Pyrolysis of Aesculus chinensis Bunge Leaves as for Extracted Bio-Oil Material
Abstract
1. Introduction
2. Material and Methods
2.1. Sample Sources
2.2. Experiment Methods
3. Results and Discussion
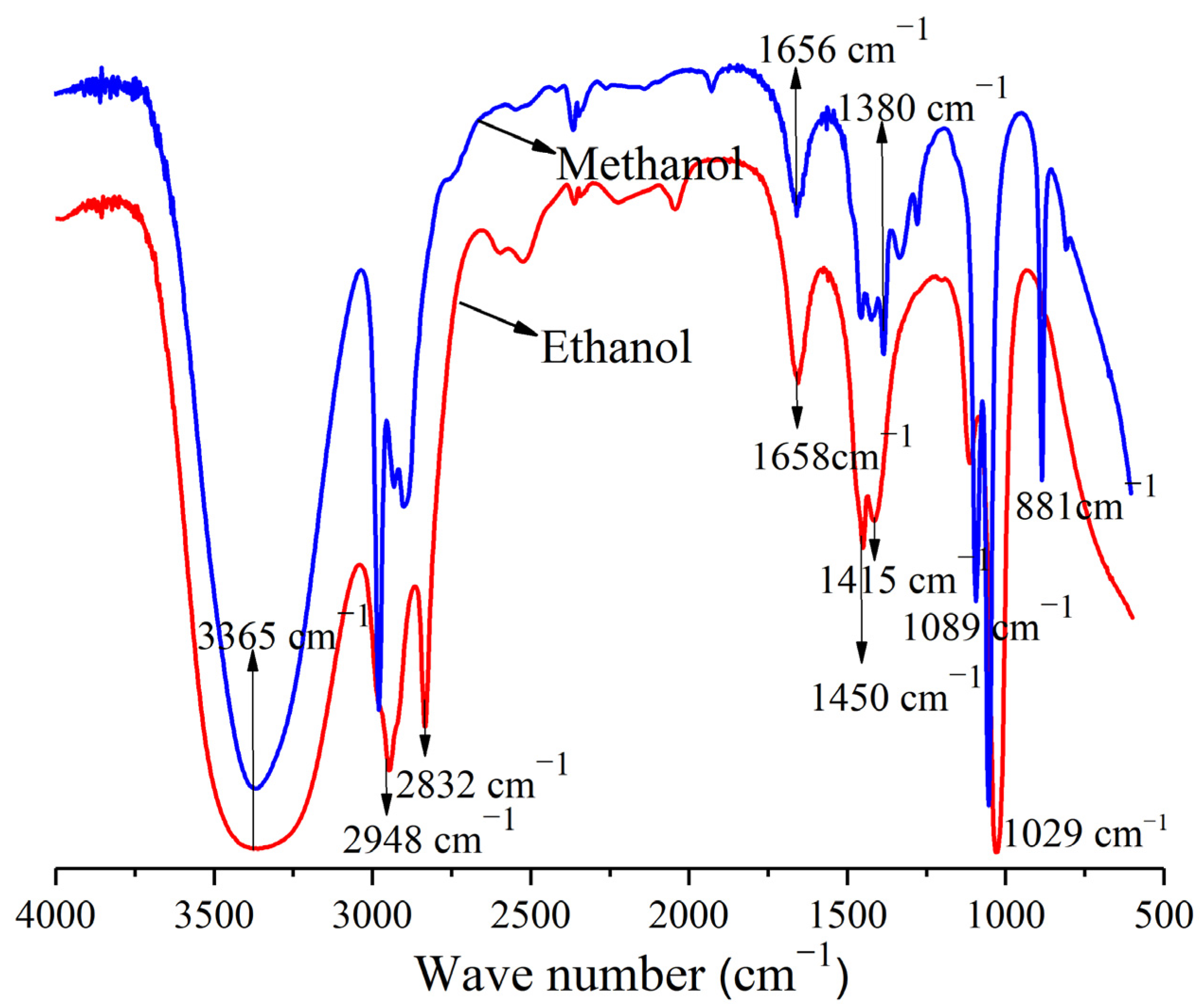
3.1. FT–IR Analysis
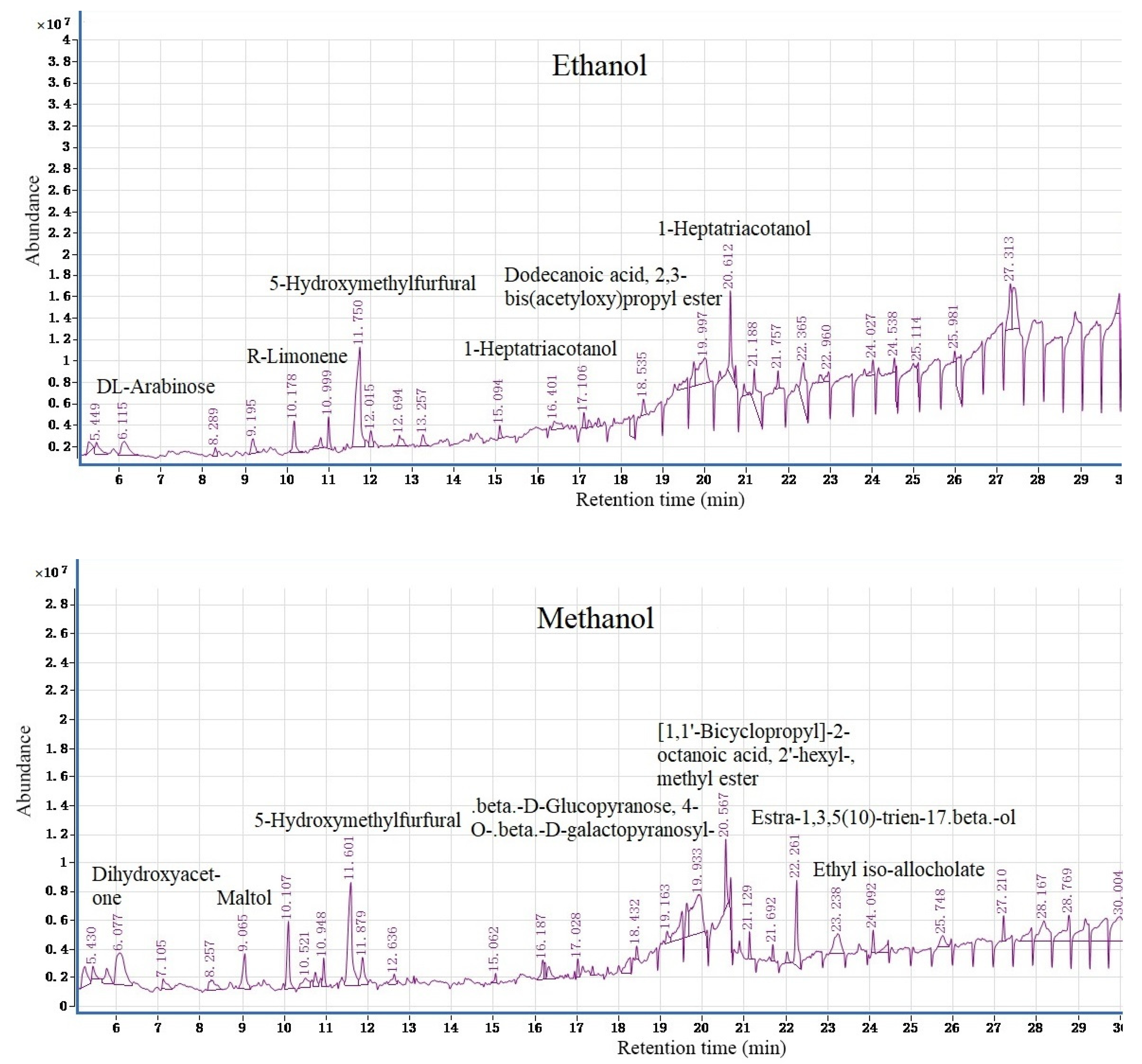
3.2. GC–MS Analysis
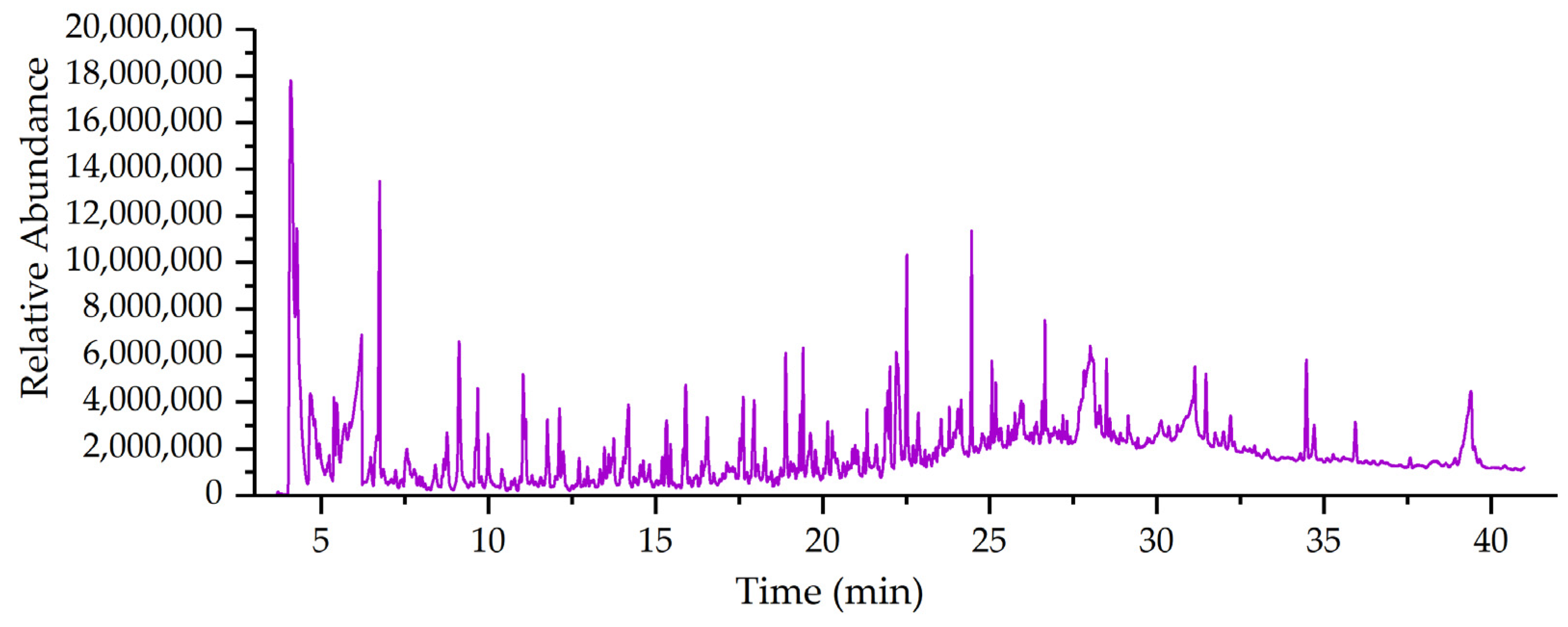
3.3. PY/GC–MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, M.H.; Mo, W.Y.; Choi, W.M.; Cheng, Z.; Man, Y.B. Recycle food wastes into high quality fish feeds for safe and quality fish production. Environ. Pollut. 2016, 219, 631–638. [Google Scholar] [CrossRef]
- Zheng, Y.P.; Zhang, Q.G.; Zhang, Z.P.; Jing, Y.Y.; Hu, J.J.; He, C.; Lu, C.Y. A review on biological recycling in agricultural waste-based biohydrogen production: Recent developments. Bioresour. Technol. 2022, 347, 126595. [Google Scholar] [CrossRef] [PubMed]
- Shan, G.C.; Xu, J.Q.; Jiang, Z.W.; Li, M.Q.; Li, Q.L. The transformation of different dissolved organic matter subfractions and distribution of heavy metals during food waste and sugarcane leaves co-composting. Waste Manag. 2019, 87, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J.; Wang, L.Z.; Li, Q.H.; Zhang, Y.G.; Zhou, H. Sustainable carbon materials from the pyrolysis of lignocellulosic biomass. Mater. Today Sustain. 2022, 19, 100209. [Google Scholar] [CrossRef]
- Ning, P.; Yang, G.; Hu, L.; Sun, J.; Shi, L.; Zhou, Y.; Wang, Z.; Yang, J. Recent advances in the valorization of plant biomass. Biotechnol. Biofuels 2021, 14, 102. [Google Scholar] [CrossRef]
- Zhang, X.K.; Li, N.N.; Han, S.; Wei, Z.; Dai, B. Terpene resin prepared from renewable turpentine oil as a new type of cold flow improver for soybean biodiesel-diesel blends. Fuel 2022, 320, 123844. [Google Scholar] [CrossRef]
- Abdulkhani, A.; Alizadeh, P.; Hedjazi, S.; Hamzeh, Y. Potential of Soya as a raw material for a whole crop biorefinery. Renew. Sust. Energ. Rev. 2017, 75, 1269–1280. [Google Scholar] [CrossRef]
- Khan, T.S.; Mubeen, U. Wheat Straw: A Pragmatic Overview. Curr. Res. J. Biol. Sci. 2012, 4, 673–675. [Google Scholar]
- Abbasi, M.; Pishvaee, M.S.; Mohseni, S. Third-generation biofuel supply chain: A comprehensive review and future research directions. J. Clean. Prod. 2021, 323, 129100. [Google Scholar] [CrossRef]
- Doren, L.G.; Posmanik, R.; Bicalho, F.A.; Tester, J.W.; Sills, D.L. Prospects for energy recovery during hydrothermal and biological processing of waste biomass. Bioresour. Technol. 2017, 225, 67–74. [Google Scholar] [CrossRef]
- Kalamdhad, A.S.; Singh, Y.K.; Ali, M.; Khwairakpam, M.; Kazmi, A.A. Rotary drum composting of vegetable waste and tree leaves. Bioresource Technol. 2009, 100, 6442–6450. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.K. Nutritional value and exploitation of common leaves. Feed Expo. 2015, 8, 57–58. [Google Scholar]
- Romero-Guiza, M.S.; Vila, J.J.; Mata-Alvarez, J.; Chimenos, J.M.; Astals, S. The role of additives on anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2016, 58, 1486–1499. [Google Scholar]
- Sánchez, Ó.J.; Ospina, D.A.; Montoya, S. Compost supplementation with nutrients and microorganisms in composting process. Waste Manag. 2017, 69, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Wang, T.; Niu, M.; Chen, X.; Liu, C.; Wang, Y.; Chen, T. Biodegradation of nonylphenol during aerobic composting of sewage sludge under two intermittent aeration treatments in a full-scale plant. Environ. Pollut. 2018, 238, 783–791. [Google Scholar] [CrossRef]
- Wang, P.; Wang, W.; Hu, J.; Zhang, G. Application of Sycamores Leaves Biomass Resources in Mushroom Cultivation. Henan Sci. 2016, 34, 517–520. [Google Scholar]
- Yang, J.; Lai, W.; Zhang, A.; Sun, J.; Xiao, H.; Fang, W. Preparation and Characterization of Leaves Activated Carbon. Adv. Mat. Chem. 2015, 03, 45–52. [Google Scholar] [CrossRef]
- Moodley, P.; Kana, E.B.G. Optimization of Operational Parameters for Biohydrogen Production from Waste Sugarcane Leaves and Semi-Pilot Scale Process Assessment. Bioresources 2017, 12, 2015–2030. [Google Scholar] [CrossRef]
- Wang, F.F.; Hu, L.; Li, Z.T.; Wang, X.H.; Yang, C. Chemical constitents of leaves from Dracaena cochinchinensis and their ameliorative effects on insulin resistance in Hep G—2 cell. J. Yunnan Uni. Nat. 2015, 24, 349–353. [Google Scholar]
- Sharma, N.; Prakash, C.; Gupta, S.A.; Rao, C.V. Pharmacognostical, phytochemical investigations and HPTLC fingerprinting of Pentapetes phoenicea L. leaves. Indian J. Nat. Prod. Res. 2015, 5, 158–163. [Google Scholar]
- Lu, Q.Q.; Shi, X.W.; Hui, H.; Zhou, J.H.; Cui, X.N. Research progress on the chemical constituents and bioactivity of Aesculus chinensis Bunge var. Chinensis. Northwest Pharm. J. 2016, 31, 651–654. [Google Scholar]
- Liu, Z. Research progress and exploitation of Aesculus chinensis Bunge. J. Anhui Agric. Sci. 2012, 40, 5986–5988. [Google Scholar]
- Yu, Z.H.; Su, P. Effect of beta-aescin extract from Chinese buckeye seed on chronic venous insufficiency. Pharmazie 2013, 68, 428–430. [Google Scholar]
- Yang, X.W.; Zhao, J.; Cui, J.; Guo, W. Studies on the biotransformation of escin Ia by human intestinal bacteria and the anti-tumor activities of desacylescin I. J. Peking Univ. Health Sci. 2004, 36, 31–35. [Google Scholar]
- Vispute, T.P.; Zhang, H.; Sanna, A.; Xiao, R.; Huber, G.W. Renewable chemical commodity feedstocks from integrated catalytic processing of pyrolysis oils. Science 2010, 330, 1222–1227. [Google Scholar] [CrossRef]
- Hashim, H.; Narayanasamy, M.; Yunus, N.A.; Shiun, L.J.; Muis, Z.A.; Ho, W.S. A cleaner and greener fuel: Biofuel blend formulation and emission assessment. J. Cleaner Prod. 2017, 146, 208–217. [Google Scholar] [CrossRef]
- Li, Y.Y.; Chen, J.; Yang, Y.; Li, C.; Peng, W. Molecular characteristics of volatile components from willow bark. J. King Saud Univ. Sci. 2020, 32, 1932–1936. [Google Scholar] [CrossRef]
- Li, Y.Y.; Shaheen, S.; M’ Rinklebe, J.; Ma, N.L.; Yang, Y.F.; Ashraf, M.A.; Chen, X.M.; Peng, W.X. Pyrolysis of Aesculus chinensis Bunge Seed with Fe2O3/NiO as nanocatalysts for the production of bio-oil material. J. Hazard. Mater. 2021, 416, 126012. [Google Scholar] [CrossRef]
- Ge, S.B.; Liang, Y.Y.; Zhou, C.X.; Sheng, Y.Q.; Zhang, M.L.; Cai, L.P.; Zhou, Y.H.; Huang, Z.H.; Manzo, M.; Wu, C.Y.; et al. The potential of Pinus armandii Franch for high-grade resource utilization. Biomass Bioenerg. 2022, 158, 106345. [Google Scholar] [CrossRef]
- Shannon, M.; Lafeuille, J.L.; Fregiere-Salmomon, A.; Lefevre, S.; Galvin-King, P.; Haughey, S.A.; Burns, D.T.; Shen, X.Q.; Kapil, A.; McGrath, T.F.; et al. The detection and determination of adulterants in turmeric using fourier-transform infrared (FTIR) spectroscopy coupled to chemometric analysis and micro-FTIR imaging. Food Control 2022, 139, 109093. [Google Scholar] [CrossRef]
- Lourençon, T.V.; Hansel, F.A.; Silva, T.A.D.; Ramos, L.P.; Muniz, G.I.B.D. Hardwood and softwood kraft lignins fractionation by simple sequential acid precipitation. Sep. Purif. Technol. 2015, 154, 82–88. [Google Scholar] [CrossRef]
- Johnson, J.B.; El Orche, A.; Naiker, M. Prediction of anthocyanin content and variety in plum extracts using ATR-FTIR spectroscopy and chemometrics. Vib. Spectrosc. 2022, 121, 103406. [Google Scholar] [CrossRef]
- Yunitasari, N.; Swasono, R.T.; Pranowo, H.D.; Raharjo, T.J. Phytochemical screening and metabolomic approach based on Fourier transform infrared (FTIR): Identification of α-amylase inhibitor metabolites in Vernonia amygdalina leaves. J. Saudi Chem. Soc. 2022, 26, 101540. [Google Scholar] [CrossRef]
- Toscano, G.; Maceratesi, V.; Leoni, E.; Stipa, P.; Laudadio, E.; Sabbatini, S. FTIR spectroscopy for determination of the raw materials used in wood pellet production. Fuel 2022, 313, 123017. [Google Scholar] [CrossRef]
- Herrera, R.; Hermoso, E.; Labidi, J.; Fernandez-Golfin, J.I. Non-destructive determination of core-transition-outer wood of Pinus nigra combining FTIR spectroscopy and prediction models. Microchem. J. 2022, 179, 107532. [Google Scholar] [CrossRef]
- Alasmary, F.A.S.; Awaad, A.S.; Alafeefy, A.M.; Alasmary, R.M.; Alqasoumi, S.I. Novel quinazoline and acetamide derivatives as safe anti-ulcerogenic agent and anti-ulcerative colitis activity. Saudi Pharm. J. 2017, 26, 138–143. [Google Scholar] [CrossRef]
- Lin, N.; Liu, T.T.; Lin, L.; Lin, S.; Zang, Q.C.; He, J.M.; Abliz, Z.P.; Li, C.; Wang, A.P.; Jin, H.T. Comparison of in vivo immunomodulatory effects of 5-hydroxymethylfurfural and 5, 50-oxydimethylenebis (2-furfural). Regul. Toxicol. Pharm. 2016, 81, 500–511. [Google Scholar] [CrossRef]
- Cao, G.; Cai, H.; Cai, B.C.; Tu, S.C. Effect of 5-hydroxymethylfurfural derived from processed Cornus officinalis on the prevention of high glucose-induced oxidative stress in human umbilical vein endothelial cells and its mechanism. Food Chem. 2013, 140, 273–279. [Google Scholar] [CrossRef]
- Santos, N.N.; Menezes, L.R.A.; Santos, J.A.F.; Meira, C.S.; Guimarhes, E.T.; Soares, M.B.P.; Nepel, A.; Barisone, A.; Costa, E.V. A New Source of (R)-Limonene and Rotundifolone from Leaves of Lippia pedunculosa (Verbenaceae) and their Trypanocidal Properties. Nat. Prod. Commun. 2014, 9, 737–739. [Google Scholar]
- Miller, J.A.; Lang, J.E.; Ley, M.; Nagle, R.; Hsu, C.; Thompson, P.A.; Cordova, C.; Waer, A.; Chow, H.S. Human Breast Tissue Disposition and Bioactivity of Limonene in Women with Early-Stage Breast Cancer. Cancer Prev. Res. 2013, 6, 577–584. [Google Scholar] [CrossRef]
- Cardoso, M.R.D.; Mota, C.M.; Ribeiro, D.P.; Santiago, F.M.; Carvalho, J.V.; Araujo, E.C.B.; Silva, N.M.; Mineo, T.W.P.; Roque-Barreira, M.C.; Mineo, J.R.; et al. ArtinM, a d-mannose-binding lectin from Artocarpus integrifolia, plays a potent adjuvant and immunostimulatory role in immunization against Neospora caninum. Vaccine 2011, 29, 9183–9193. [Google Scholar] [CrossRef] [PubMed]
- Braunberger, T.L.; Nahhas, A.F.; Katz, L.M.; Sadrieh, N.; Lim, H.M. Dihydroxyacetone: A Review. J. Drugs Dermatol. 2018, 17, 387–391. [Google Scholar] [PubMed]
- Wang, W.; Xu, Y.; Liu, Y.; Sun, S.; Zhu, S.; Mao, T. Yishen Granules in Treating 54 Cases of Leukopenia of Heart and Spleen Deficiency Pattern. J. Tradit. Chin. Med. 2017, 30, 72–73. [Google Scholar]
- An, B.; Chal, L. Determination of Batilol Tablets by HPLC—ELSD. Chin. J. Biochem Pharm. 2014, 45, 282–283. [Google Scholar]
- Vallée, M.; Vitiello, S.; Bellocchio, L.; Hébert-Chatelain, E.; Monlezun, S.; Martin-Garcia, E.; Kasanetz, F.; Baillie, G.L.; Panin, F. Pregnenolone can protect the brain from cannabis intoxication. Science 2014, 343, 94. [Google Scholar] [CrossRef]
- Shirakawa, H.; Katsuki, H.; Kume, T.; Kaneko, S.; Akaike, A. Pregnenolone sulphate attenuates AMPA cytotoxicity on rat cortical neurons. Eur. J. Neurosci. 2015, 21, 2329–2335. [Google Scholar] [CrossRef]
- Ritsner, M.S.; Bawakny, H.; Kreinin, A. Pregnenolone treatment reduces severity of negative symptoms in recent-onset schizophrenia: An 8-week, double-blind, randomized add-on two-center trial. Psychiat. Clin. Neuros. 2014, 68, 432–440. [Google Scholar] [CrossRef]
- Liu, X.F.; Zheng, C.J.; Sun, L.P.; Liu, X.K.; Piao, H.R. Synthesis of new chalcone derivatives bearing 2,4-thiazolidinedione and benzoic acid moieties as potential anti-bacterial agents. Eur. J. Med. Chem. 2011, 346, 3469–3473. [Google Scholar] [CrossRef]
- Mahiwal, K.; Kumar, P.; Narasimhan, B. Synthesis, antimicrobial evaluation, ot-QSAR and mt-QSAR studies of 2-amino benzoic acid derivatives. Med. Chem. Res. 2012, 21, 293–307. [Google Scholar] [CrossRef]
- Klimko, P.G.; Hellberg, M.R.; Bingaman, D.P.; Gamache, D.A. 5,6,7-trihydroxyheptanoic Acid and Analogs for the Treatment of Ocular Diseases and Diseases Associated with Hyperproliferative and Angiogenic Responses. U.S. Patent 7,879,905, 1 February 2011. [Google Scholar]
- Zou, Z.; Du, D.; Zhang, Y.; Zhang, J.; Niu, G.; Tan, H. A γ-butyrolactone-sensing activator/repressor, JadR3, controls a regulatory mini-network for jadomycin biosynthesis. Mol. Microbiol. 2015, 94, 490–505. [Google Scholar] [CrossRef]
- Tan, G.Y.; Peng, Y.; Lu, C.; Bai, L.; Zhong, J.J. Engineering validamycin production by tandem deletion of γ-butyrolactone receptor genes in Streptomyces hygroscopicus 5008. Metab. Eng. 2015, 28, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Haroon, M.H.; Premaratne, S.R.; Choudhry, M.I.; Dharmaratne, H.R. A new β-glucuronidase inhibiting butyrolactone from the marine endophytic fungus Aspergillus terreus. Nat. Prod. Res. 2013, 27, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.Z.; Gu, L. Comparison of Efficacy of Salmeterol-flutiacasone Alone versus Fluticasone Propionate Combined with Mon-telukast Sodium in the Treatment of Children with Moderate Persistent Asthma. Chin. J. Clin. Pharm. 2015, 4, 247–249. [Google Scholar]
- Xu, Y.H.; Li, X.; Han, Y.Y. Effect of Tiotropium Bromide combined with Salmeterol/Fluticasone Propionate treatment on lung function of COPD patients. Chin. Med. Herald. 2016, 13, 146–149. [Google Scholar]
- Shafaghat, H.; Kim, J.M.; Lee, I.G.; Jae, J.; Jung, S.C.; Park, Y.K. Catalytic hydrodeoxygenation of crude bio-oil in supercritical methanol using supported nickel catalysts. Renew. Energ. 2019, 44, 159–166. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Ma, Q.; Li, G.; Lou, J.; Chen, X.; He, Y.; Peng, W. Pyrolysis of Aesculus chinensis Bunge Leaves as for Extracted Bio-Oil Material. Polymers 2022, 14, 5003. https://doi.org/10.3390/polym14225003
Li Y, Ma Q, Li G, Lou J, Chen X, He Y, Peng W. Pyrolysis of Aesculus chinensis Bunge Leaves as for Extracted Bio-Oil Material. Polymers. 2022; 14(22):5003. https://doi.org/10.3390/polym14225003
Chicago/Turabian StyleLi, Yiyang, Qian Ma, Guanyan Li, Junwei Lou, Xiangmeng Chen, Yifeng He, and WanXi Peng. 2022. "Pyrolysis of Aesculus chinensis Bunge Leaves as for Extracted Bio-Oil Material" Polymers 14, no. 22: 5003. https://doi.org/10.3390/polym14225003
APA StyleLi, Y., Ma, Q., Li, G., Lou, J., Chen, X., He, Y., & Peng, W. (2022). Pyrolysis of Aesculus chinensis Bunge Leaves as for Extracted Bio-Oil Material. Polymers, 14(22), 5003. https://doi.org/10.3390/polym14225003





