The Effect of Chitosan on Plant Physiology, Wound Response, and Fruit Quality of Tomato
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Plant Physiological Parameters
2.3. Phytohormone Analysis
2.4. Real-Time qPCR
2.5. Fruit Physico-Chemical Parameters
2.6. Metabolomics Analysis
2.7. Sugar Content
2.8. α-Tocopherol Content
2.9. Lycopene Content
2.10. Total Flavonoid Content
2.11. Macronutrient Content
2.12. Statistical Analysis
3. Results
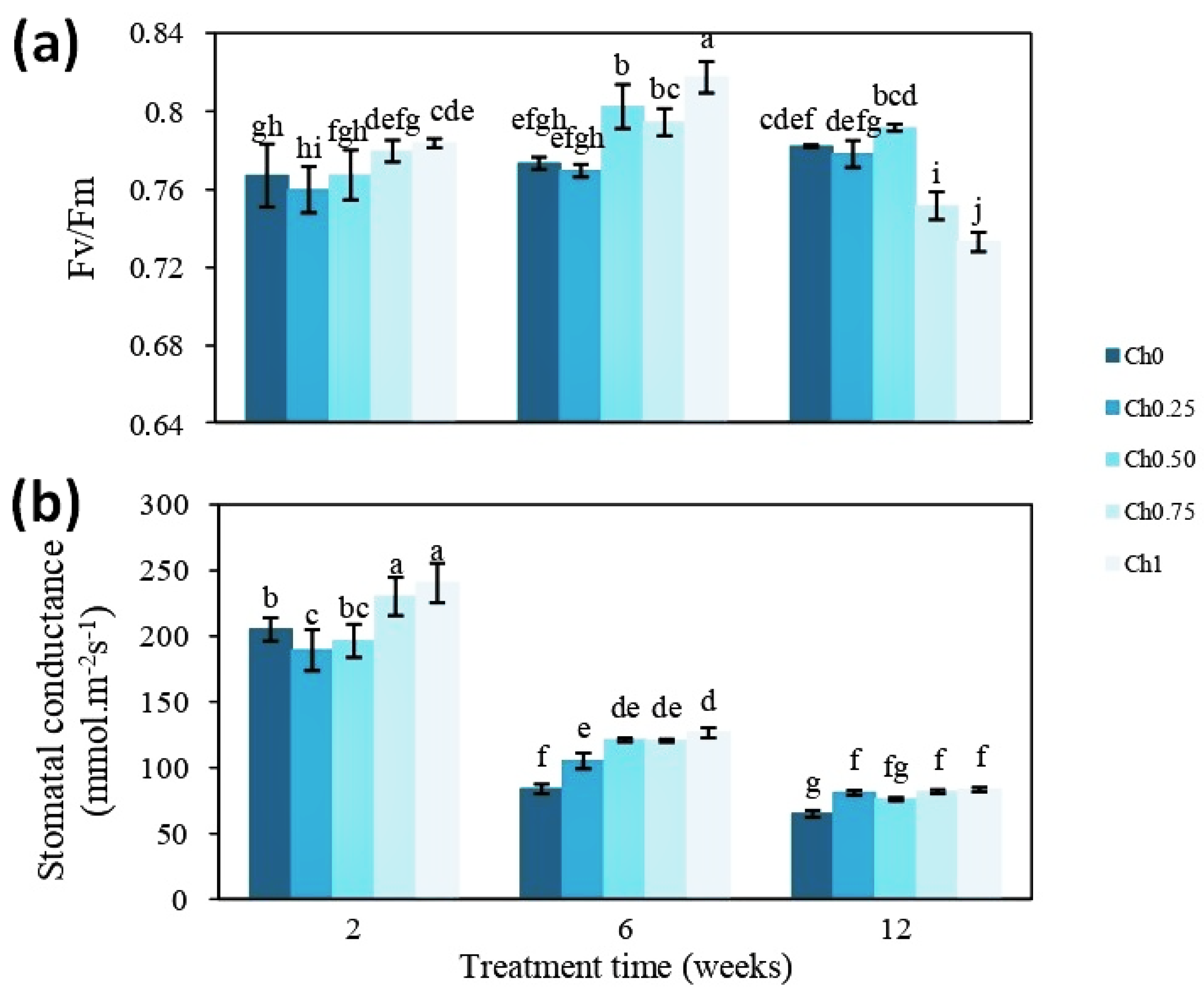
3.1. Chitosan Effects on Plant Growth and Physiological Parameters
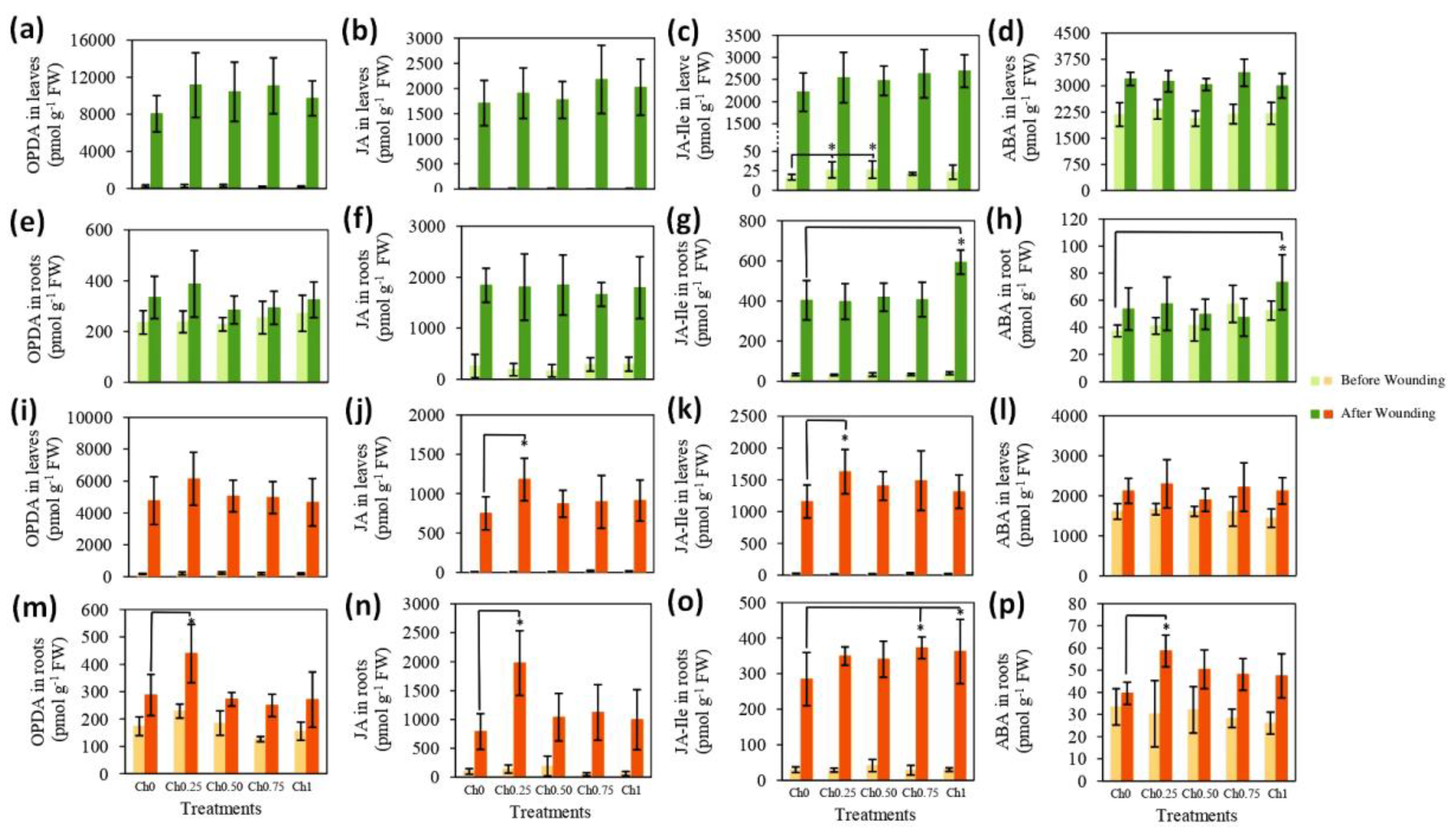
3.2. Chitosan Effects on Plant’s Response to Wounding
3.3. Chitosan Effects on Tomato Fruits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADP | adenosine diphosphate |
| ALA | alanine |
| AMP | adenosine monophosphate |
| ARG | arginine |
| ASC | ascorbate |
| ASP | aspartic acid |
| Ch | Chitosan |
| CIT | citrate |
| CoA | coenzyme A |
| DHA | dehydroascorbic acid |
| DHO | dihydroorotate |
| GDP | guanosine diphosphate |
| GMP | guanosine monophosphate |
| GUO | guanosine |
| HIS | histidine |
| IP4 | sum of inositol tetrakisphosphates |
| IP5 | sum of inositol pentakisphosphates |
| IP6 | inositol hexakisphosphate |
| ISOC | isocitrate |
| LAC | lactate |
| MET | methionine |
| NADP | nicotinamide adenine dinucleotide phosphate (oxidized) |
| PAN | pantothenate |
| PRPP | phosphoribosyl pyrophosphate |
| SUC | sucrose |
| RiBU-1,5-bP | ribulose1,5-bisphosphate |
| UMP | uridine monophosphate |
References
- Coppola, D.; Lauritano, C.; Palma Esposito, F.; Riccio, G.; Rizzo, C.; de Pascale, D. Fish waste: From problem to valuable resource. Mar. Drugs 2021, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Negi, T.; Sagar, N.A.; Kumar, Y.; Tarafdar, A.; Sirohi, R.; Sindhu, R.; Pandey, A. Sustainable processes for treatment and management of seafood solid waste. Sci. Total Environ. 2022, 817, 152951. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, D.K.; Jadhav, S.J.; Yu, M.H. Quality and nutritional composition of tomato fruit as influenced by certain biochemical and physiological changes. Qual. Plant. 1974, 24, 85–113. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and Livestock Products. 2019. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 29 September 2021).
- Fróna, D.; Szenderák, J.; Harangi-Rákos, M. The challenge of feeding the world. Sustainability 2019, 11, 5816. [Google Scholar] [CrossRef]
- Goetz, M.; Hooper, L.C.; Johnson, S.D.; Rodrigues, J.C.M.; Vivian-Smith, A.; Koltunow, A.M. Expression of aberrant forms of AUXIN RESPONSE FACTOR8 stimulates parthenocarpy in Arabidopsis and tomato. Plant Physiol. 2007, 145, 351–366. [Google Scholar] [CrossRef]
- Huang, W.; Liao, S.; Lv, H.; Khaldun, A.B.M.; Wang, Y. Characterization of the growth and fruit quality of tomato grafted on a woody medicinal plant, Lyciumchinense. Sci. Hortic 2015, 197, 447–453. [Google Scholar] [CrossRef]
- Kariathi, V.; Kassim, N.; Kimanya, M. Pesticide exposure from fresh tomatoes and its relationship with pesticide application practices in Meru district. Cogent Food Agric. 2016, 2, 1196808. [Google Scholar] [CrossRef]
- Elmer, W.H.; White, J.C. The use of metallic oxide nanoparticles to enhance growth of tomatoes and eggplants in disease infested soil or soilless medium. Environ. Sci. Nano 2016, 3, 1072–1079. [Google Scholar] [CrossRef]
- Koleška, I.; Hasanagić, D.; Todorović, V.; Murtić, S.; Klokić, I.; Parađiković, N.; Kukavica, B. Biostimulant prevents yield loss and reduces oxidative damage in tomato plants grown on reduced NPK nutrition. J. Plant Interact. 2017, 12, 209–218. [Google Scholar] [CrossRef]
- Benaffari, W.; Boutasknit, A.; Anli, M.; Ait-El-Mokhtar, M.; Ait-Rahou, Y.; Ben-Laouane, R.; Ben Ahmed, H.; Mitsui, T.; Baslam, M.; Meddich, A. The native arbuscular mycorrhizal fungi and vermicompost-based organic amendments enhance soil fertility, growth performance, and the drought stress tolerance of quinoa. Plants 2022, 11, 393. [Google Scholar] [CrossRef]
- El Amerany, F.; Aboudamia, F.Z.; Janah, I.; Taourirte, M.; Rhazi, M. Polymer-Based Nanoparticles (NPs): A Promising Approach for Crop Productivity. In Plant and Nanoparticles, 1st ed.; Chen, J.T., Ed.; Springer: Singapore, 2022; pp. 119–154. [Google Scholar]
- Abo Elsoud, M.M.; El Kady, E.M. Current trends in fungal biosynthesis of chitin and chitosan. Bull. Natl. Res. Cent. 2019, 43, 59. [Google Scholar] [CrossRef]
- Iriti, M.; Picchi, V.; Rossoni, M.; Gomarasca, S.; Ludwig, N.; Gargano, M.; Faoro, F. Chitosan antitranspirant activity is due to abscisic acid-dependent stomatal closure. Environ. Exp. Bot. 2009, 66, 493–500. [Google Scholar] [CrossRef]
- Xing, K.; Zhu, X.; Peng, X.; Qin, S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agron. Sustain. Dev. 2015, 35, 569–588. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Chitosan effects on plant systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef]
- Kipkoech, C.; Kinyuru, J.N.; Imathiu, S.; Meyer-Rochow, V.B.; Roos, N. In Vitro Study of Cricket Chitosan’s Potential as a Prebiotic and a Promoter of Probiotic Microorganisms to Control Pathogenic Bacteria in the Human Gut. Foods 2021, 10, 2310. [Google Scholar] [CrossRef]
- Chamnanmanoontham, N.; Pongprayoon, W.; Pichayangkura, R.; Roytrakul, S.; Chadchawan, S. Chitosan enhances rice seedling growth via gene expression network between nucleus and chloroplast. Plant Growth Regul. 2015, 75, 101–114. [Google Scholar] [CrossRef]
- Saharan, V.; Pal, A. Chitosan Based Nanomaterials in Plant Growth and Protection; Springer: New Delhi, India, 2016; pp. 33–41. [Google Scholar]
- Lopez-Moya, F.; Escudero, N.; Zavala-Gonzalez, E.A.; Esteve-Bruna, D.; Blázquez, M.A.; Alabadí, D.; Lopez-Llorca, L.V. Induction of auxin biosynthesis and WOX5 repression mediate changes in root development in Arabidopsis exposed to chitosan. Sci. Rep. 2017, 7, 16813. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and its potential use as a scaffold for tissue engineering in regenerative medicine. BioMed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef] [PubMed]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [CrossRef] [PubMed]
- El Amerany, F.; Meddich, A.; Wahbi, S.; Porzel, A.; Taourirte, M.; Rhazi, M.; Hause, B. Foliar application of chitosan increases tomato growth and influences mycorrhization and expression of endochitinase-encoding genes. Int. J. Mol. Sci. 2020, 21, 535. [Google Scholar] [CrossRef] [PubMed]
- El Amerany, F.; Rhazi, M.; Wahbi, S.; Taourirte, M.; Meddich, A. The effect of chitosan, arbuscular mycorrhizal fungi, and compost applied individually or in combination on growth, nutrient uptake, and stem anatomy of tomato. Sci. Hortic. 2020, 261, 109015. [Google Scholar] [CrossRef]
- Colman, S.L.; Salcedo, M.F.; Mansilla, A.Y.; Iglesias, M.J.; Fiol, D.F.; Martín-Saldaña, S.; Alvarez, V.A.; Chevalier, A.A.; Casalongué, C.A. Chitosan microparticles improve tomato seedling biomass and modulate hormonal, redox and defense pathways. Plant Physiol. Biochem. 2019, 143, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Asgari-Targhi, G.; Iranbakhsh, A.; Ardebili, Z.O. Potential benefits and phytotoxicity of bulk and nano-chitosan on the growth, morphogenesis, physiology, and micropropagation of Capsicum annuum. Plant Physiol. Biochem. 2018, 127, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Pichyangkura, R.; Chadchawan, S. Biostimulant activity of chitosan in horticulture. Sci. Hortic. 2015, 196, 49–65. [Google Scholar] [CrossRef]
- Savatin, D.V.; Gramegna, G.; Modesti, V.; Cervone, F. Wounding in the plant tissue: The defense of a dangerous passage. Front. Plant Sci. 2014, 5, 470. [Google Scholar] [CrossRef]
- Halitschke, R.; Schittko, U.; Pohnert, G.; Boland, W.; Baldwin, I.T. Molecular interactions between the specialist herbivore Manducasexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol. 2001, 125, 711–717. [Google Scholar] [CrossRef]
- Suttle, J.C.; Lulai, E.C.; Huckle, L.L.; Neubauer, J.D. Wounding of potato tubers induces increases in ABA biosynthesis and catabolism and alters expression of ABA metabolic genes. J. Plant Physiol. 2013, 170, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Peña-Cortés, H.; Fisahn, J.; Willmitzer, L. Signals involved in wound-induced proteinase inhibitor II gene expression in tomato and potato plants. Proc. Natl. Acad. Sci. USA 1995, 92, 4106–4113. [Google Scholar] [CrossRef]
- Koo, A.J.; Howe, G.A. The wound hormone jasmonate. Phytochemistry 2009, 70, 1571–1580. [Google Scholar] [CrossRef]
- Rakwal, R.; Tamogami, S.; Agrawal, G.K.; Iwahashi, H. Octadecanoid signaling component “burst” in rice (Oryza sativa L.) seedling leaves upon wounding by cut and treatment with fungal elicitor chitosan. Biochem. Biophys. Res. Commun. 2002, 295, 1041–1045. [Google Scholar] [CrossRef]
- Ozeretskovskaya, O.L.; Vasyukova, N.I.; Chalenko, G.I.; Gerasimova, N.G.; Revina, T.A.; Valueva, T.A. Wound healing and induced resistance in potato tubers. Appl. Biochem. Microbiol. 2009, 45, 199–203. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Sobeih, W.Y.; Dodd, I.C.; Bacon, M.A.; Grierson, D.; Davies, W.J. Long-distance signals regulating stomatal conductance and leaf growth in tomato (Lycopersicon esculentum) plants subjected to partial root-zone drying. J. Exp. Bot. 2004, 55, 2353–2363. [Google Scholar] [CrossRef] [PubMed]
- Balcke, G.U.; Handrick, V.; Bergau, N.; Fichtner, M.; Henning, A.; Stellmach, H.; Tissier, A.; Hause, B.; Frolov, A. An UPLC-MS/MS method for highly sensitive high-throughput analysis of phytohormones in plant tissues. Plant Methods 2012, 8, 47. [Google Scholar] [CrossRef]
- Chafi, A.; Benabbes, R.; Bouakka, M.; Hakkou, A.; Kouddane, N.; Berrichi, A. Pomological study of dates of some date palm varieties cultivated in Figuig oasis. J. Mater. Environ. Sci. 2015, 6, 1266–1275. [Google Scholar]
- El Amerany, F.; Taourirte, M.; Wahbi, S.; Meddich, A.; Rhazi, M. Use of metabolomics data analysis to identify fruit quality markers enhanced by the application of an aminopolysaccharide. RSC Adv. 2021, 11, 35514–35524. [Google Scholar] [CrossRef]
- Schaarschmidt, S.; Qu, N.; Strack, D.; Sonnewald, U.; Hause, B. Local induction of the alc gene switch in transgenic tobacco plants by acetaldehyde. Plant Cell Physiol. 2004, 45, 1566–1577. [Google Scholar] [CrossRef]
- Sadler, G.; Davis, J.; Dezman, D. Rapid extraction of lycopene and β-carotene from reconstituted tomato paste and pink grapefruit homogenates. J. Food Sci. 1990, 55, 1460–1461. [Google Scholar] [CrossRef]
- Hilares, R.T.; Dos Santos, J.G.; Shiguematsu, N.B.; Ahmed, M.A.; da Silva, S.S.; Santos, J.C. Low-pressure homogenization of tomato juice using hydrodynamic cavitation technology: Effects on physical properties and stability of bioactive compounds. Ultrason. Sonochem. 2019, 54, 192–197. [Google Scholar] [CrossRef]
- Fu, X.; Cheng, S.; Liao, Y.; Huang, B.; Du, B.; Zeng, W.; Jiang, Y.; Duan, X.; Yang, Z. Comparative analysis of pigments in red and yellow banana fruit. Food Chem. 2018, 239, 1009–1018. [Google Scholar] [CrossRef]
- Segarra, G.; Casanova, E.; Borrero, C.; Avilés, M.; Trillas, I. The suppressive effects of composts used as growth media against Botrytis cinerea in cucumber plants. Euro J. Plant Pathol. 2007, 117, 393–402. [Google Scholar] [CrossRef]
- Stenzel, I.; Hause, B.; Maucher, H.; Pitzschke, A.; Miersch, O.; Ziegler, J.; Ryan, C.; Wasternack, C. Allene oxide cyclase dependence of the wound response and vascular bundle-specific generation of jasmonates in tomato—Amplification in wound signaling. Plant J. 2003, 33, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Taylor, I.B.; Burbidge, A.; Thompson, A.J. Control of abscisic acid synthesis. J. Exp. Bot. 2000, 51, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Drevinskas, T.; Naujokaitytė, G.; Maruška, A.; Kaya, M.; Sargin, I.; Daubaras, R.; Česonienė, L. Effect of molecular weight of chitosan on the shelf life and other quality parameters of three different cultivars of Actinidia kolomikta (kiwifruit). Carbohydr. Polym. 2017, 173, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Liu, S.; Xing, R.; Chen, X.; Li, P. Protective effect of chitosan on photosynthesis and antioxidative defense system in edible rape (Brassica rapa L.) in the presence of cadmium. Ecotoxicol. Environ. Saf. 2017, 138, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Al-Tawaha, A.R.; Turk, M.A.; Al-Tawaha, A.R.M.; Alu’datt, M.H.; Wedyan, M.; Al-Ramamneh, E.A.D.M.; Hoang, A.T. Using chitosan to improve growth of maize cultivars under salinity conditions. Bulg. J. Agric. Sci. 2018, 24, 437–442. [Google Scholar]
- Stasińska-Jakubas, M.; Hawrylak-Nowak, B. Protective, Biostimulating, and Eliciting Effects of Chitosan and Its Derivatives on Crop Plants. Molecules 2022, 27, 2801. [Google Scholar] [CrossRef]
- Barka, E.A.; Eullaffroy, P.; Clément, C.; Vernet, G. Chitosan improves development, and protects Vitis vinifera L. against Botrytis cinerea. Plant Cell Rep. 2004, 22, 608–614. [Google Scholar] [CrossRef]
- Ahmad, B.; Jaleel, H.; Shabbir, A.; Khan, M.M.A.; Sadiq, Y. Concomitant application of depolymerized chitosan and GA3 modulates photosynthesis, essential oil and menthol production in peppermint (Mentha piperita L.). Sci. Hortic. 2019, 246, 371–379. [Google Scholar] [CrossRef]
- Whitehead, D.; Barbour, M.M.; Griffin, K.L.; Turnbull, M.H.; Tissue, D.T. Effects of leaf age and tree size on stomatal and mesophyll limitations to photosynthesis in mountain beech (Nothofagus solandrii var. cliffortiodes). Tree Physiol. 2011, 31, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Peguero-Pina, J.J.; Mendoza-Herrer, Ó.; Gil-Pelegrín, E.; Sancho-Knapik, D. Cavitation limits the recovery of gas exchange after severe drought stress in holm oak (Quercus ilex L.). Forests 2018, 9, 443. [Google Scholar] [CrossRef]
- Ahmad, B.; Khan, M.M.A.; Jaleel, H.; Sadiq, Y.; Shabbir, A.; Uddin, M. Exogenously sourced γ-irradiated chitosan-mediated regulation of growth, physiology, quality attributes and yield in Mentha piperita L. Turk. J. Biol. 2017, 41, 388–401. [Google Scholar] [CrossRef]
- Shangguan, Z.; Shao, M.; Dyckmans, J. Effects of nitrogen nutrition and water deficit on net photosynthetic rate and chlorophyll fluorescence in winter wheat. J. Plant Physiol. 2000, 156, 46–51. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant 2015, 153, 284–298. [Google Scholar] [CrossRef]
- Czékus, Z.; Iqbal, N.; Pollák, B.; Martics, A.; Ördög, A.; Poór, P. Role of ethylene and light in chitosan-induced local and systemic defence responses of tomato plants. J. Plant Physiol. 2021, 263, 153461. [Google Scholar] [CrossRef]
- Vaseva, I.I.; Qudeimat, E.; Potuschak, T.; Du, Y.; Genschik, P.; Vandenbussche, F.; Van Der Straeten, D. The plant hormone ethylene restricts Arabidopsis growth via the epidermis. Proc. Natl. Acad. Sci. USA 2018, 115, E4130–E4139. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Trivellini, A.; Fatma, M.; Masood, A.; Francini, A.; Iqbal, N.; Ferrante, A.; Khan, N.A. Role of ethylene in responses of plants to nitrogen availability. Front. Plant Sci. 2015, 6, 927. [Google Scholar] [CrossRef]
- Vega-Muñoz, I.; Duran-Flores, D.; Fernández-Fernández, Á.D.; Heyman, J.; Ritter, A.; Stael, S. Breaking Bad News: Dynamic molecular mechanisms of wound response in plants. Front. Plant Sci. 2020, 11, 1959. [Google Scholar] [CrossRef]
- Dammann, C.; Rojo, E.; Sánchez-Serrano, J.J. Abscisic acid and jasmonic acid activate wound-inducible genes in potato through separate, organ-specific signal transduction pathways. Plant J. 1997, 11, 773–782. [Google Scholar] [CrossRef]
- Kang, J.N.; Lee, W.H.; Won, S.Y.; Chang, S.; Hong, J.P.; Oh, T.J.; Lee, S.M.; Kang, S.H. Systemic Expression of Genes Involved in the Plant Defense Response Induced by Wounding in Senna tora. Int. J. Mol. Sci. 2021, 22, 10073. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, Y.; Martinoia, E.; Geisler, M. Plant hormone transporters: What we know and what we would like to know. BMC Biol. 2017, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, F.G. Auxin distribution in fruits and its significance in fruit development. Am. J. Bot. 1939, 26, 189–194. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Nascimento, V.L.; Medeiros, D.B.; Nunes-Nesi, A.; Ribeiro, D.M.; Zsögön, A.; Araújo, W.L. Modifications in organic acid profiles during fruit development and ripening: Correlation or causation? Front. Plant Sci. 2018, 9, 1689. [Google Scholar] [CrossRef]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.P.; Lutts, S. Tomato fruit development and metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef]
- Farouk, S.; Mosa, A.A.; Taha, A.A.; El-Gahmery, A.M. Protective effect of humic acid and chitosan on radish (Raphanus sativus, L. var. sativus) plants subjected to cadmium stress. J. Stress Physiol. Biochem. 2011, 7, 99–116. [Google Scholar]
- Choudhary, R.C.; Joshi, A.; Kumari, S.; Kumaraswamy, R.V.; Saharan, V. Preparation of Cu-chitosan nanoparticle and its effect on growth and enzyme activity during seed germination in maize. J. Pharmacogn. Phytochem. 2017, 6, 669–673. [Google Scholar]
- Sajid, M.; Basit, A.; Ullah, Z.; Shah, S.T.; Ullah, I.; Mohamed, H.I.; Ullah, I. Chitosan-based foliar application modulated the yield and biochemical attributes of peach (Prunus persica L.) cv. Early grand. Bull. Natl. Res. Cent. 2020, 44, 150. [Google Scholar] [CrossRef]
- Raman, S.B.; Rathinasabapathi, B. Pantothenate synthesis in plants. Plant Sci. 2004, 167, 961–968. [Google Scholar] [CrossRef]
- Wang, S.Y.; Gao, H. Effect of chitosan-based edible coating on antioxidants, antioxidant enzyme system, and postharvest fruit quality of strawberries (Fragaria x aranassa Duch.). LWT-Food Sci. Technol. 2013, 52, 71–79. [Google Scholar] [CrossRef]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of chitosan coating on the postharvest quality and antioxidant enzyme system response of strawberry fruit during cold storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Mostofa, M.G.; Diaz-Vivancos, P.; Burritt, D.J.; Fujita, M.; Tran, L.S.P. Glutathione in Plant Growth, Development, and Stress Tolerance; Springer: Cham, Switzerland, 2017; pp. 1–421. [Google Scholar]
- Li, Z.; Zhang, Y.; Zhang, X.; Merewitz, E.; Peng, Y.; Ma, X.; Huang, L.; Yan, Y. Metabolic pathways regulated by chitosan contributing to drought resistance in white clover. J. Proteome Res. 2017, 16, 3039–3052. [Google Scholar] [CrossRef] [PubMed]



| Ch0 | Ch0.25 | Ch1 | ||||
|---|---|---|---|---|---|---|
| BW 1 | AW 1 | BW | AW | BW | AW | |
| AOC | 0.007 ± 0.004 a | 0.011 ± 0.009 a | 0.005 ± 0.003 a | 0.007 ± 0.005 a | 0.005 ± 0.002 a | 0.006 ± 0.003 a |
| NCED1 | 0.012 ± 0.006 c | 0.053 ± 0.033 b | 0.010 ± 0.003 c | 0.055 ± 0.035 b | 0.025 ± 0.010 c | 0.084 ± 0.046 a |
| Parameters | Ch0 | Ch0.25 | Ch0.50 | Ch0.75 | Ch1 |
|---|---|---|---|---|---|
| Total fruit weight (kg/plant) A | 0.14 ± 0.02 c | 0.21 ± 0.01 a | 0.22 ± 0.01 a | 0.19 ± 0.01 ab | 0.18 ± 0.01 bc |
| Transverse diameter (mm) A | |||||
| Min | 48.17 ± 5.52 a | 50.50 ± 2.29 a | 46.17 ± 1.61 a | 50.00 ± 0.87 a | 43.67 ±0.58 a |
| Max | 51.17 ± 5.39 a | 52.33 ± 3.75 a | 46.67 ± 1.26 ab | 51.17 ± 1.04 a | 43.67 ± 0.58 b |
| Longitudinal diameter (mm) A | 40.33 ± 6.79 a | 47.50 ± 3.04 a | 44.67 ± 3.01 a | 44.17 ± 1.75 a | 41.00 ± 2.5 a |
| Shape index (SI = DL/DT) A | |||||
| Min | 0.836 ± 0.01 b | 0.940 ± 0.04 ab | 0.970 ± 0.09 a | 0.884 ± 0.04 ab | 0.939 ± 0.06 ab |
| Max | 0.785 ± 0.06 b | 0.910 ± 0.08 ab | 0.959 ± 0.09 a | 0.864 ± 0.05 ab | 0.939 ± 0.06 a |
| Water content (%) B | 93.50 ± 0.08 a | 93.87 ± 0.19 a | 93.28 ± 0.41 a | 93.47 ± 0.11 a | 91.77 ± 0.56 b |
| Total soluble sugar (°Brix) A | 4.61 ± 0.87 b | 4.41 ± 0.70 b | 4.13 ± 0.07 b | 3.89 ± 0.49 b | 6.04 ± 0.54 a |
| pH A | 4.55 ± 0.01 c | 4.58 ± 0.02 c | 4.59 ± 0.10 bc | 4.83 ± 0.13 a | 4.79 ± 0.13 ab |
| Parameters | Ch0 | Ch0.25 | Ch0.50 | Ch0.75 | Ch1 |
|---|---|---|---|---|---|
| Glucose (mg g−1 FW) A | 2.48 ± 0.06 b | 2.31 ± 0.14 b | 2.48 ± 0.17 b | 2.37 ± 0.05 b | 3.15 ± 0.36 a |
| Fructose (mg g−1 FW) A | 4.18 ± 0.08 b | 3.94 ± 0.19 b | 4.13 ± 0.23 b | 4.10 ± 0.13 b | 5.35 ± 0.44 a |
| Sucrose (mg g−1 FW) A | 4.76 ± 0.81 b | 4.58 ± 0.58 b | 5.15 ± 1.17 b | 4.85 ± 1.35 b | 7.49 ± 1.05 a |
| α-Tocopherol (µg g−1 DW) A | 76.93 ± 21.64 a | 109.94 ± 31.37 a | 115.70 ± 41.88 a | 103.11 ± 13.18 a | 104.79 ± 25.29 a |
| Lycopene (mg kg−1 FW) B | 16.40 ± 0.76 c | 24.73 ± 1.22 b | 25.67 ± 1.31 b | 30.20 ± 2.02 a | 31.18 ±1.38 a |
| Total flavonoids (mg g−1 DW) B | 1.62 ± 0.11 d | 1.97 ± 0.22 c | 2.09 ± 0.09 bc | 2.44 ± 0.15 a | 2.25 ± 0.06 b |
| Macronutrients (mg g−1 DW) B | |||||
| N | 25.29 ± 1.19 c | 26.31 ± 1.85 c | 29.89 ± 3.28 b | 36.25 ± 2.38 a | 34.89 ± 2.82 a |
| P | 5.57 ± 0.79 a | 6.66 ± 1.09 a | 6.47 ± 1.31 a | 6.58 ± 0.79 a | 6.70 ± 0.78 a |
| K | 26.99 ± 3.70 a | 26.71 ± 3.25 a | 29.16 ± 1.90 a | 29.93 ± 3.25 a | 28.42 ± 1.74 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Amerany, F.; Rhazi, M.; Balcke, G.; Wahbi, S.; Meddich, A.; Taourirte, M.; Hause, B. The Effect of Chitosan on Plant Physiology, Wound Response, and Fruit Quality of Tomato. Polymers 2022, 14, 5006. https://doi.org/10.3390/polym14225006
El Amerany F, Rhazi M, Balcke G, Wahbi S, Meddich A, Taourirte M, Hause B. The Effect of Chitosan on Plant Physiology, Wound Response, and Fruit Quality of Tomato. Polymers. 2022; 14(22):5006. https://doi.org/10.3390/polym14225006
Chicago/Turabian StyleEl Amerany, Fatima, Mohammed Rhazi, Gerd Balcke, Said Wahbi, Abdelilah Meddich, Moha Taourirte, and Bettina Hause. 2022. "The Effect of Chitosan on Plant Physiology, Wound Response, and Fruit Quality of Tomato" Polymers 14, no. 22: 5006. https://doi.org/10.3390/polym14225006
APA StyleEl Amerany, F., Rhazi, M., Balcke, G., Wahbi, S., Meddich, A., Taourirte, M., & Hause, B. (2022). The Effect of Chitosan on Plant Physiology, Wound Response, and Fruit Quality of Tomato. Polymers, 14(22), 5006. https://doi.org/10.3390/polym14225006







