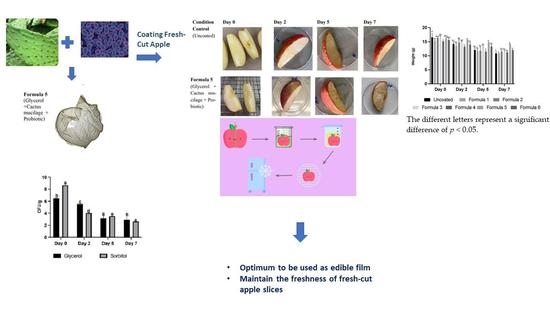
Development of Bioactive Opuntia ficus-indica Edible Films Containing Probiotics as a Coating for Fresh-Cut Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Cactus Mucilage Extraction
2.2. Analysis of the Composition of Cactus Mucilage
2.2.1. Total Sugar Content
2.2.2. Total Phenolic Content
2.2.3. Vitamin C Content
- A is the amount of ascorbic acid in the sample solution (mg)
- VI2 is Volume of iodine used for titration (mL)
2.2.4. Radical Scavenging Effect
- Asample is the absorbance of the sample + DPPH
- ADPPH is the absorbance of DPPH
2.3. Preparation of Probiotic Cells
2.4. Preparation of Probiotic Cactus Film
2.5. Characterization of Film Physical Properties
2.5.1. Film Thickness
2.5.2. Moisture Content
2.5.3. Film Solubility
2.5.4. Water Vapor Permeability
- WVTR (g/m2) is the water vapor permeability rate. It is obtained as the slope (g/min) divided by the transmission area (m2).
- X (mm) is the film thickness.
- Δp (kpa) is the difference in water vapor pressure between the films (Δp = p(RH2 − RH1) = 337.60 where p is the saturation pressure of water at 35 °C, RH2 = 60% and RH1 = 0%)
2.5.5. Mechanical Properties
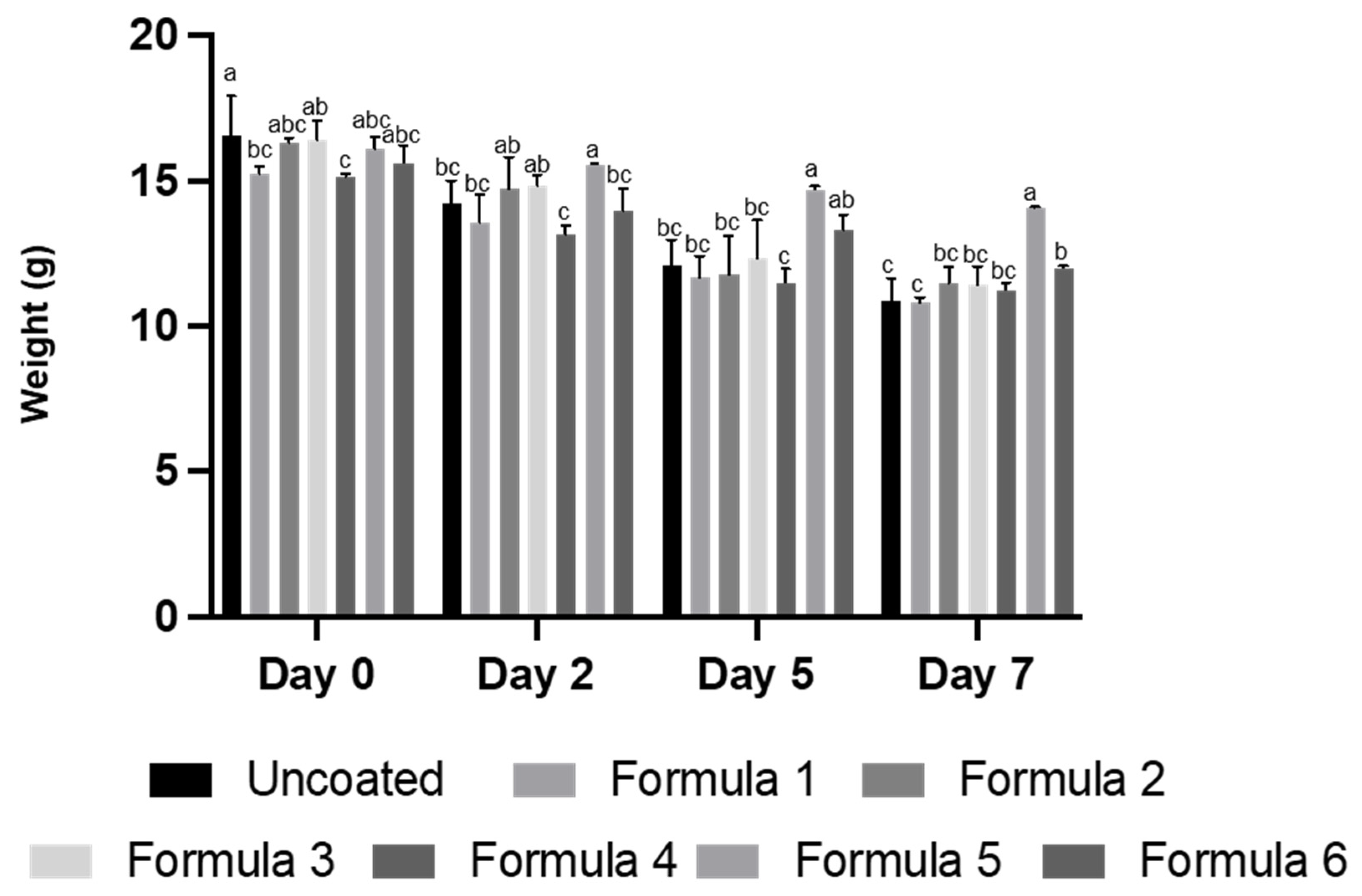
2.6. Properties of Film Coating on Fresh-Cut Apple
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gebrechristos, H.Y.; Ma, X.; Xiao, F.; He, Y.; Zheng, S.; Oyungerel, G.; Chen, W. Potato peel extracts as an antimicrobial and potential antioxidant in active edible film. Food Sci. Nutr. 2020, 8, 6338–6345. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Izquierdo, V.; Krochta, J. Thermoplastic processing of proteins for film formation—A review. J. Food Sci. 2008, 73, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Chhikara, S.; Kumar, D. Edible Coating and Edible Film as Food Packaging Material: A Review. J. Packag. Technol. Res. 2021, 6, 1–10. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Brito-De La Fuente, E.; Torrestiana-Sanchez, B.; Katthain, R. Rheological properties of the mucilage gum (Opuntia ficus indica). Food Hydrocoll. 2000, 14, 417–424. [Google Scholar] [CrossRef]
- Cai, W.; Gu, X.; Tang, J. Extraction, purification, and characterization of the polysaccharides from Opuntia milpa alta. Carbohydr. Polym. 2008, 71, 403–410. [Google Scholar] [CrossRef]
- Park, E.; Kahng, H.J.H.; Paek, E.A. Studies on the pharmacological actions of cactus: Identification of its anti-inflammatory effect. Arch. Pharm. Res. 1998, 21, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, S.M.; Ha, H.J.; Moon, C.J.; Shin, T.K.; Kim, J.M.; Lee, N.H.; Kim, H.C.; Jang, K.J.; Wie, M.B. Opuntia ficus-indica attenuates neuronal injury in in vitro and in vivo models of cerebral ischemia. J. Ethnopharmacol. 2006, 104, 257–262. [Google Scholar] [CrossRef]
- López, A.D. Use of the fruits and stems of the prickly pear cactus (Opuntia spp.) into human food. Food Sci. Technol. Int. 1995, 1, 65–74. [Google Scholar] [CrossRef]
- Dhall, R. Advances in edible coatings for fresh fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef]
- González Sandoval, D.C.; Luna Sosa, B.; Martínez-Ávila, G.C.G.; Rodríguez Fuentes, H.; Avendaño Abarca, V.H.; Rojas, R. Formulation and characterization of edible films based on organic mucilage from Mexican Opuntia ficus-indica. Coatings 2019, 9, 506. [Google Scholar] [CrossRef]
- Allegra, A.; Sortino, G.; Inglese, P.; Settanni, L.; Todaro, A.; Gallotta, A. The effectiveness of Opuntia ficus-indica mucilage edible coating on post-harvest maintenance of ‘Dottato’fig (Ficus carica L.) fruit. Food Packag. Shelf Life 2017, 12, 135–141. [Google Scholar] [CrossRef]
- Del-Valle, V.; Hernández-Muñoz, P.; Guarda, A.; Galotto, M.J. Development of a cactus-mucilage edible coating (Opuntia ficus indica) and its application to extend strawberry (Fragaria ananassa) shelf-life. Food Chem. 2005, 91, 751–756. [Google Scholar] [CrossRef]
- Morais, M.A.D.S.; Fonseca, K.S.; Viégas, E.K.D.; de Almeida, S.L.; Maia, R.K.M.; Silva, V.N.S.; Simões, A.D.N. Mucilage of spineless cactus in the composition of an edible coating for minimally processed yam (Dioscorea spp.). J. Food Meas. Character. 2019, 13, 2000–2008. [Google Scholar] [CrossRef]
- Otoni, C.G.; Espitia, P.J.; Avena-Bustillos, R.J.; McHugh, T.H. Trends in antimicrobial food packaging systems: Emitting sachets and absorbent pads. Food Res. Int. 2016, 83, 60–73. [Google Scholar] [CrossRef]
- Tapia, M.S.; Rojas-Graü, M.A.; Rodríguez, F.J.; Ramírez, J.; Carmona, A.; Martin-Belloso, O. Alginate-and gellan-based edible films for probiotic coatings on fresh-cut fruits. J. Food Sci. 2007, 72, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Lim, S.T. Development and characterization of novel probiotic-residing pullulan/starch edible films. Food Chem. 2013, 141, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.J.; Cedran, M.F.; Garcia, S. Influence of linseed mucilage incorporated into an alginate-base edible coating containing probiotic bacteria on shelf-life of fresh-cut yacon (Smallanthus sonchifolius). Food Bioprocess Technol. 2018, 11, 1605–1614. [Google Scholar] [CrossRef]
- Gheribi, R.; Puchot, L.; Verge, P.; Jaoued-Grayaa, N.; Mezni, M.; Habibi, Y.; Khwaldia, K. Development of plasticized edible films from Opuntia ficus-indica mucilage: A comparative study of various polyol plasticizers. Carbohydr. Polym. 2018, 190, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Otálora, M.C.; Wilches-Torres, A.; Castaño, J.A.G. Extraction and Physicochemical Characterization of Dried Powder Mucilage from Opuntia ficus-indica Cladodes and Aloe Vera Leaves: A Comparative Study. Polymers 2021, 13, 1689. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S. Total carbohydrate by phenol-sulfuric acid method. In Food Analysis Laboratory Manual; Springer: Berlin/Heidelberg, Germany, 2017; pp. 137–141. [Google Scholar]
- Euawongkul, N.; Rattanamalee, C.; Daduang, S. Effect of Tricholoma crassum on growth and productivity of pineapple (Ananas comosus cv. Pattawia). Prog. Appl. Sci. Technol. 2017, 7, 232–240. [Google Scholar]
- Tatsaporn, T.; Kornkanok, K. Using potential lactic acid bacteria biofilms and their compounds to control biofilms of foodborne pathogens. Biotechnol. Rep. 2020, 26, e00477. [Google Scholar] [CrossRef]
- Tanwar, R.; Gupta, V.; Kumar, P.; Kumar, A.; Singh, S.; Gaikwad, K.K. Development and characterization of PVA-starch incorporated with coconut shell extract and sepiolite clay as an antioxidant film for active food packaging applications. Int. J. Biol. Macromol. 2021, 185, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Jouki, M.; Yazdi, F.T.; Mortazavi, S.A.; Koocheki, A. Physical, barrier and antioxidant properties of a novel plasticized edible film from quince seed mucilage. Int. J. Biol. Macromol. 2013, 62, 500–507. [Google Scholar] [CrossRef]
- ASTM. E96/E96M-16, Standard Test Methods for Water Vapor Transmission of Materials. In Annual Book of ASTM Standards; American Society for Testing and Materials: West Conshohocken, PA, USA, 2016; pp. 719–725. [Google Scholar]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.H.S.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef] [PubMed]
- Jorge, A.J.; De La Garza, T.H.; Alejandro, Z.C.; Ruth, B.C.; Noé, A.C. The optimization of phenolic compounds extraction from cactus pear (Opuntia ficus-indica) skin in a reflux system using response surface methodology. Asian Pac. J. Trop. Biomed. 2013, 3, 436–442. [Google Scholar] [CrossRef]
- Suderman, N.; Isa, M.; Sarbon, N. The effect of plasticizers on the functional properties of biodegradable gelatin-based film: A review. Food Biosci. 2018, 24, 111–119. [Google Scholar] [CrossRef]
- Thomazine, M.; Carvalho, R.A.; Sobral, P.J. Physical properties of gelatin films plasticized by blends of glycerol and sorbitol. J. Food Sci. 2005, 70, E172–E176. [Google Scholar] [CrossRef]
- Dai, H.; Li, X.; Du, J.; Ma, L.; Yu, Y.; Zhou, H.; Guo, T.; Zhang, Y. Effect of interaction between sorbitol and gelatin on gelatin properties and its mechanism under different citric acid concentrations. Food Hydrocoll. 2020, 101, 105557. [Google Scholar] [CrossRef]
- Espitia, P.J.; Batista, R.A.; Azeredo, H.M.; Otoni, C.G. Probiotics and their potential applications in active edible films and coatings. Food Res. Int. 2016, 90, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Soukoulis, C.; Yonekura, L.; Gan, H.H.; Behboudi-Jobbehdar, S.; Parmenter, C.; Fisk, I. Probiotic edible films as a new strategy for developing functional bakery products: The case of pan bread. Food Hydrocoll. 2014, 39, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Galindez, A.; Daza, L.D.; Homez-Jara, A.; Eim, V.S.; Váquiro, H.A. Characterization of ulluco starch and its potential for use in edible films prepared at low drying temperature. Carbohydr. Polym. 2019, 215, 143–150. [Google Scholar] [CrossRef]
- Sancakli, A.; Basaran, B.; Arican, F.; Polat, O. Effects of bovine gelatin viscosity on gelatin-based edible film mechanical, physical and morphological properties. SN Appl. Sci. 2021, 3, 8. [Google Scholar] [CrossRef]
- Fadini, A.L.; Rocha, F.S.; Alvim, I.D.; Sadahira, M.S.; Queiroz, M.B.; Alves, R.M.V.; Silva, L.B. Mechanical properties and water vapour permeability of hydrolysed collagen–cocoa butter edible films plasticised with sucrose. Food Hydrocoll. 2013, 30, 625–631. [Google Scholar] [CrossRef]
- Soukoulis, C.; Behboudi-Jobbehdar, S.; Yonekura, L.; Parmenter, C.; Fisk, I.D. Stability of Lactobacillus rhamnosus GG in prebiotic edible films. Food Chem. 2014, 159, 302–308. [Google Scholar] [CrossRef]
- Faust, S.; Foerster, J.; Lindner, M.; Schmid, M. Effect of glycerol and sorbitol on the mechanical and barrier properties of films based on pea protein isolate produced by high-moisture extrusion processing. Polym. Eng. Sci. 2022, 62, 95–102. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of starch type on the physico-chemical properties of edible films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, G.F.; Fakhouri, F.M.; de Oliveira, R.A. Extraction and characterization of arrowroot (Maranta arundinaceae L.) starch and its application in edible films. Carbohydr. Polym. 2018, 186, 64–72. [Google Scholar] [CrossRef]
- Fan, X.; Niemira, B.A.; Doona, C.J.; Feeherry, F.E.; Gravani, R.B. (Eds.) Microbial Safety of Fresh Produce; John Wiley & Sons: Hoboken, NJ, USA, 2009; Volume 41. [Google Scholar]
- Aldana, D.S.; Andrade-Ochoa, S.; Aguilar, C.N.; Contreras-Esquivel, J.C.; Nevárez-Moorillón, G.V. Antibacterial activity of pectic-based edible films incorporated with Mexican lime essential oil. Food Control 2015, 50, 907–912. [Google Scholar] [CrossRef]
- Bhowmik, S.R.; Pan, J.C. Shelf life of mature green tomatoes stored in controlled atmosphere and high humidity. J. Food Sci. 1992, 57, 948–953. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, Y.; Wang, D.; Zhang, L.; Sun, J.; Sun, L.; Zhang, B. Effect of alginate coating combined with yeast antagonist on strawberry (Fragaria × ananassa) preservation quality. Postharvest Biol. Technol. 2009, 53, 84–90. [Google Scholar] [CrossRef]
- Do Esiroto, S.A.; Perego, P. Influence of food matrices on probiotic viability–A review focusing on the fruity bases. Trends Food Sci. Technol. 2011, 22, 377–385. [Google Scholar]
- Soukoulis, C.; Singh, P.; Macnaughtan, W.; Parmenter, C.; Fisk, I.D. Compositional and physicochemical factors governing the viability of Lactobacillus rhamnosus GG embedded in starch-protein based edible films. Food Hydrocoll. 2016, 52, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Gebrechristos, H.Y.; Ma, X.; Xiao, F.; He, Y.; Zheng, S.; Oyungerel, G.; Chen, W. Physical properties and prebiotic activity of maize starch-based functional films. Starch-Stärke 2015, 67, 124–131. [Google Scholar]
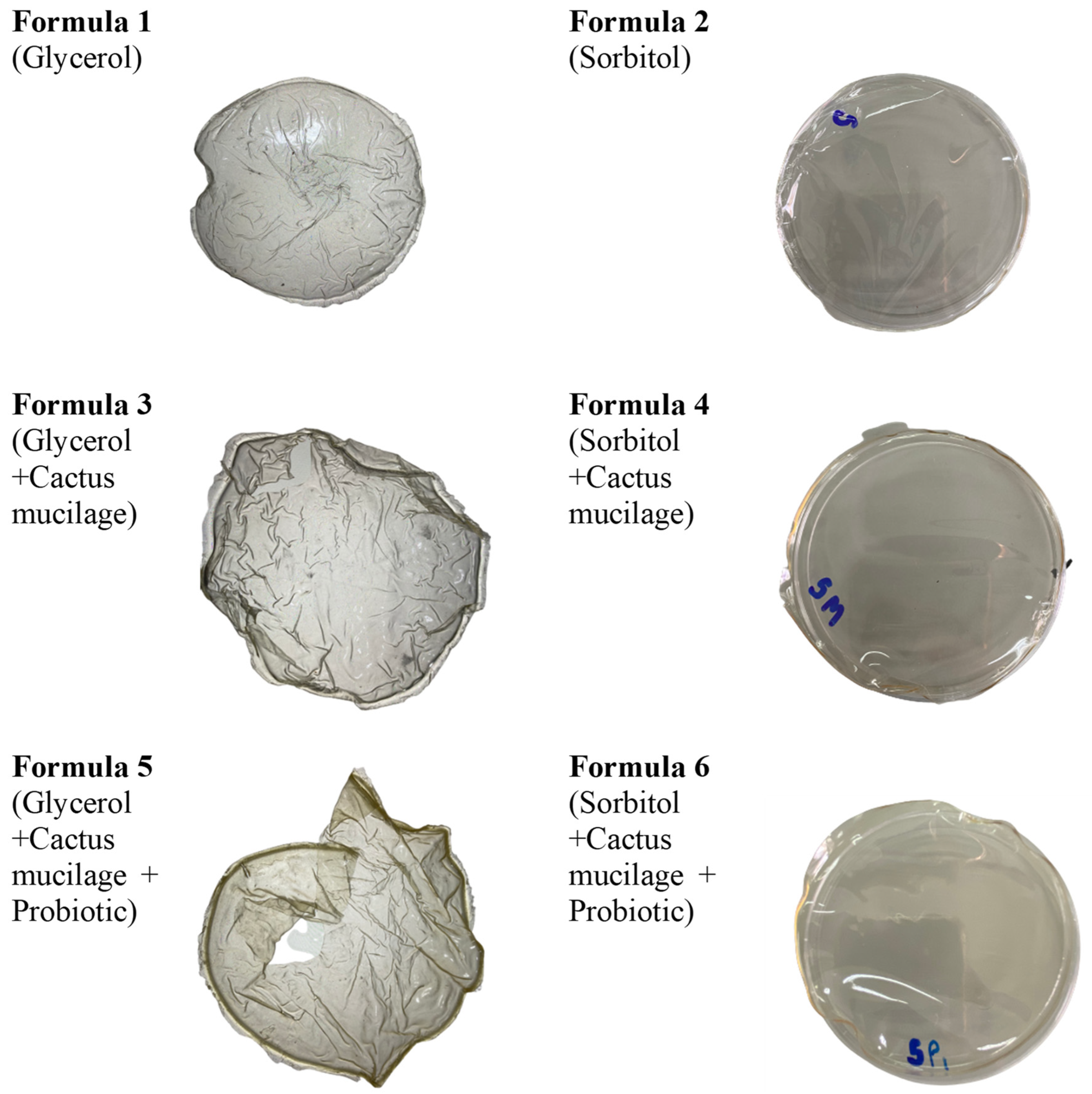
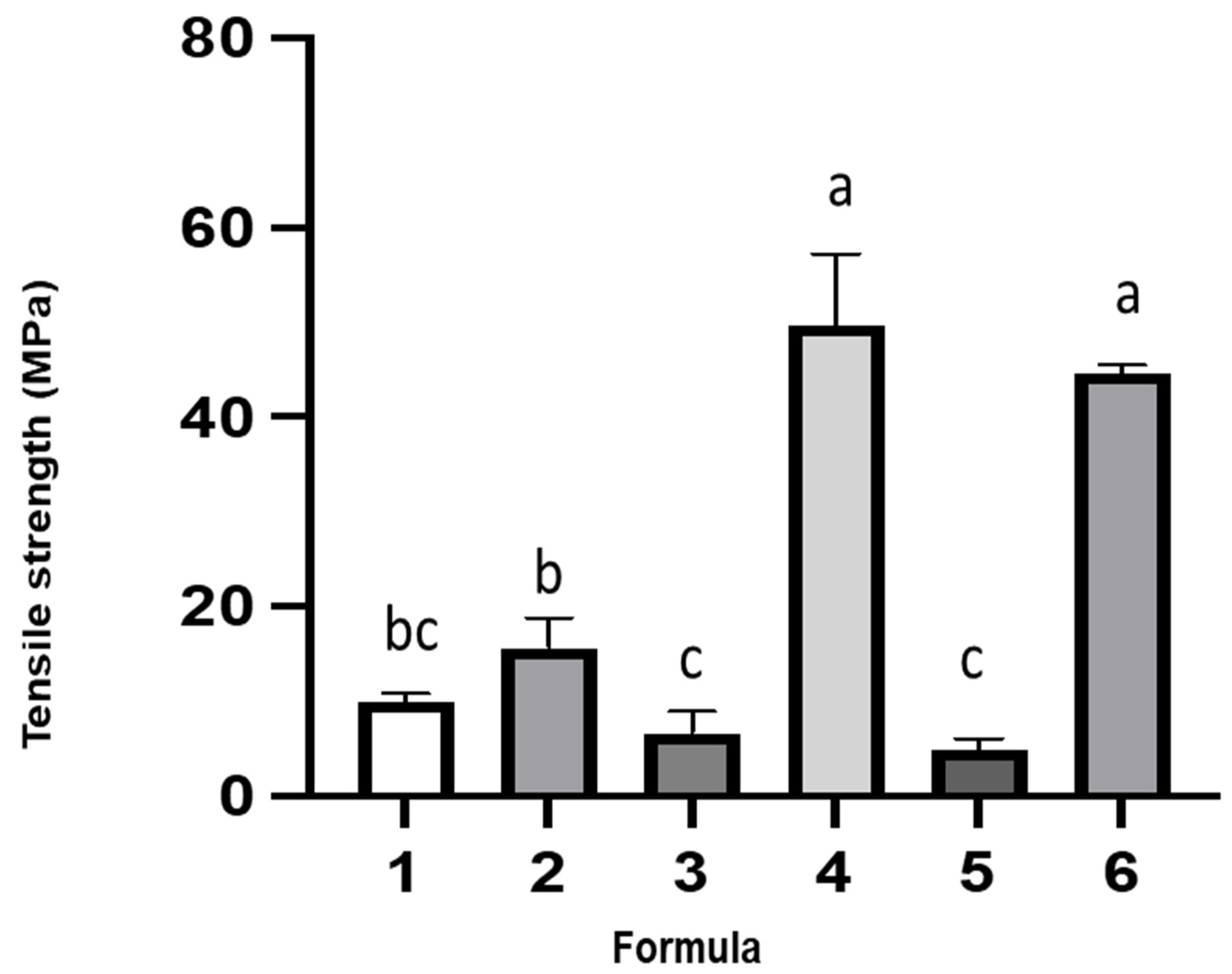
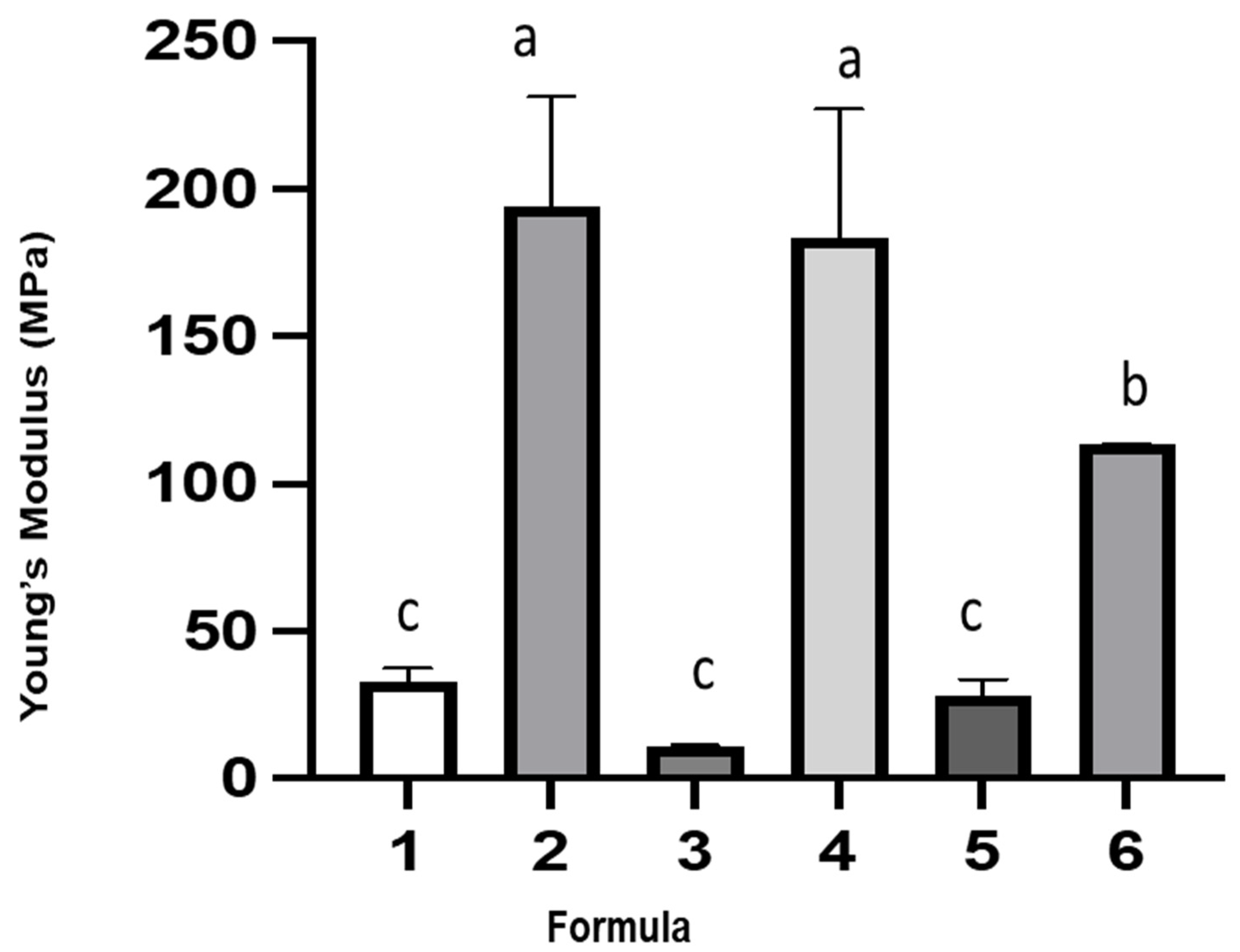




| Formula No. | Distilled Water (mL) | Cactus Mucilage (mL) | Gelatin (g) | Plasticizer (mL) | Probiotic Added | |
|---|---|---|---|---|---|---|
| Glycerol | Sorbitol | |||||
| 1 | 170 | - | 5 | 5.7 | - | - |
| 2 | 170 | - | 5 | - | 5.7 | - |
| 3 | 85 | 85 | 5 | 5.7 | - | - |
| 4 | 85 | 85 | 5 | - | 5.7 | - |
| 5 | 85 | 85 | 5 | 5.7 | - | + |
| 6 | 85 | 85 | 5 | - | 5.7 | + |
| Total Sugar (mg/g) | Phenolic Content (mg AGE/mL MUC) | Vitamin C (mg/mL) | Percent DPPH Radical Scavenging (%) |
|---|---|---|---|
| 0.47 ± 0.06 | 0.33 ± 0.06 | 0.14 | 35.51 ± 1.88 |
| Formula | Film Thickness (mm) | Moisture Content (%) | Film Solubility (%) | Water Vapor Permeability (g·mm/m2·min·kpa) |
|---|---|---|---|---|
| 1 (Glycerol) | 0.01 ± 0.01 c | 0.36 ± 0.03 a | 60.65 ± 3.27 a | 0.05 ± 0.001 c |
| 2 (Sorbitol) | 0.06 ± 0.03 b | 0.18 ± 0.01 b | 40.97 ± 4.91 c | 0.32 ± 0.001 b |
| 3 (Glycerol + Cactus mucilage) | 0.02 ± 0.02 bc | 0.24 ± 0.07 b | 59.41 ± 6.89 a | 0.15 ± 0.070 c |
| 4 (Sorbitol + Cactus mucilage) | 0.02 ± 0.01 c | 0.21 ± 0.04 b | 45.43 ± 2.73 bc | 0.16 ± 0.071 b |
| 5 (Glycerol + Cactus mucilage + Probiotic) | 0.02 ± 0.01 bc | 0.21 ± 0.05 b | 53.15 ± 3.79 ab | 0.31 ± 0.008 b |
| 6 (Sorbitol + Cactus mucilage + Probiotic) | 0.11 ± 0.04 a | 0.19 ± 0.01 b | 30.66 ± 7.23 d | 1.50 ± 0.009 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todhanakasem, T.; Boonchuai, P.; Itsarangkoon Na Ayutthaya, P.; Suwapanich, R.; Hararak, B.; Wu, B.; Young, B.M. Development of Bioactive Opuntia ficus-indica Edible Films Containing Probiotics as a Coating for Fresh-Cut Fruit. Polymers 2022, 14, 5018. https://doi.org/10.3390/polym14225018
Todhanakasem T, Boonchuai P, Itsarangkoon Na Ayutthaya P, Suwapanich R, Hararak B, Wu B, Young BM. Development of Bioactive Opuntia ficus-indica Edible Films Containing Probiotics as a Coating for Fresh-Cut Fruit. Polymers. 2022; 14(22):5018. https://doi.org/10.3390/polym14225018
Chicago/Turabian StyleTodhanakasem, Tatsaporn, Pratana Boonchuai, Pavarunya Itsarangkoon Na Ayutthaya, Rachit Suwapanich, Bongkot Hararak, Bo Wu, and Briana M. Young. 2022. "Development of Bioactive Opuntia ficus-indica Edible Films Containing Probiotics as a Coating for Fresh-Cut Fruit" Polymers 14, no. 22: 5018. https://doi.org/10.3390/polym14225018
APA StyleTodhanakasem, T., Boonchuai, P., Itsarangkoon Na Ayutthaya, P., Suwapanich, R., Hararak, B., Wu, B., & Young, B. M. (2022). Development of Bioactive Opuntia ficus-indica Edible Films Containing Probiotics as a Coating for Fresh-Cut Fruit. Polymers, 14(22), 5018. https://doi.org/10.3390/polym14225018