Non-Isocyanate Polyurethane Bio-Foam with Inherent Heat and Fire Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
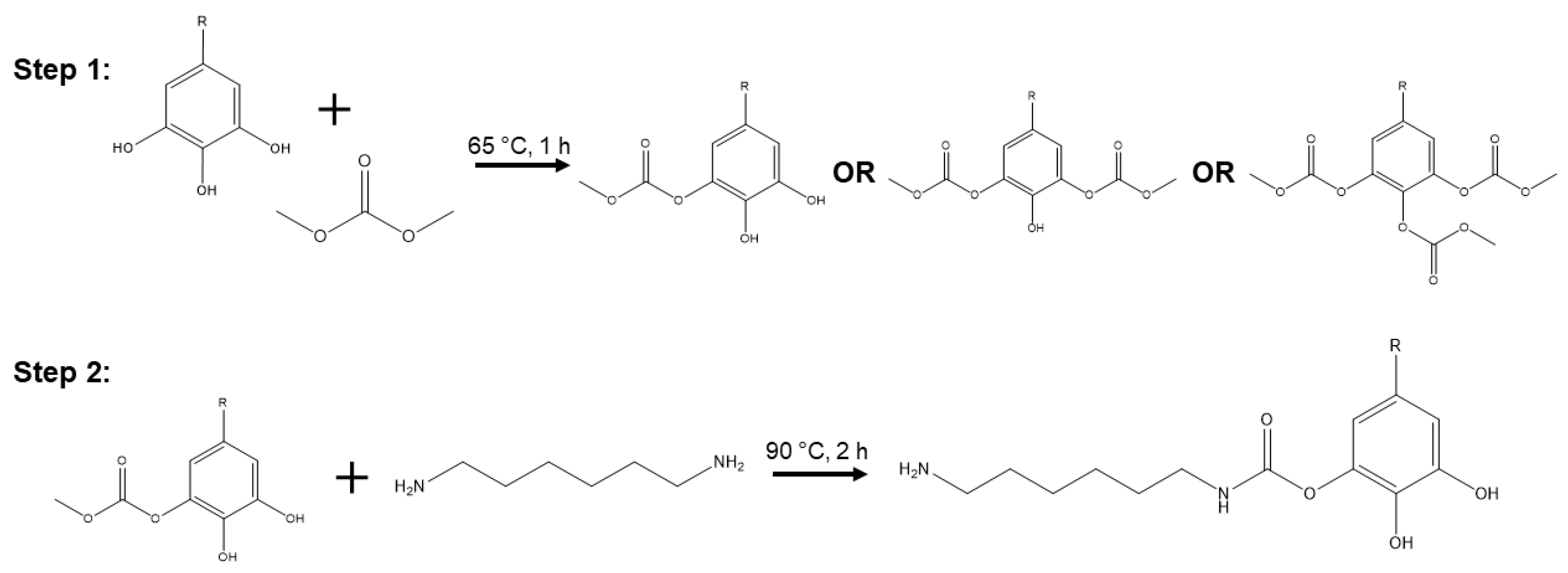
2.2. Resin Synthesis and Foaming
2.3. Characterization
3. Results and Discussion
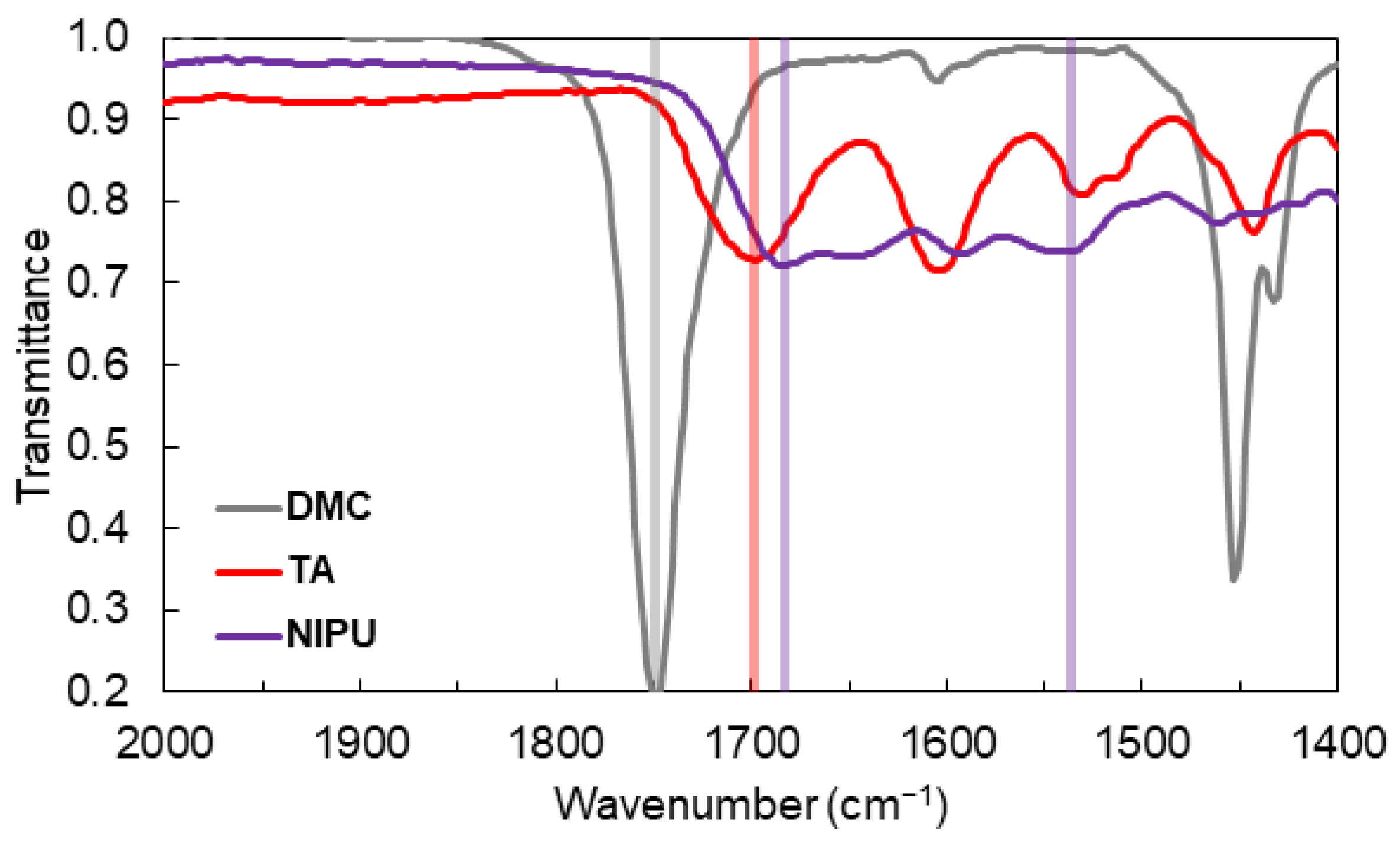
3.1. NIPU Synthesis
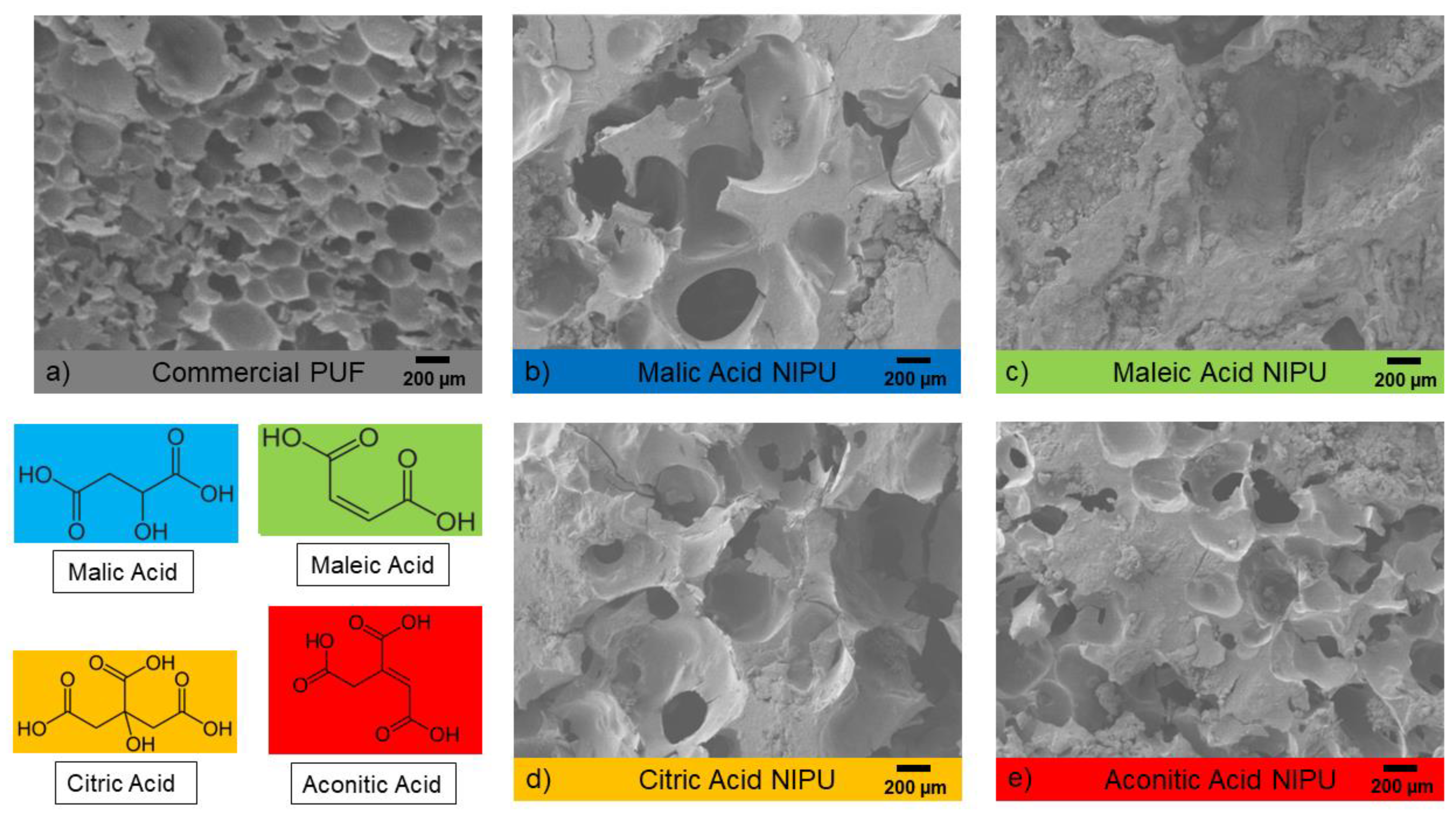
3.2. Foam Structure
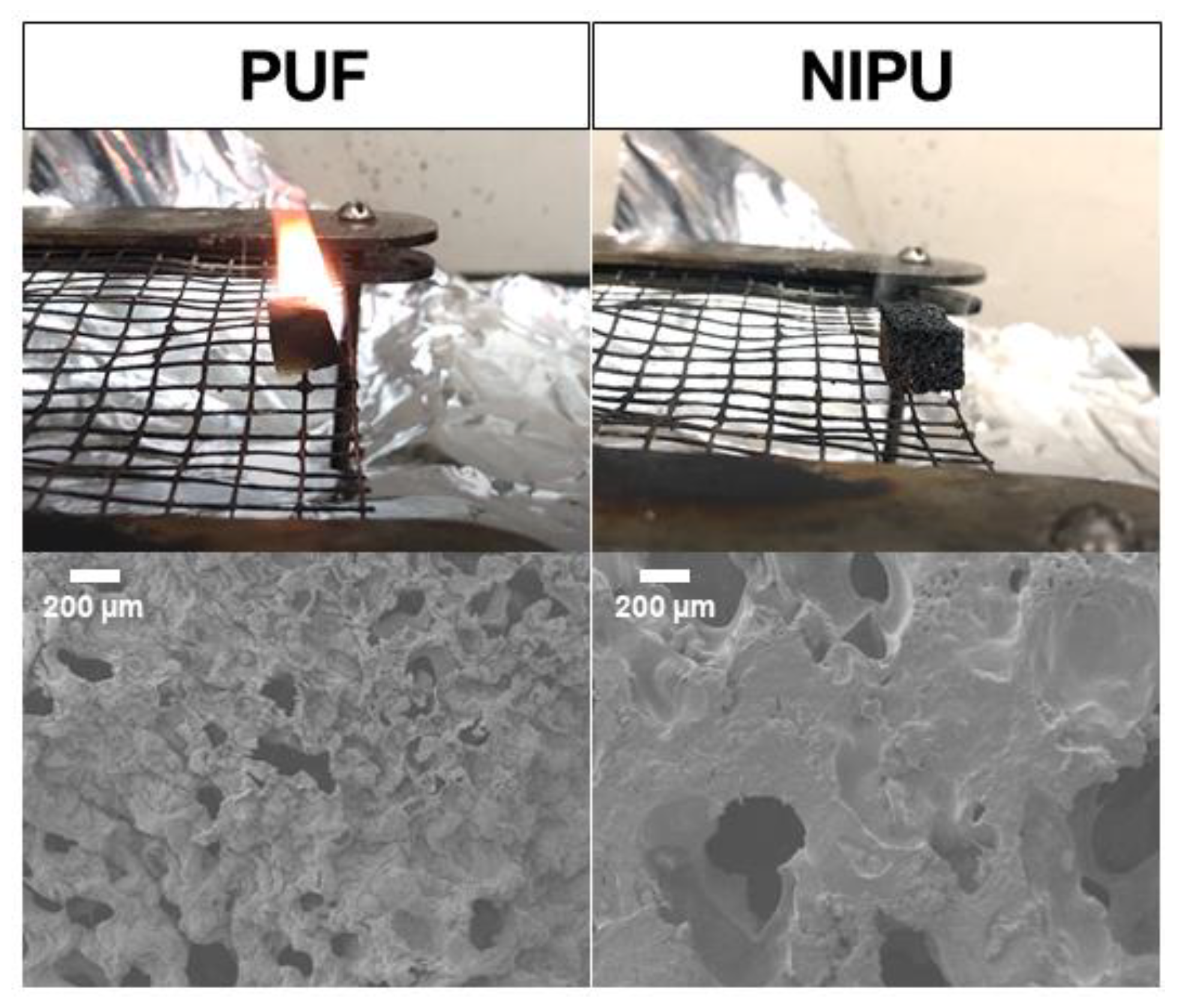
3.3. Thermal Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane Types, Synthesis and Applications—A Review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Liang, C.; Gracida-Alvarez, U.R.; Gallant, E.T.; Gillis, P.A.; Marques, Y.A.; Abramo, G.P.; Hawkins, T.R.; Dunn, J.B. Material Flows of Polyurethane in the United States. Environ. Sci. Technol. 2021, 55, 14215–14224. [Google Scholar] [CrossRef]
- Sonnenschein, M.F. Polyurethanes: Science, Technology, Markets, and Trends; John Wiley & Sons: Hoboken, NJ, USA, 2021; ISBN 978-1-119-66941-8. [Google Scholar]
- Twitchett, H.J. Chemistry of the Production of Organic Isocyanates. Chem. Soc. Rev. 1974, 3, 209–230. [Google Scholar] [CrossRef]
- Isocyanates|NIOSH|CDC. Available online: https://www.cdc.gov/niosh/topics/isocyanates/default.html (accessed on 21 June 2022).
- Bello, D.; Herrick, C.A.; Smith, T.J.; Woskie, S.R.; Streicher, R.P.; Cullen, M.R.; Liu, Y.; Redlich, C.A. Skin Exposure to Isocyanates: Reasons for Concern. Environ. Health Perspect. 2007, 115, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Isocyanates—Overview|Occupational Safety and Health Administration. Available online: https://www.osha.gov/isocyanates (accessed on 21 June 2022).
- Cornille, A.; Auvergne, R.; Figovsky, O.; Boutevin, B.; Caillol, S. A Perspective Approach to Sustainable Routes for Non-Isocyanate Polyurethanes. Eur. Polym. J. 2017, 87, 535–552. [Google Scholar] [CrossRef]
- Khatoon, H.; Iqbal, S.; Irfan, M.; Darda, A.; Rawat, N.K. A Review on the Production, Properties and Applications of Non-Isocyanate Polyurethane: A Greener Perspective. Prog. Org. Coat. 2021, 154, 106124. [Google Scholar] [CrossRef]
- Sternberg, J.; Sequerth, O.; Pilla, S. Green Chemistry Design in Polymers Derived from Lignin: Review and Perspective. Prog. Polym. Sci. 2021, 113, 101344. [Google Scholar] [CrossRef]
- More, A.; Gadenne, B.; Alfos, C.; Cramail, H. AB Type Polyaddition Route to Thermoplastic Polyurethanes from Fatty Acid Derivatives. Polym. Chem. 2012, 3, 1594–1605. [Google Scholar] [CrossRef]
- Dyer, E.; Scott, H. The Preparation of Polymeric and Cyclic Urethans and Ureas from Ethylene Carbonate and Amines. J. Am. Chem. Soc. 1957, 79, 672–675. [Google Scholar] [CrossRef]
- Rokicki, G.; Piotrowska, A. A New Route to Polyurethanes from Ethylene Carbonate, Diamines and Diols. Polymer 2002, 43, 2927–2935. [Google Scholar] [CrossRef]
- Thébault, M.; Pizzi, A.; Dumarçay, S.; Gerardin, P.; Fredon, E.; Delmotte, L. Polyurethanes from Hydrolysable Tannins Obtained without Using Isocyanates. Ind. Crop. Prod. 2014, 59, 329–336. [Google Scholar] [CrossRef]
- Ochiai, B.; Satoh, Y.; Endo, T. Nucleophilic Polyaddition in Water Based on Chemo-Selective Reaction of Cyclic Carbonate with Amine. Green Chem. 2005, 7, 765–767. [Google Scholar] [CrossRef]
- Sternberg, J.; Pilla, S. Materials for the Biorefinery: High Bio-Content, Shape Memory Kraft Lignin-Derived Non-Isocyanate Polyurethane Foams Using a Non-Toxic Protocol. Green Chem. 2020, 22, 6922–6935. [Google Scholar] [CrossRef]
- Clark, J.H.; Farmer, T.J.; Ingram, I.D.V.; Lie, Y.; North, M. Renewable Self-Blowing Non-Isocyanate Polyurethane Foams from Lysine and Sorbitol. Eur. J. Org. Chem. 2018, 2018, 4265–4271. [Google Scholar] [CrossRef]
- Choong, P.S.; Chong, N.X.; Wai Tam, E.K.; Seayad, A.M.; Seayad, J.; Jana, S. Biobased Nonisocyanate Polyurethanes as Recyclable and Intrinsic Self-Healing Coating with Triple Healing Sites. ACS Macro Lett. 2021, 10, 635–641. [Google Scholar] [CrossRef]
- Saha, P.; Goswami, L.; Kim, B.S. Novel Biobased Non-Isocyanate Polyurethanes from Microbially Produced 7,10-Dihydroxy-8(E)-Octadecenoic Acid for Potential Packaging and Coating Applications. ACS Sustain. Chem. Eng. 2022, 10, 4623–4633. [Google Scholar] [CrossRef]
- Cornille, A.; Dworakowska, S.; Bogdal, D.; Boutevin, B.; Caillol, S. A New Way of Creating Cellular Polyurethane Materials: NIPU Foams. Eur. Polym. J. 2015, 66, 129–138. [Google Scholar] [CrossRef]
- Blattmann, H.; Lauth, M.; Mülhaupt, R. Flexible and Bio-Based Nonisocyanate Polyurethane (NIPU) Foams. Macromol. Mater. Eng. 2016, 301, 944–952. [Google Scholar] [CrossRef]
- Cornille, A.; Guillet, C.; Benyahya, S.; Negrell, C.; Boutevin, B.; Caillol, S. Room Temperature Flexible Isocyanate-Free Polyurethane Foams. Eur. Polym. J. 2016, 84, 873–888. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Gerardin, C.; Du, G. Glucose-Biobased Non-Isocyanate Polyurethane Rigid Foams. J. Renew. Mater. 2021, 7, 301–312. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Gerardin, C.; Lei, H.; Chen, X.; Amirou, S. Preparation and Evaluation of Glucose Based Non-Isocyanate Polyurethane Self-Blowing Rigid Foams. Polymers 2019, 11, 1802. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xi, X.; Pizzi, A.; Fredon, E.; Zhou, X.; Li, J.; Gerardin, C.; Du, G. Preparation and Characterization of Condensed Tannin Non-Isocyanate Polyurethane (NIPU) Rigid Foams by Ambient Temperature Blowing. Polymers 2020, 12, 750. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, J.; Xi, X.; Pizzi, A.; Zhou, X.; Fredon, E.; Du, G.; Gerardin, C. Condensed Tannin-Glucose-Based NIPU Bio-Foams of Improved Fire Retardancy. Polym. Degrad. Stab. 2020, 175, 109121. [Google Scholar] [CrossRef]
- Lazar, S.; Carosio, F.; Davesne, A.-L.; Jimenez, M.; Bourbigot, S.; Grunlan, J. Extreme Heat Shielding of Clay/Chitosan Nanobrick Wall on Flexible Foam. ACS Appl. Mater. Interfaces 2018, 10, 31686–31696. [Google Scholar] [CrossRef] [PubMed]
- Laufer, G.; Kirkland, C.; Cain, A.A.; Grunlan, J.C. Clay–Chitosan Nanobrick Walls: Completely Renewable Gas Barrier and Flame-Retardant Nanocoatings. ACS Publ. 2012, 4, 1643–1649. [Google Scholar] [CrossRef]
- Nam, S.; Condon, B.D.; Xia, Z.; Nagarajan, R.; Hinchliffe, D.J.; Madison, C.A. Intumescent Flame-Retardant Cotton Produced by Tannic Acid and Sodium Hydroxide. J. Anal. Appl. Pyrolysis 2017, 126, 239–246. [Google Scholar] [CrossRef]
- Pizzi, A. Tannin-Based Biofoams-A Review. J. Renew. Mater. 2019, 7, 477–492. [Google Scholar] [CrossRef]
- Thébault, M.; Pizzi, A.; Essawy, H.A.; Barhoum, A.; Van Assche, G. Isocyanate Free Condensed Tannin-Based Polyurethanes. Eur. Polym. J. 2015, 67, 513–526. [Google Scholar] [CrossRef]
- Tundo, P.; Selva, M. The Chemistry of Dimethyl Carbonate. Acc. Chem. Res. 2002, 35, 706–716. [Google Scholar] [CrossRef]
- Ishida, T.; Kitagaki, R.; Watanabe, R.; Hagihara, H.; Elakneswaran, Y.; Shinzawa, H. A Study of Molecular Architectural Dynamics of Crosslinked Urethane during Photo-Aging by Two-Dimensional Infrared Correlation Spectroscopy. Polym. Degrad. Stab. 2020, 179, 109242. [Google Scholar] [CrossRef]
- Ketata, N.; Sanglar, C.; Waton, H.; Alamercery, S.; Delolme, F.; Raffin, G.; Grenier-Loustalot, M.F. Thermal Degradation of Polyurethane Bicomponent Systems in Controlled Atmospheres. Polym. Polym. Compos. 2005, 13, 1–26. [Google Scholar] [CrossRef]
- Lattimer, R.P.; Williams, R.C. Low-Temperature Pyrolysis Products from a Polyether-Based Urethane. J. Anal. Appl. Pyrolysis 2002, 63, 85–104. [Google Scholar] [CrossRef]
- Kim, Y.-O.; Cho, J.; Yeo, H.; Lee, B.W.; Moon, B.J.; Ha, Y.-M.; Jo, Y.R.; Jung, Y.C. Flame Retardant Epoxy Derived from Tannic Acid as Biobased Hardener. ACS Sustain. Chem. Eng. 2019, 7, 3858–3865. [Google Scholar] [CrossRef]
- Sonnier, R. Microscale Forced Combustion: Pyrolysis-Combustion Flow Calorimetry (PCFC). In Analysis of Flame Retardancy in Polymer Science; Elsevier: Amsterdam, The Netherlands, 2022; p. 107. [Google Scholar]
- Laufer, G.; Kirkland, C.; Morgan, A.B.; Grunlan, J.C. Intumescent Multilayer Nanocoating, Made with Renewable Polyelectrolytes, for Flame-Retardant Cotton. Biomacromolecules 2012, 13, 2843–2848. [Google Scholar] [CrossRef] [PubMed]


| Sample | Tmax (°C) | Mass at 400 °C (%) | Mass at 700 °C (%) |
|---|---|---|---|
| PUF | 312, 542 | 53.7 | 1.5 |
| Malic | 229, 426, 556 | 67.8 | 1.9 |
| Maleic | 218, 435, 548 | 66.4 | 1.7 |
| Citric | 217, 438, 554 | 63.9 | 1.4 |
| Aconitic | 155, 209, 437, 553 | 65.0 | 2.1 |
| Sample | THR (kJ/g) | pHRR (W/g) | TpHRR (°C) |
|---|---|---|---|
| PUF | 16.9 ± 0.1 | 144.2 ± 3.5 | 342.5 ± 1.0 |
| Malic | 16.4 ± 0.2 | 160.3 ± 5.5 | 458.6 ± 4.9 |
| Maleic | 18.5 ± 0.5 | 198 ± 25 | 456.0 ± 3.2 |
| Citric | 15.9 ± 0.7 | 158.3 ± 7.5 | 463.5 ± 3.5 |
| Aconitic | 17.4 ± 0.9 | 171 ± 20 | 485.7 ± 1.8 |
| Sample | Mass Residue (%) | Afterburn (s) |
|---|---|---|
| PUF | 51.4 ± 7.1 | 4.3 ± 0.7 |
| Malic | 88.7 ± 1.6 | 0.8 ± 0.2 |
| Maleic | 89.9 ± 3.1 | 5.1 ± 5.9 |
| Citric | 90.1 ± 0.1 | 1.0 ± 0.2 |
| Aconitic | 84.7 ± 1.6 | 1.0 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, D.L.; Rodriguez-Melendez, D.; Cotton, S.M.; Quan, Y.; Wang, Q.; Grunlan, J.C. Non-Isocyanate Polyurethane Bio-Foam with Inherent Heat and Fire Resistance. Polymers 2022, 14, 5019. https://doi.org/10.3390/polym14225019
Smith DL, Rodriguez-Melendez D, Cotton SM, Quan Y, Wang Q, Grunlan JC. Non-Isocyanate Polyurethane Bio-Foam with Inherent Heat and Fire Resistance. Polymers. 2022; 14(22):5019. https://doi.org/10.3390/polym14225019
Chicago/Turabian StyleSmith, Dallin L., Danixa Rodriguez-Melendez, Sidney M. Cotton, Yufeng Quan, Qingsheng Wang, and Jaime C. Grunlan. 2022. "Non-Isocyanate Polyurethane Bio-Foam with Inherent Heat and Fire Resistance" Polymers 14, no. 22: 5019. https://doi.org/10.3390/polym14225019
APA StyleSmith, D. L., Rodriguez-Melendez, D., Cotton, S. M., Quan, Y., Wang, Q., & Grunlan, J. C. (2022). Non-Isocyanate Polyurethane Bio-Foam with Inherent Heat and Fire Resistance. Polymers, 14(22), 5019. https://doi.org/10.3390/polym14225019








