Fabrication of Flexible Wiring with Intrinsically Conducting Polymers Using Blue-Laser Microstereolithography
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Stereolithography System
2.4. Preparation of Resin for Conductive Fabrication Objects
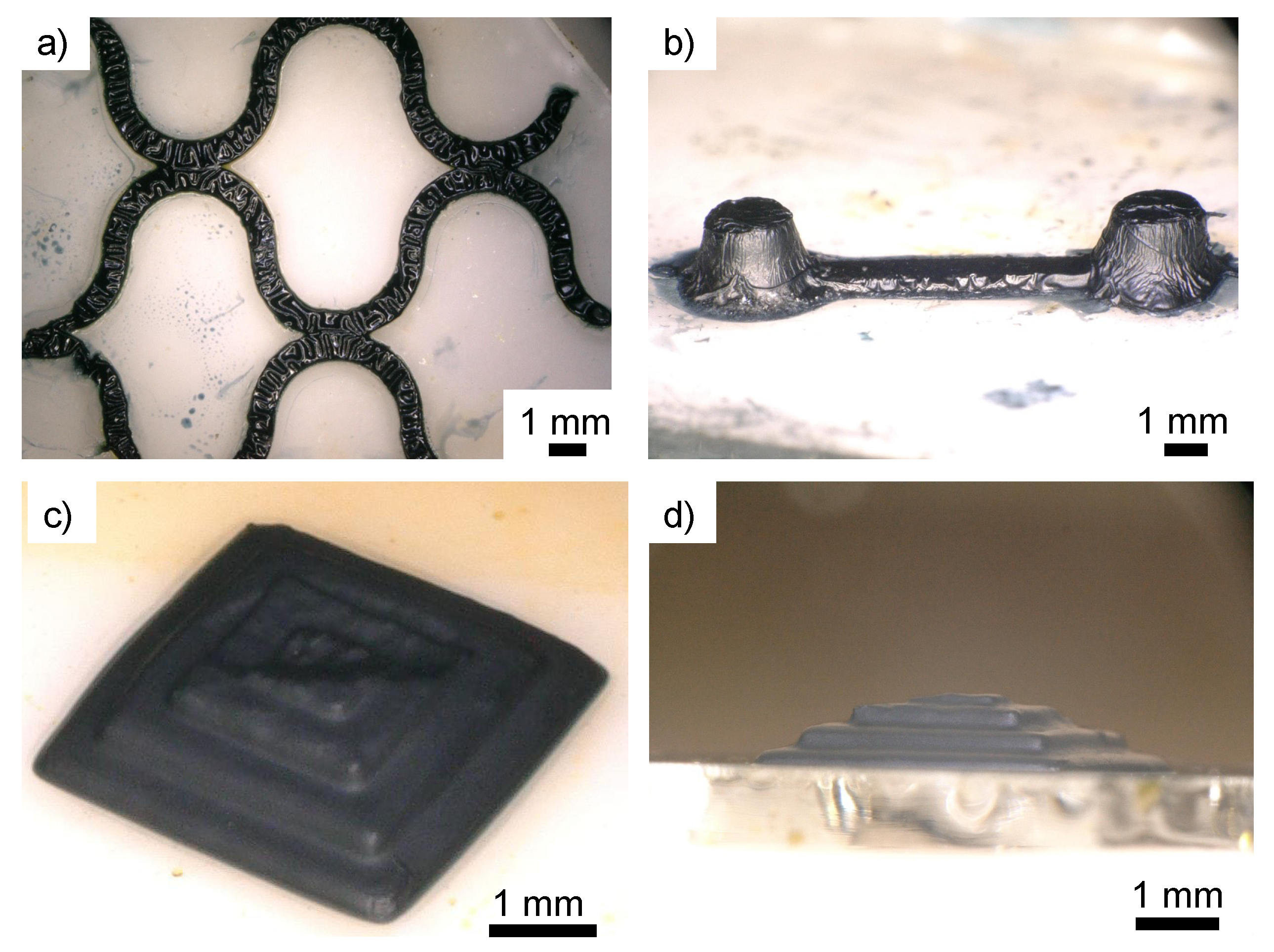
3. Results and Discussion
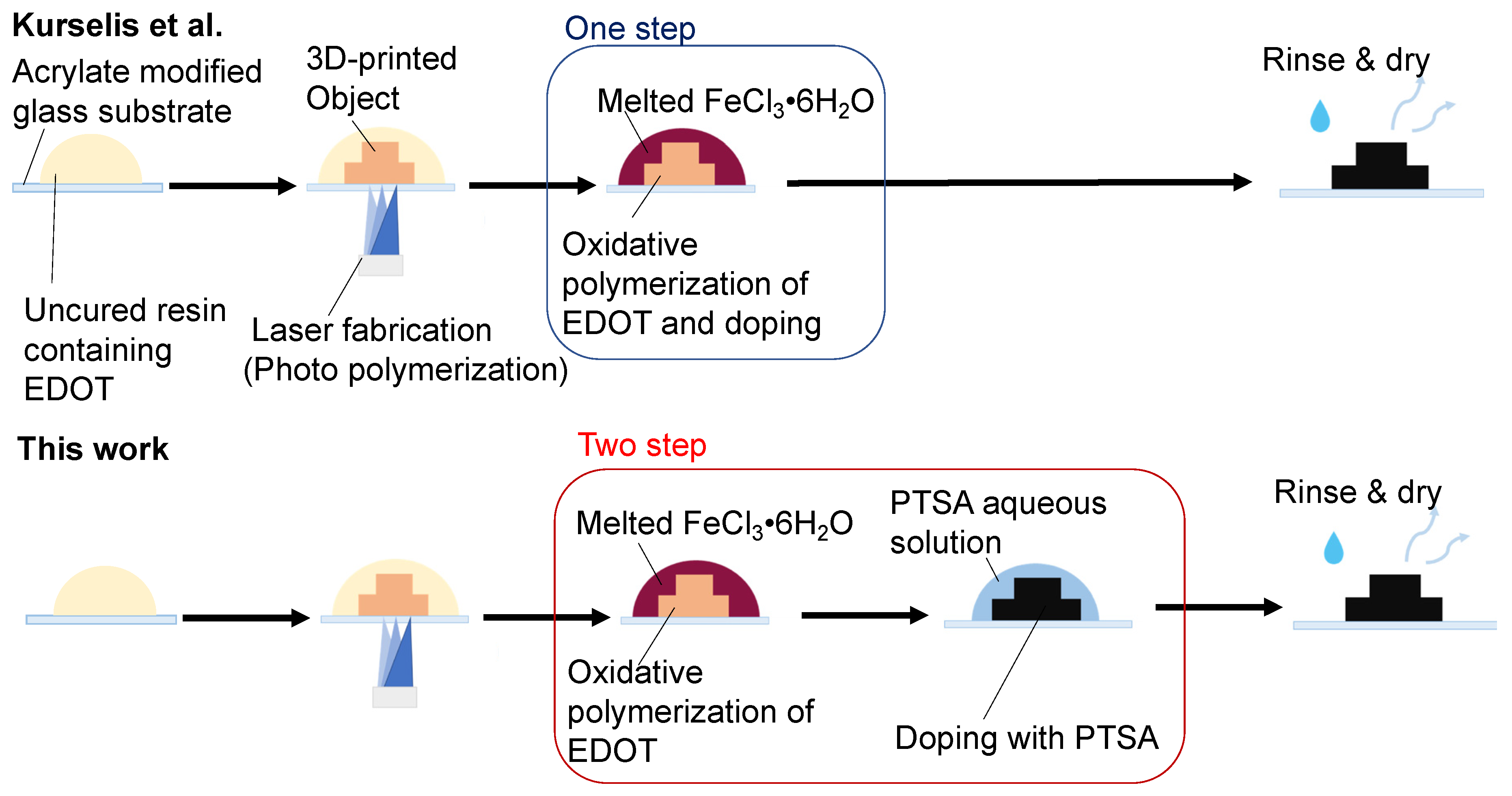
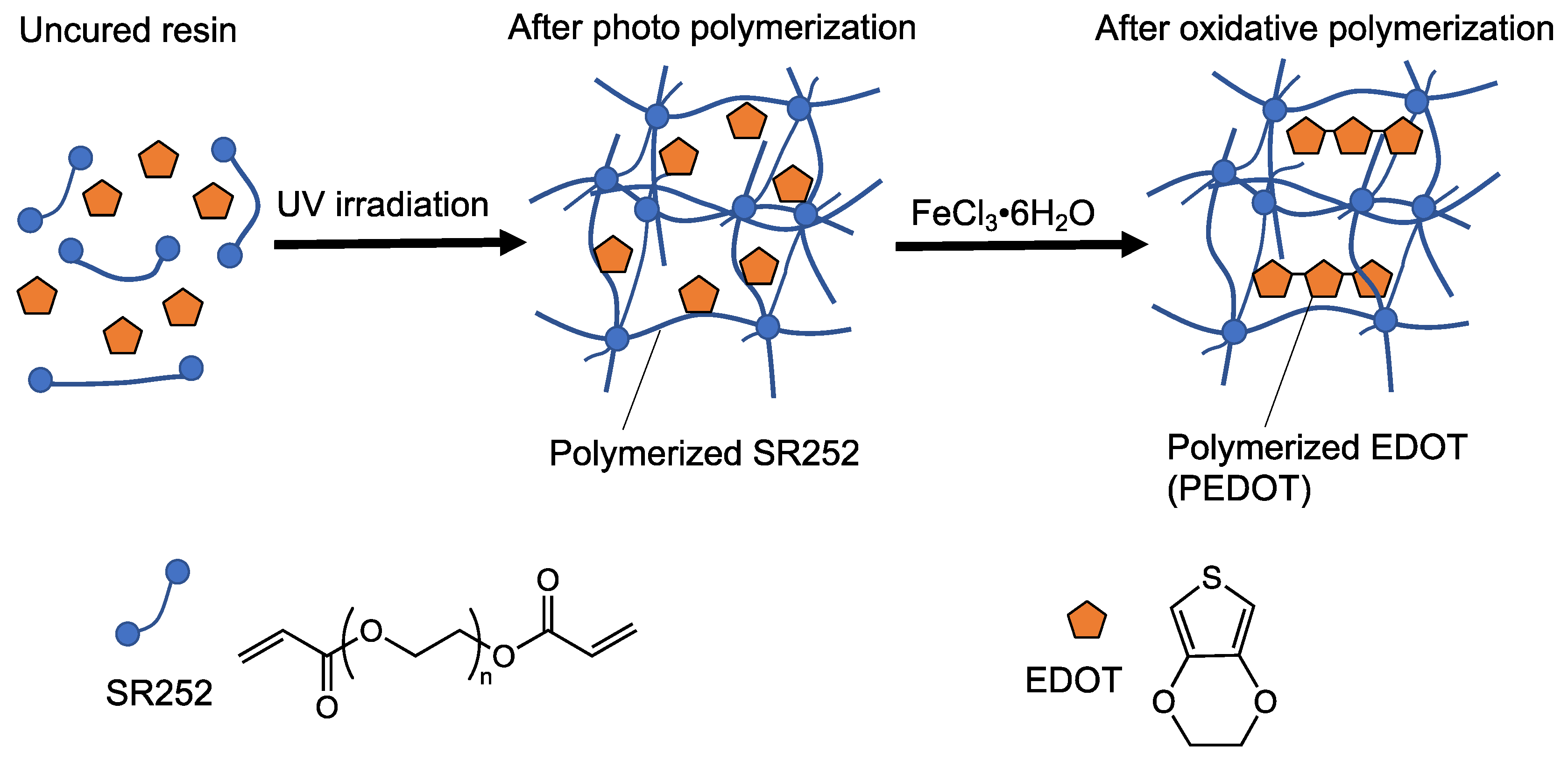
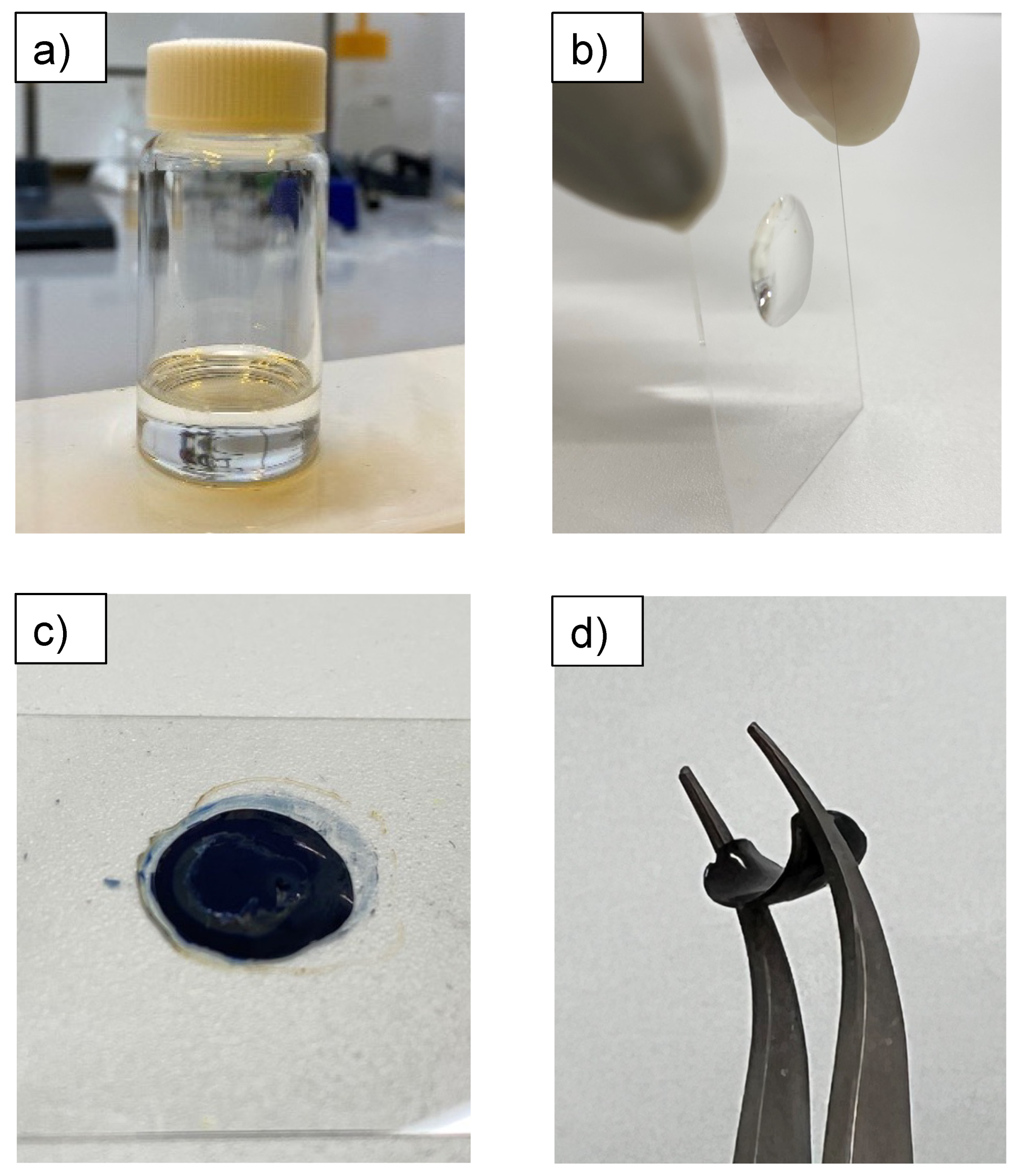
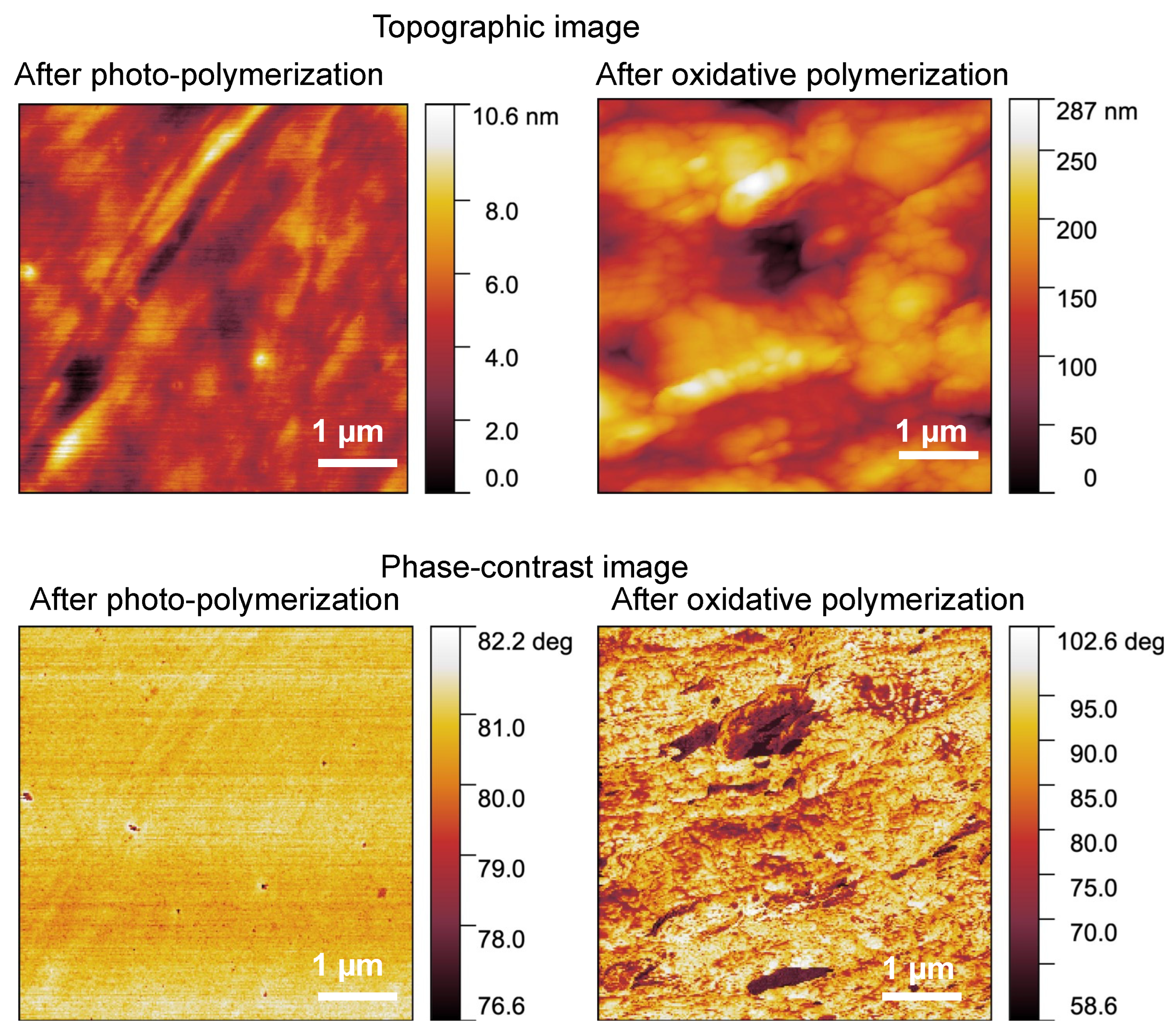
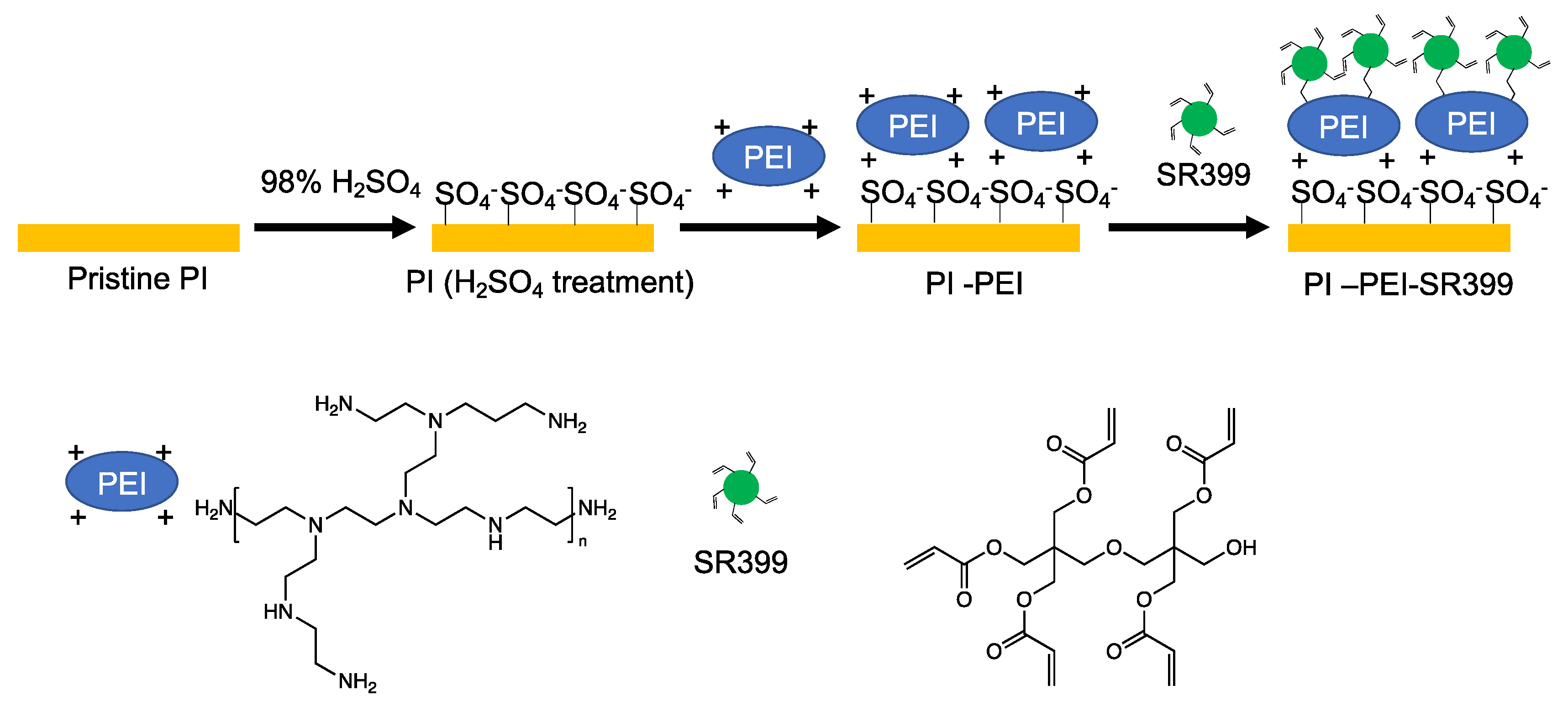
3.1. Conductive Treatment of Resin Droplet
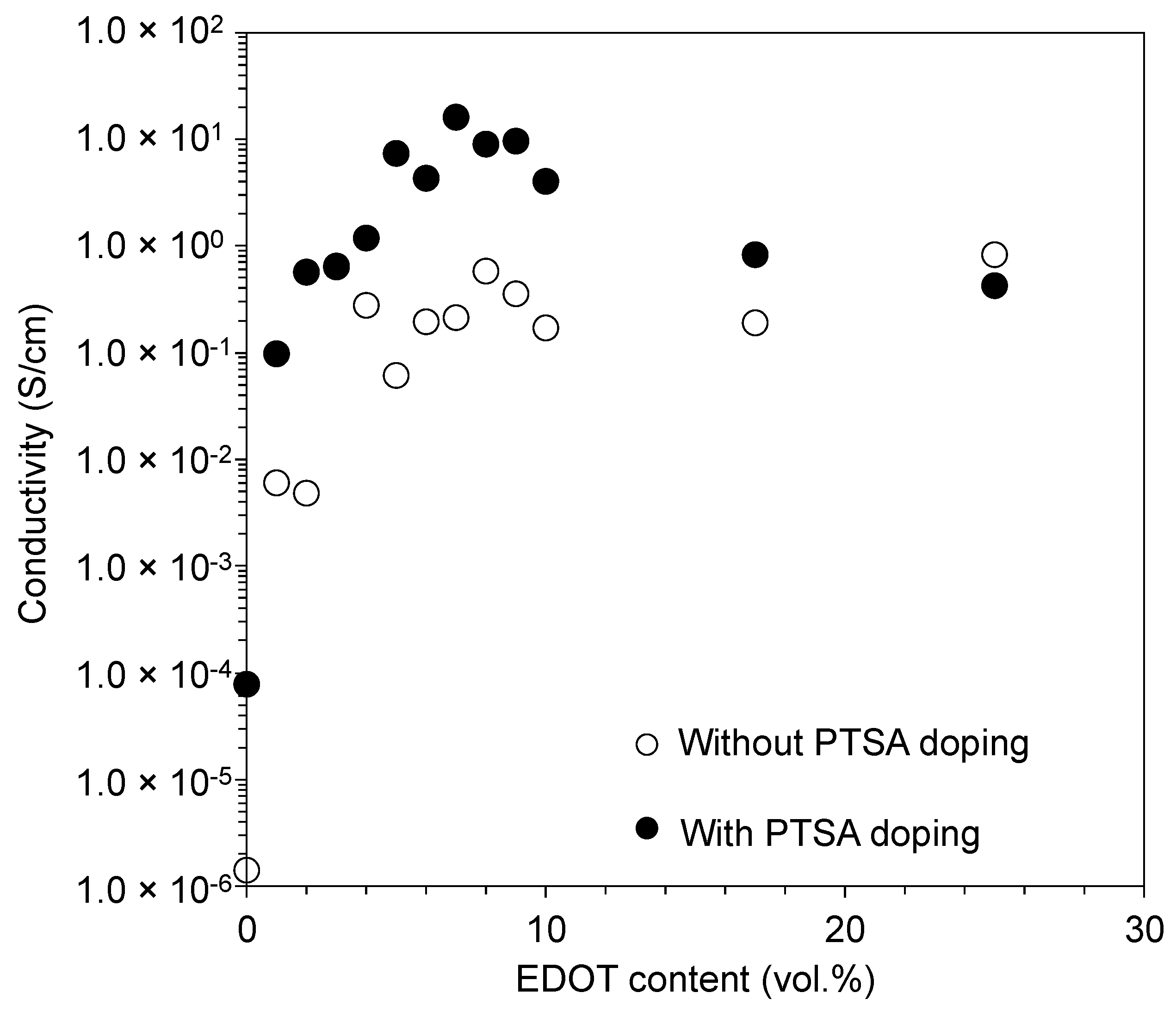
3.2. Tuning of the Resin Composition to Improve Conductivity with PTSA Doping
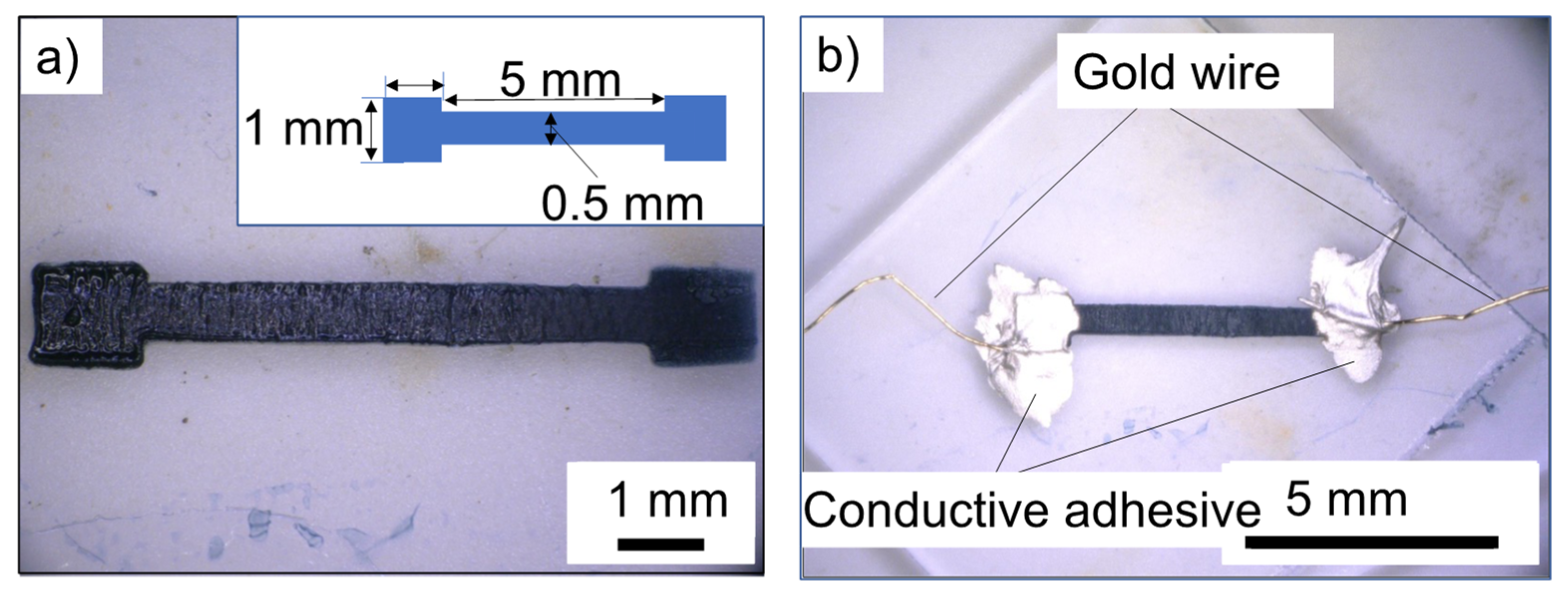
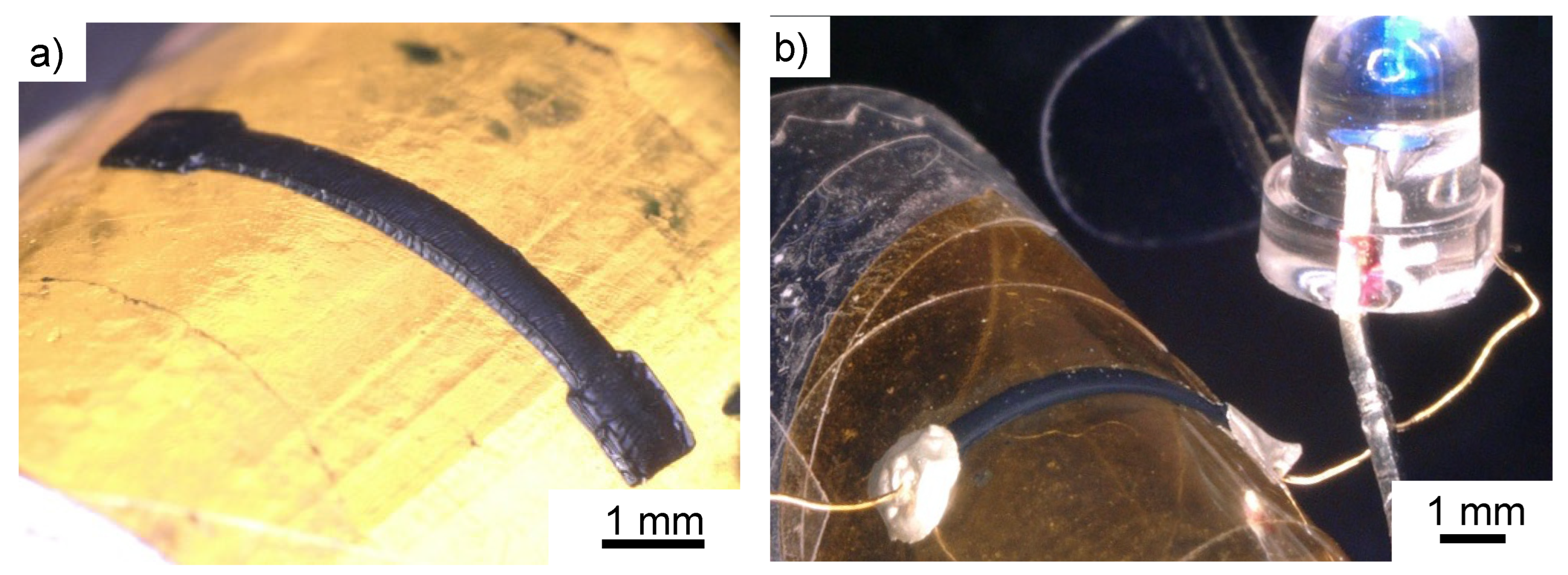
3.3. Flexible Wiring Using PI Film as a Substrate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH). J. Chem. Soc. Chem. Commun. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- Naarmann, H. Polymers, Electrically Conducting. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Louwet, F.; Groenendaal, L.; Dhaen, J.; Manca, J.; Van Luppen, J.; Verdonck, E.; Leenders, L. PEDOT/PSS: Synthesis, characterization, properties and applications. Synth. Met. 2003, 135–136, 115–117. [Google Scholar] [CrossRef]
- Elschner, A.; Kirchmeyer, S.; Lovenich, W.; Merker, U.; Reuter, K. PEDOT: Principles and Applications of an Intrinsically Conductive Polymer; CRC Press: New York, NY, USA, 2010. [Google Scholar]
- Mukai, M.; Cheng, C.-H.; Ma, W.; Chin, M.; Lin, C.-H.; Luo, S.-C.; Takahara, A. Synthesis of a conductive polymer thin film having a choline phosphate side group and its bioadhesive properties. Chem. Commun. 2020, 56, 2691–2694. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, Z.; Wang, Y.-X.; Li, D.; Coen, C.-T.; Hwaun, E.; Chen, G.; Wu, H.-C.; Zhong, D.; Niu, S.; et al. Topological supramolecular network enabled high-conductivity, stretchable organic bioelectronics. Science 2022, 375, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yang, G.; Li, R.; Pan, T. Photopatternable PEDOT:PSS/PEG hybrid thin film with moisture stability and sensitivity. Microsyst. Nanoeng. 2017, 3, 17004. [Google Scholar] [CrossRef]
- Cui, N.; Tang, Q.; Ren, H.; Zhao, X.; Tong, Y.; Liu, Y. A photolithographic stretchable transparent electrode for an all-solution-processed fully transparent conformal organic transistor array. J. Mater. Chem. C 2019, 7, 5385–5393. [Google Scholar] [CrossRef]
- Pal, R.K.; Farghaly, A.A.; Collinson, M.M.; Kundu, S.C.; Yadavalli, V.K. Photolithographic Micropatterning of Conducting Polymers on Flexible Silk Matrices. Adv. Mater. 2016, 28, 1406–1412. [Google Scholar] [CrossRef]
- Pal, R.K.; Pradhan, S.; Narayanan, L.; Yadavalli, V.K. Micropatterned conductive polymer biosensors on flexible PDMS films. Sens. Actuators B Chem. 2018, 259, 498–504. [Google Scholar] [CrossRef]
- Basak, I.; Nowicki, G.; Ruttens, B.; Desta, D.; Prooth, J.; Jose, M.; Nagels, S.; Boyen, H.-G.; D’Haen, J.; Buntinx, M.; et al. Inkjet Printing of PEDOT:PSS Based Conductive Patterns for 3D Forming Applications. Polymers 2020, 12, 2915. [Google Scholar] [CrossRef]
- Tseghai, G.A.-O.; Malengier, B.A.-O.; Fante, K.A.-O.; Nigusse, A.B.; Van Langenhove, L. Development of a Flex and Stretchy Conductive Cotton Fabric Via Flat Screen Printing of PEDOT:PSS/PDMS Conductive Polymer Composite. Sensors 2020, 20, 1742. [Google Scholar] [CrossRef]
- Zucca, A.; Yamagishi, K.; Fujie, T.; Takeoka, S.; Mattoli, V.; Greco, F. Roll to roll processing of ultraconformable conducting polymer nanosheets. J. Mater. Chem. C 2015, 3, 6539–6548. [Google Scholar] [CrossRef]
- Kayser, L.V.; Lipomi, D.J. Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT:PSS. Adv. Mater. 2019, 31, 1806133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, W.; Zhang, H.; Xie, M.; Duan, X. PEDOT:PSS: From conductive polymers to sensors. Nanotechnol. Precis. Eng. 2021, 4, 045004. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D printing of conducting polymers. Nat. Commun. 2020, 11, 1604. [Google Scholar] [CrossRef]
- Tao, Y.; Wei, C.; Liu, J.; Deng, C.; Cai, S.; Xiong, W. Nanostructured electrically conductive hydrogels obtained via ultrafast laser processing and self-assembly. Nanoscale 2019, 11, 9176–9184. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Sone, J. A New 3D Printing System of Poly(3,4-ethylenedioxythiophene) for Realizing a High Electrical Conductivity and Fine Processing Resolution. Micromachines 2020, 11, 1120. [Google Scholar] [CrossRef]
- Kurselis, K.; Kiyan, R.; Bagratashvili, V.N.; Popov, V.K.; Chichkov, B.N. 3D fabrication of all-polymer conductive microstructures by two photon polymerization. Opt. Express 2013, 21, 31029–31035. [Google Scholar] [CrossRef]
- Gueye, M.N.; Carella, A.; Massonnet, N.; Yvenou, E.; Brenet, S.; Faure-Vincent, J.; Pouget, S.; Rieutord, F.; Okuno, H.; Benayad, A.; et al. Structure and Dopant Engineering in PEDOT Thin Films: Practical Tools for a Dramatic Conductivity Enhancement. Chem. Mater. 2016, 28, 3462–3468. [Google Scholar] [CrossRef]
- Opwis, K.; Knittel, D.; Gutmann, J.S. Oxidative in situ deposition of conductive PEDOT:PTSA on textile substrates and their application as textile heating element. Synth. Met. 2012, 162, 1912–1918. [Google Scholar] [CrossRef]
- Sapp, S.; Luebben, S. New Conducting and Semiconducting Polymers for Organic Photovoltaics. MRS Online Proc. Libr. 2010, 1270, 1440. [Google Scholar] [CrossRef]
- Fantino, E.; Chiappone, A.; Roppolo, I.; Manfredi, D.; Bongiovanni, R.; Pirri, C.F.; Calignano, F. 3D Printing of Conductive Complex Structures with in Situ Generation of Silver Nanoparticles. Adv. Mater. 2016, 28, 3712–3717. [Google Scholar] [CrossRef] [PubMed]
- Sciancalepore, C.; Moroni, F.; Messori, M.; Bondioli, F. Acrylate-based silver nanocomposite by simultaneous polymerization–reduction approach via 3D stereolithography. Compos. Commun. 2017, 6, 11–16. [Google Scholar] [CrossRef]
- Lebel, L.L.; Aissa, B.; Khakani, M.A.E.; Therriault, D. Ultraviolet-Assisted Direct-Write Fabrication of Carbon Nanotube/Polymer Nanocomposite Microcoils. Adv. Mater. 2010, 22, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Wang, L.; Dunn, C.K.; Kuang, X.; Duan, F.; Zhang, Z.; Qi, H.J.; Wang, T. Digital light processing 3D printing of conductive complex structures. Addit. Manuf. 2017, 18, 74–83. [Google Scholar] [CrossRef]
- Gonzalez, G.; Chiappone, A.; Roppolo, I.; Fantino, E.; Bertana, V.; Perrucci, F.; Scaltrito, L.; Pirri, F.; Sangermano, M. Development of 3D printable formulations containing CNT with enhanced electrical properties. Polymer 2017, 109, 246–253. [Google Scholar] [CrossRef]
- Maruyama, T.; Hirata, H.; Furukawa, T.; Maruo, S. Multi-material microstereolithography using a palette with multicolor photocurable resins. Opt. Mater. Express 2020, 10, 2522–2532. [Google Scholar] [CrossRef]
- Maruyama, T.; Mukai, M.; Sato, R.; Iijima, M.; Sato, M.; Furukawa, T.; Maruo, S. Multifunctional 3D Printing of Heterogeneous Polymer Structures by Laser-Scanning Micro-Stereolithography Using Reversible Addition-Fragmentation Chain-Transfer Polymerization. ACS Appl. Polym. Mater. 2022, 4, 5515–5523. [Google Scholar] [CrossRef]
- Maruo, S.; Nakamura, O.; Kawata, S. Three-dimensional microfabrication with two-photon-absorbed photopolymerization. Opt. Lett. 1997, 22, 132–134. [Google Scholar] [CrossRef]
- Arita, R.; Iijima, M.; Fujishiro, Y.; Morita, S.; Furukawa, T.; Tatami, J.; Maruo, S. Rapid three-dimensional structuring of transparent SiO2 glass using interparticle photo-cross-linkable suspensions. Commun. Mater. 2020, 1, 30. [Google Scholar] [CrossRef]
- Ghosh, S.; Remita, H.; Ramos, L.; Dazzi, A.; Deniset-Besseau, A.; Beaunier, P.; Goubard, F.; Aubert, P.-H.; Brisset, F.; Remita, S. PEDOT nanostructures synthesized in hexagonal mesophases. New J. Chem. 2014, 38, 1106–1115. [Google Scholar] [CrossRef]
- Selvaganesh, S.V.; Mathiyarasu, J.; Phani, K.L.N.; Yegnaraman, V. Chemical Synthesis of PEDOT–Au Nanocomposite. Nanoscale Res. Lett. 2007, 2, 546. [Google Scholar] [CrossRef]
- Turbiez, M.; Frère, P.; Roncali, J. Stable and Soluble Oligo(3,4-ethylenedioxythiophene)s End-Capped with Alkyl Chains. J. Org. Chem. 2003, 68, 5357–5360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, W.; Zhang, G.; Bai, Y.; Chen, S.; Xu, J.; Yu, Z.; Sun, K. p-Toluenesulfonic acid catalytic polymerization of EDOT without oxidants. Mater. Lett. 2018, 222, 105–108. [Google Scholar] [CrossRef]
- Riaz, U.; Ashraf, S.M. Chapter 15-Conductive Polymer Composites and Blends: Recent Trends. In Nanostructured Polymer Blends; Thomas, S., Shanks, R., Chandrasekharakurup, S., Eds.; William Andrew Publishing: Oxford, UK, 2014; pp. 509–538. [Google Scholar]
- Jiu, J.; Nogi, M.; Sugahara, T.; Tokuno, T.; Araki, T.; Komoda, N.; Suganuma, K.; Uchida, H.; Shinozaki, K. Strongly adhesive and flexible transparent silver nanowire conductive films fabricated with a high-intensity pulsed light technique. J. Mater. Chem. 2012, 22, 23561–23567. [Google Scholar] [CrossRef]
- Uddin, M.A.; Alam, M.O.; Chan, Y.C.; Chan, H.P. Plasma cleaning of the flex substrate for flip-chip bonding with anisotropic conductive adhesive film. J. Electron. Mater. 2003, 32, 1117–1124. [Google Scholar] [CrossRef]
- Baldacchini, T.; LaFratta, C.N.; Farrer, R.A.; Teich, M.C.; Saleh, B.E.A.; Naughton, M.J.; Fourkas, J.T. Acrylic-based resin with favorable properties for three-dimensional two-photon polymerization. J. Appl. Phys. 2004, 95, 6072–6076. [Google Scholar] [CrossRef]
- Ishida, H.; Wellinghoff, S.T.; Baer, E.; Koenig, J.L. Spectroscopic Studies of Poly[N,N′-bis(phenoxyphenyl)pyromellitimide]. 1. Structures of the Polyimide and Three Model Compounds. Macromolecules 1980, 13, 826–834. [Google Scholar] [CrossRef]
- Liu, B.; Robertson, G.P.; Kim, D.-S.; Guiver, M.D.; Hu, W.; Jiang, Z. Aromatic Poly(ether ketone)s with Pendant Sulfonic Acid Phenyl Groups Prepared by a Mild Sulfonation Method for Proton Exchange Membranes. Macromolecules 2007, 40, 1934–1944. [Google Scholar] [CrossRef]
- Hosseini, E.; Sabet, N.; Arjmand, M.; Sundararaj, U.; Hassanzadeh, H.; Zarifi, M.H.; Karan, K. Multilayer polymeric nanocomposite thin film heater and electromagnetic interference shield. Chem. Eng. J. 2022, 435, 134598. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takenouchi, M.; Mukai, M.; Furukawa, T.; Maruo, S. Fabrication of Flexible Wiring with Intrinsically Conducting Polymers Using Blue-Laser Microstereolithography. Polymers 2022, 14, 4949. https://doi.org/10.3390/polym14224949
Takenouchi M, Mukai M, Furukawa T, Maruo S. Fabrication of Flexible Wiring with Intrinsically Conducting Polymers Using Blue-Laser Microstereolithography. Polymers. 2022; 14(22):4949. https://doi.org/10.3390/polym14224949
Chicago/Turabian StyleTakenouchi, Mai, Masaru Mukai, Taichi Furukawa, and Shoji Maruo. 2022. "Fabrication of Flexible Wiring with Intrinsically Conducting Polymers Using Blue-Laser Microstereolithography" Polymers 14, no. 22: 4949. https://doi.org/10.3390/polym14224949
APA StyleTakenouchi, M., Mukai, M., Furukawa, T., & Maruo, S. (2022). Fabrication of Flexible Wiring with Intrinsically Conducting Polymers Using Blue-Laser Microstereolithography. Polymers, 14(22), 4949. https://doi.org/10.3390/polym14224949





