scholar] [CrossRef]
- Lin, X.; Jiang, K.; Liu, X.; Han, D.; Zhang, Q. Review on Development of Ionic Liquids in Lignocellulosic Biomass Refining. J. Mol. Liq. 2022, 359, 119326. [Google Scholar] [CrossRef]
- Sun, W.; Tajvidi, M.; Hunt, C.G.; Cole, B.J.W.; Howell, C.; Gardner, D.J.; Wang, J. Fungal and Enzymatic Pretreatments in Hot-Pressed Lignocellulosic Bio-Composites: A Critical Review. J. Clean. Prod. 2022, 353, 131659. [Google Scholar] [CrossRef]
- Cavalaglio, G.; Gelosia, M.; Giannoni, T.; Barros Lovate Temporim, R.; Nicolini, A.; Cotana, F.; Bertini, A. Acid-Catalyzed Steam Explosion for High Enzymatic Saccharification and Low Inhibitor Release from Lignocellulosic Cardoon Stalks. Biochem. Eng. J. 2021, 174, 108121. [Google Scholar] [CrossRef]
- Poursorkhabi, V.; Misra, M.; Mohanty, A.K. Extraction of Lignin from a Coproduct of the Cellulosic Ethanol Industry and Its Thermal Characterization. BioResources 2013, 8, 5083–5101. [Google Scholar] [CrossRef]
- Phirom-on, K.; Apiraksakorn, J. Development of Cellulose-Based Prebiotic Fiber from Banana Peel by Enzymatic Hydrolysis. Food Biosci. 2021, 41, 101083. [Google Scholar] [CrossRef]
- Sarker, T.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K.; Meda, V.; Naik, S. Hydrothermal Pretreatment Technologies for Lignocellulosic Biomass: A Review of Steam Explosion and Subcritical Water Hydrolysis. Chemosphere 2021, 284, 131372. [Google Scholar] [CrossRef] [PubMed]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Stoffel, R.B.; Neves, P.V.; Felissia, F.E.; Ramos, L.P.; Gassa, L.M.; Area, M.C. Hemicellulose Extraction from Slash Pine Sawdust by Steam Explosion with Sulfuric Acid. Biomass Bioenergy 2017, 107, 93–101. [Google Scholar] [CrossRef]
- Ferrández-García, A.; Ferrández-Villena, M.; Ferrández-García, C.E.; García-Ortuño, T.; Ferrández-García, M.T. Potential Use of Phoenix canariensis Biomass in Binderless Particleboards at Low Temperature and Pressure. BioResources 2017, 12, 6698–6712. [Google Scholar] [CrossRef]
- Blasco, M.P.C.; Limiñana, M.Á.P.; Silvestre, C.R.; Calpena, E.O.; Aís, F.A. Sustainable Reactive Polyurethane Hot Melt Adhesives Based on Vegetable Polyols for Footwear Industry. Polymers 2022, 14, 284. [Google Scholar] [CrossRef]
- Carbonell-Blasco, P.; Martín-Martínez, J.M.; Antoniac, I.V. Synthesis and Characterization of Polyurethane Sealants Containing Rosin Intended for Sealing Defect in Annulus for Disc Regeneration. Int. J. Adhes. Adhes. 2013, 42, 11–20. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Silvestre, C.R.; Blasco, M.P.C.; López, S.R.; Aguilar, H.P.; Limiñana, M.Á.P.; Gil, E.B.; Calpena, E.O.; Ais, F.A. Hydrophobic Leather Coating for Footwear Applications by a Low-Pressure Plasma Polymerisation Process. Polymers 2021, 13, 3549. [Google Scholar] [CrossRef]
- EN 828; Adhesives—Wettability—Determination by Measurement of Contact Angle and Surface Free Energy of Solid Surface. European Committee for Standardization: Brussels, Belgium, 2013.
- Ruzafa-Silvestre, C.; Juan-Fernández, B.; Carbonell-Blasco, M.P.; Bañón-Gil, E.; Orgilés-Calpena, E.; Arán-Ais, F. Organosilicon-Based Plasma Nanocoating on Crust Leather for Water-Repellent Footwear. Materials 2022, 15, 7255. [Google Scholar] [CrossRef]
- ISO 2417:2016; Leather—Physical and Mechanical Tests—Determination of the Static Absorption of Water. International Organization for Standardization: Geneva, Switzerland, 2019.
- Asif, M.; Ahmed, D.; Ahmad, N.; Qamar, M.T.; Alruwaili, N.K.; Bukhari, S.N.A. Extraction and Characterization of Microcrystalline Cellulose from Lagenaria Siceraria Fruit Pedicles. Polymers 2022, 14, 1867. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Jaisan, C.; Klunklin, W.; Phongthai, S.; Rawdkuen, S.; Tongdeesoontorn, W. Mechanical and Physicochemical Properties of Composite Biopolymer Films Based on Carboxymethyl Cellulose from Young Palmyra Palm Fruit Husk and Rice Flour. Polymers 2022, 14, 1872. [Google Scholar] [CrossRef]
- Biswas, S.; Rahaman, T.; Gupta, P.; Mitra, R.; Dutta, S.; Kharlyngdoh, E.; Guha, S.; Ganguly, J.; Pal, A.; Das, M. Cellulose and Lignin Profiling in Seven, Economically Important Bamboo Species of India by Anatomical, Biochemical, FTIR Spectroscopy and Thermogravimetric Analysis. Biomass Bioenergy 2022, 158, 106362. [Google Scholar] [CrossRef]
- Azizan, A.; Jusri, N.A.A.; Azmi, I.S.; Abd Rahman, M.F.; Ibrahim, N.; Jalil, R. Emerging Lignocellulosic Ionic Liquid Biomass Pretreatment Criteria/Strategy of Optimization and Recycling Short Review with Infrared Spectroscopy Analytical Know-How. Mater. Today Proc. 2022, 63, S359–S367. [Google Scholar] [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.-M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric Analysis as a New Method to Determine the Lignocellulosic Composition of Biomass. Biomass Bioenergy 2011, 35, 298–307. [Google Scholar] [CrossRef]
- Lupoi, J.S.; Singh, S.; Parthasarathi, R.; Simmons, B.A.; Henry, R.J. Recent Innovations in Analytical Methods for the Qualitative and Quantitative Assessment of Lignin. Renew. Sustain. Energy Rev. 2015, 49, 871–906. [Google Scholar] [CrossRef]
- Lu, X.; Zhu, X.; Guo, H.; Que, H.; Wang, D.; Liang, D.; He, T.; Hu, C.; Xu, C.; Gu, X. Investigation on the Thermal Degradation Behavior of Enzymatic Hydrolysis Lignin with or without Steam Explosion Treatment Characterized by TG-FTIR and Py-GC/MS. Biomass Convers. Biorefinery 2020, 12, 5825–5834. [Google Scholar] [CrossRef]
- Saffe, A.; Fernandez, A.; Mazza, G.; Rodriguez, R. Prediction of Regional Agro-Industrial Wastes Characteristics by Thermogravimetric Analysis to Obtain Bioenergy Using Thermal Process. Energy Explor. Exploit. 2019, 37, 544–557. [Google Scholar] [CrossRef]
- Akhlamadi, G.; Goharshadi, E.K.; Saghir, S.V. Extraction of Cellulose Nanocrystals and Fabrication of High Alumina Refractory Bricks Using Pencil Chips as a Waste Biomass Source. Ceram. Int. 2021, 47, 27042–27049. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Pattnaik, F.; Nanda, S.; Kumar, V.; Naik, S.; Dalai, A.K. Isolation of Cellulose Fibers from Wetland Reed Grass through an Integrated Subcritical Water Hydrolysis-Pulping-Bleaching Process. Fuel 2022, 311, 122618. [Google Scholar] [CrossRef]
- Kalpana, V.P.; Perarasu, V.T. Analysis on Cellulose Extraction from Hybrid Biomass for Improved Crystallinity. J. Mol. Struct. 2020, 1217, 128350. [Google Scholar] [CrossRef]
- Midhun Dominic, C.D.; Raj, V.; Neenu, K.V.; Begum, P.M.S.; Formela, K.; Saeb, M.R.; Prabhu, D.D.; Poornima Vijayan, P.; Ajithkumar, T.G.; Parameswaranpillai, J. Chlorine-Free Extraction and Structural Characterization of Cellulose Nanofibers from Waste Husk of Millet (Pennisetum glaucum). Int. J. Biol. Macromol. 2022, 206, 92–104. [Google Scholar] [CrossRef]
- Wahib, S.A.; Da’na, D.A.; Al-Ghouti, M.A. Insight into the Extraction and Characterization of Cellulose Nanocrystals from Date Pits. Arab. J. Chem. 2022, 15, 103650. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; de Freitas Rosa, M.; Cioffi, M.O.H.; de Carvalho Benini , K.C.C.; Milanese, A.C.; Voorwald, H.J.C.; Mulinari, D.R. Vegetal Fibers in Polymeric Composites: A Review. Polímeros 2015, 25, 9–22. [Google Scholar] [CrossRef]
- Shaikh, H.M.; Anis, A.; Poulose, A.M.; Al-Zahrani, S.M.; Madhar, N.A.; Alhamidi, A.; Alam, M.A. Isolation and Characterization of Alpha and Nanocrystalline Cellulose from Date Palm (Phoenix dactylifera L.) Trunk Mesh. Polymers 2021, 13, 1893. [Google Scholar] [CrossRef]
- Oh, J.E.; Park, N.-M. Hydrophilic, Transparent, and Stretchable Film Using Unmodified Cellulose Fibers. Mater. Lett. 2022, 309, 131385. [Google Scholar] [CrossRef]
- Brenelli, L.B.; Bhatia, R.; Djajadi, D.T.; Thygesen, L.G.; Rabelo, S.C.; Leak, D.J.; Franco, T.T.; Gallagher, J.A. Xylo-Oligosaccharides, Fermentable Sugars, and Bioenergy Production from Sugarcane Straw Using Steam Explosion Pretreatment at Pilot-Scale. Bioresour. Technol. 2022, 357, 127093. [Google Scholar] [CrossRef]
- Khan, N.; Mohan, S.; Dinesha, P. Regimes of Hydrochar Yield from Hydrothermal Degradation of Various Lignocellulosic Biomass: A Review. J. Clean. Prod. 2021, 288, 125629. [Google Scholar] [CrossRef]
- Mihiretu, G.T.; Chimphango, A.F.; Görgens, J.F. Steam Explosion Pre-Treatment of Alkali-Impregnated Lignocelluloses for Hemicelluloses Extraction and Improved Digestibility. Bioresour. Technol. 2019, 294, 122121. [Google Scholar] [CrossRef]


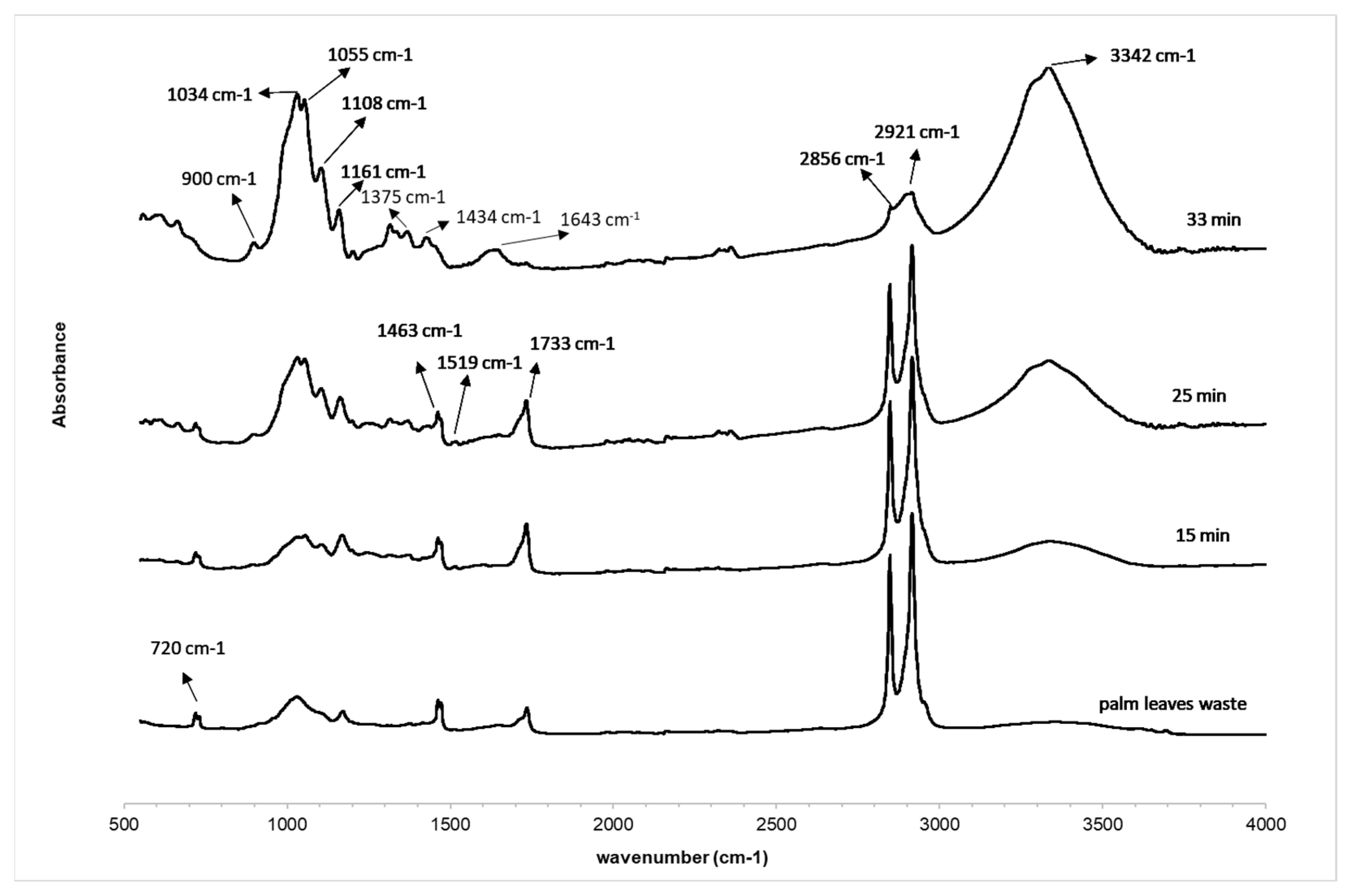

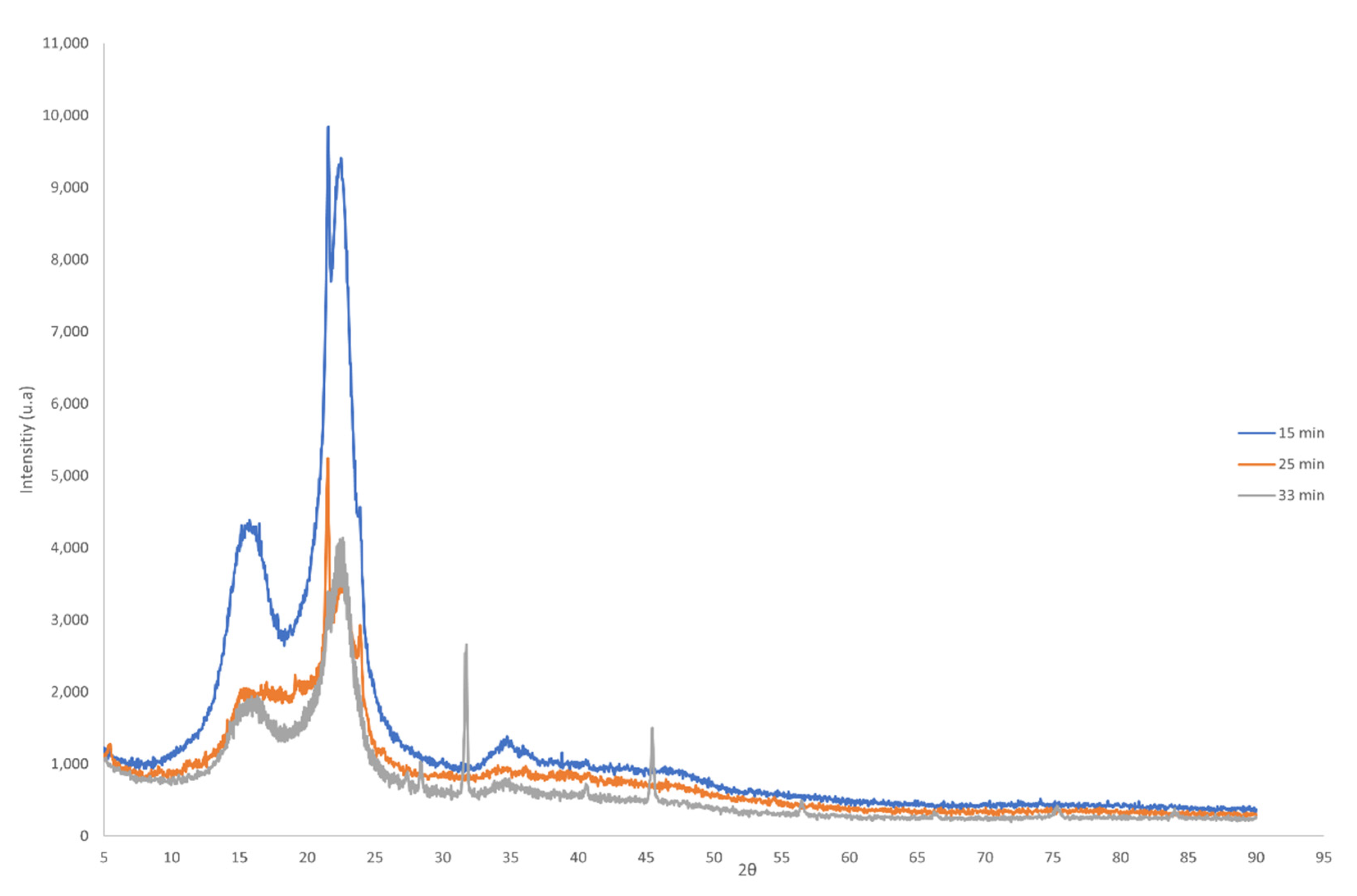
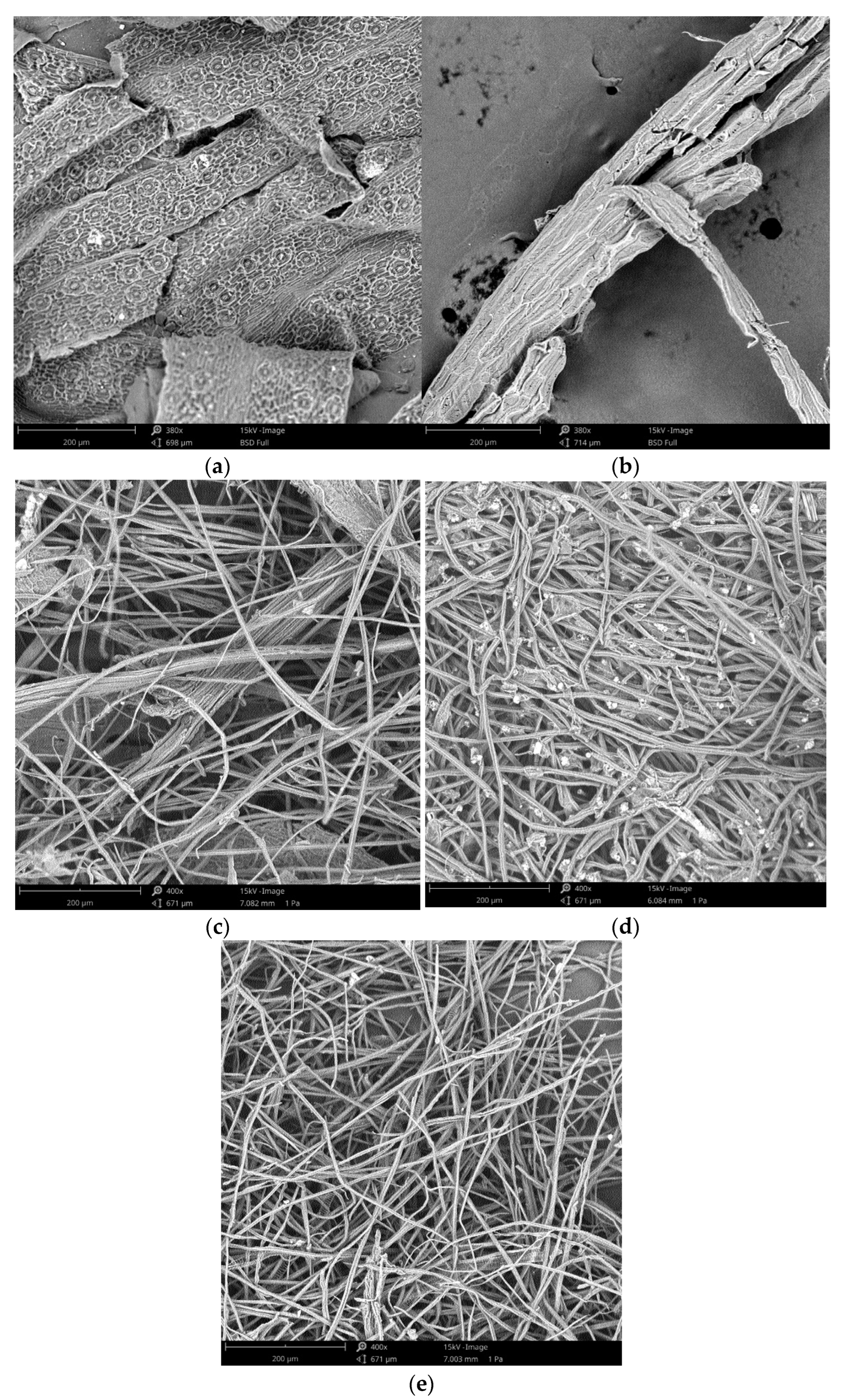
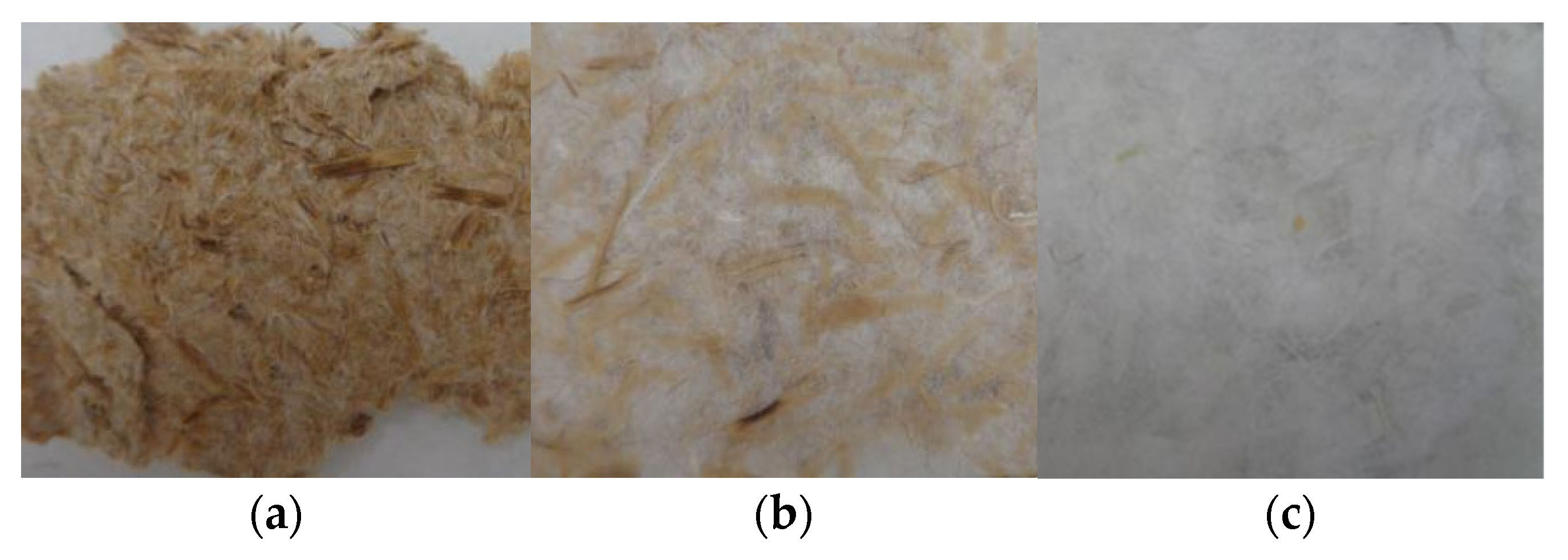
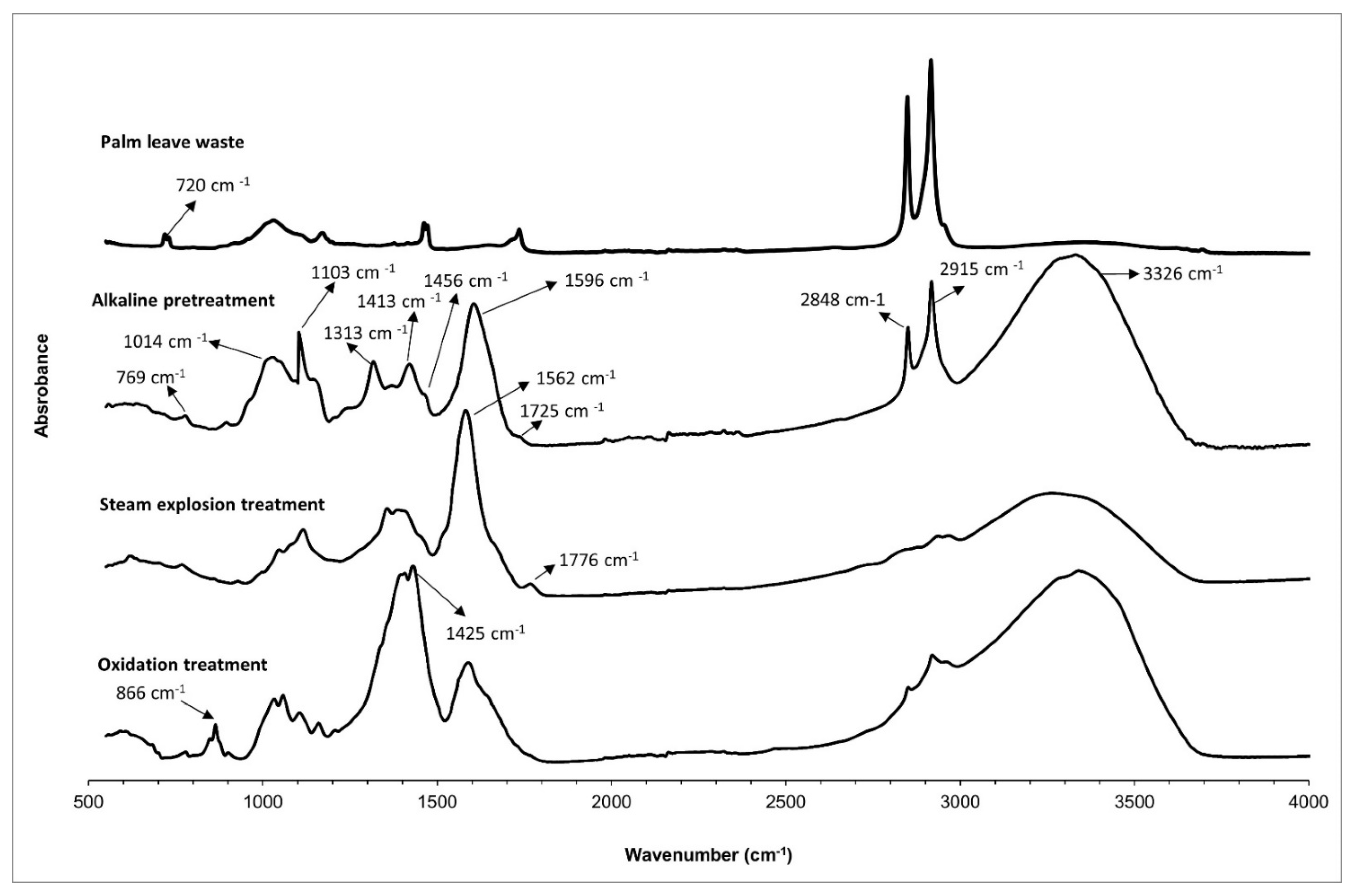
| Wavenumber (cm−1) | Assignment |
|---|---|
| 3600–3100 | Stretching of OH groups on cellulose, hemicellulose and lignin |
| 2990–2850 | C-H stretching vibration |
| 1733 | C=O stretching of carbonyl related to hemicelluloses, lignin and extractives |
| 1643 | Absorbed water |
| 1600–1500 | Aromatic skeletal vibration corresponds to the presence of pure hemicellulose and lignin |
| 1463 | C-H2 δ in lignin, carbohydrates and waxes |
| 1434 | A symmetric CH2 bending vibration attributed to crystalline cellulose |
| 1375 | C-H3 δ sy |
| 1161 | C-O asymmetric bending cellulose |
| 1108 | C-OH skeletal vibration |
| 1055 | C-O-C skeletal vibration |
| 1034 | C-O stretching vibration |
| 900 | β-glycosidic C-H deformation cellulose |
| 720 | (CH2)n out of plane deformation n > 4 (oils and waxes) |
| Peak 1 | Peak 2 | Peak 3 | Peak 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | T (°C) | Weight-Loss (%) | T (°C) | Weight-Loss (%) | T (°C) | Weight-Loss (%) | T (°C) | Weight-Loss (%) | Residue (wt%) |
| Pruning waste | 94.7 | 4.8 | 281 | 20.8 | 335 | 20.6 | 410 °C | 20.1 | 33.7 |
| 15 min | 361 | 68.8 | 17.0 | ||||||
| 25 min | 361 | 67.7 | 17.7 | ||||||
| 33 min | 338 | 56.6 | 380–500 | 14.6 | 19.0 | ||||
| Time | Cellulose Content (wt%) 1 |
|---|---|
| Waste | 4.8 |
| 15 min | 68.8 |
| 25 min | 67.7 |
| 33 min | 56.6 |
| Time | Nitrogen % | Carbon % | Hydrogen % | Sulfur % |
|---|---|---|---|---|
| 15 min | 0.29 | 50.67 | 7.18 | 0.00 |
| 25 min | 0.29 | 57.31 | 8.35 | 0.00 |
| 33 min | 0.13 | 47.37 | 6.88 | 0.00 |
| Steam-Explosion Time | Water Absorbed (wt%) |
|---|---|
| 15 min | 701.5 |
| 25 min | 897.5 |
| 33 min | 1561.0 |
| Step | Dry Matter (wt%) |
|---|---|
| Alkaline pretreatment | 24.11 |
| Steam-explosion | 20.36 |
| Oxidation treatment with H2O2 | 30.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Limiñana, M.A.; Pérez-Aguilar, H.; Ruzafa-Silvestre, C.; Orgilés-Calpena, E.; Arán-Ais, F. Effect of Processing Time of Steam-Explosion for the Extraction of Cellulose Fibers from Phoenix canariensis Palm Leaves as Potential Renewable Feedstock for Materials. Polymers 2022, 14, 5206. https://doi.org/10.3390/polym14235206
Pérez-Limiñana MA, Pérez-Aguilar H, Ruzafa-Silvestre C, Orgilés-Calpena E, Arán-Ais F. Effect of Processing Time of Steam-Explosion for the Extraction of Cellulose Fibers from Phoenix canariensis Palm Leaves as Potential Renewable Feedstock for Materials. Polymers. 2022; 14(23):5206. https://doi.org/10.3390/polym14235206
Chicago/Turabian StylePérez-Limiñana, Maria Angeles, Henoc Pérez-Aguilar, Carlos Ruzafa-Silvestre, Elena Orgilés-Calpena, and Francisca Arán-Ais. 2022. "Effect of Processing Time of Steam-Explosion for the Extraction of Cellulose Fibers from Phoenix canariensis Palm Leaves as Potential Renewable Feedstock for Materials" Polymers 14, no. 23: 5206. https://doi.org/10.3390/polym14235206
APA StylePérez-Limiñana, M. A., Pérez-Aguilar, H., Ruzafa-Silvestre, C., Orgilés-Calpena, E., & Arán-Ais, F. (2022). Effect of Processing Time of Steam-Explosion for the Extraction of Cellulose Fibers from Phoenix canariensis Palm Leaves as Potential Renewable Feedstock for Materials. Polymers, 14(23), 5206. https://doi.org/10.3390/polym14235206






