Hollow TiO2/Poly (Vinyl Pyrrolidone) Fibers Obtained via Coaxial Electrospinning as Easy-to-Handle Photocatalysts for Effective Nitrogen Oxide Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
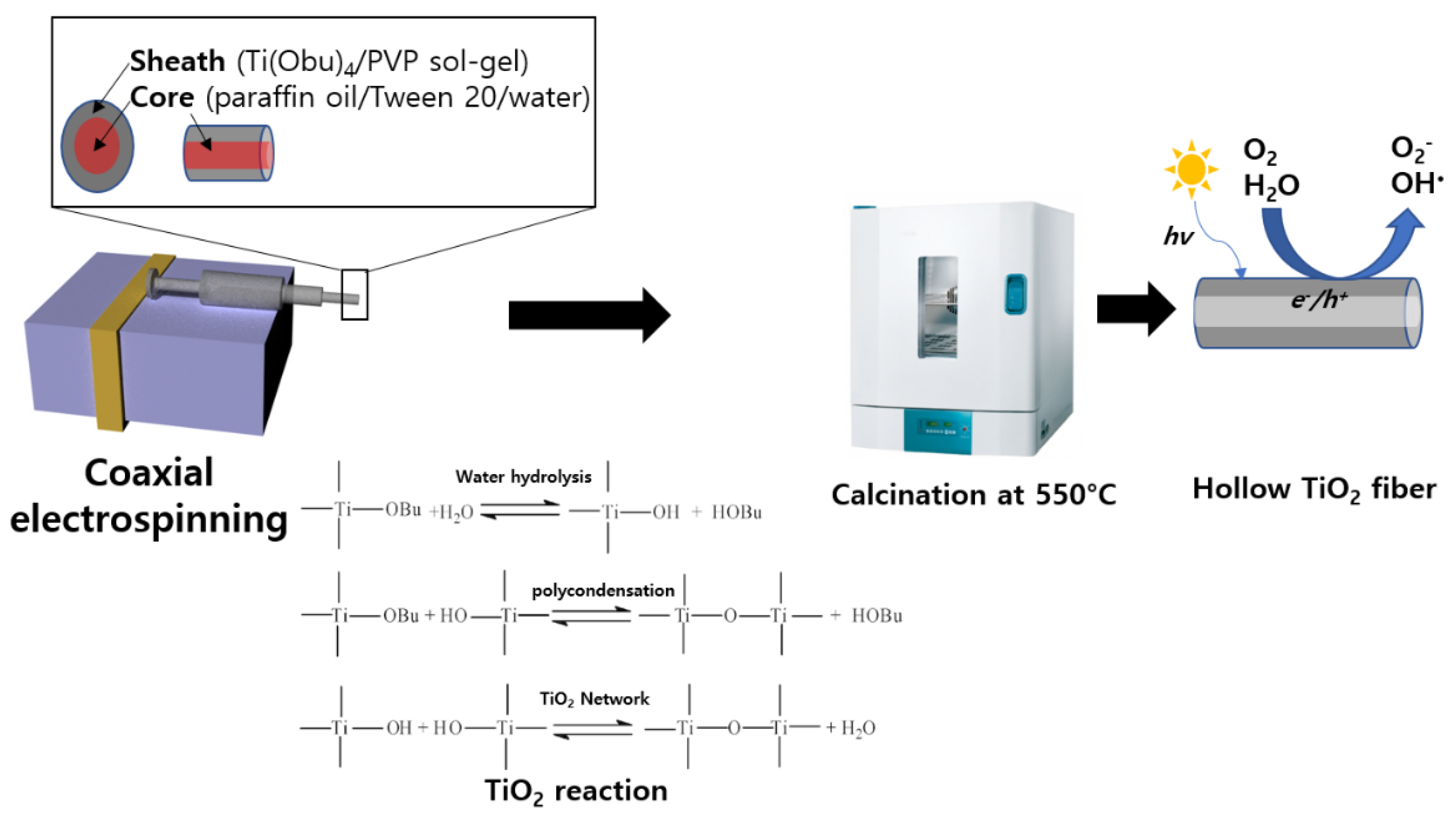
2.2. Preparation of Core-Sheath Sol-Gel
2.3. Fabrication of Hollow TiO2 Fibers
2.4. Chemical Structures and Physical Properties of TiO2 Fibers
2.5. Characterization of Synthesized TiO2 Fibers
2.6. Photocatalytic Performance of TiO2 Fibers for NO Removal
3. Results and Discussion
3.1. Morphology of TiO2@C1, TiO2@C2, TiO2@C3, and TiO2@C0
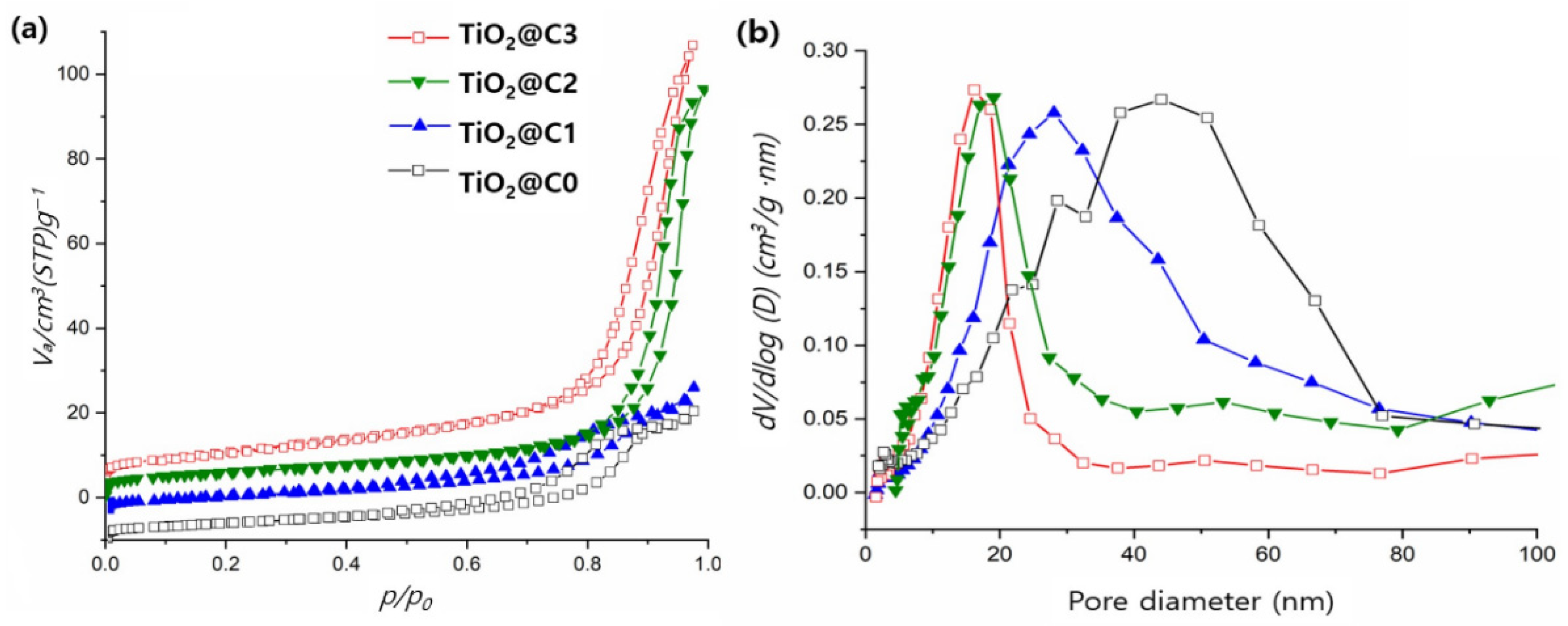
3.2. BET Analysis of TiO2@C1, TiO2@C2, TiO2@C3, and TiO2@C0
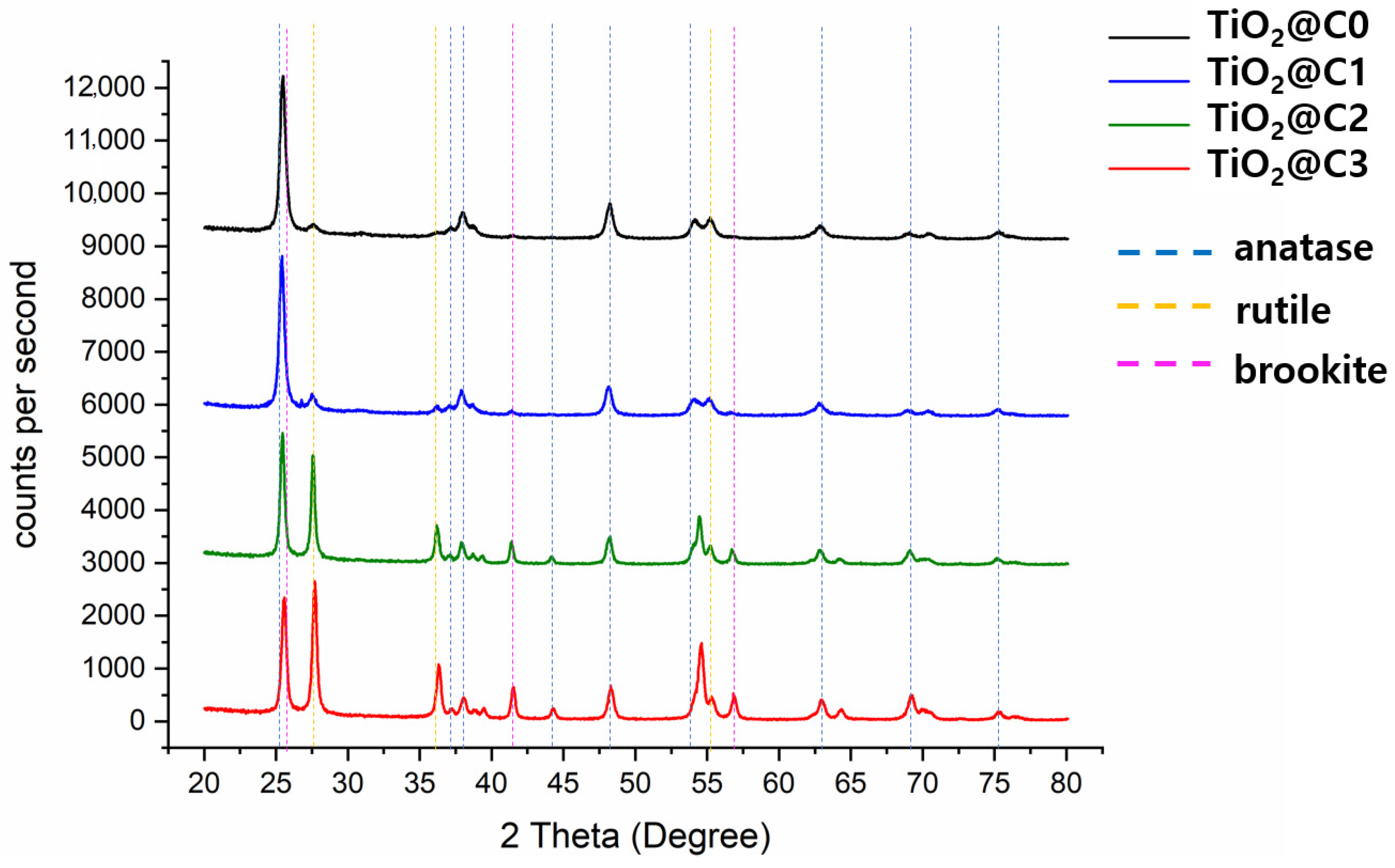
3.3. XRD Analysis of TiO2@C1, TiO2@C2, TiO2@C3, and TiO2@C0
3.4. Photocatalytic Performance of NO Removal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garin, F. Mechanism of NOx decomposition. Appl. Catal. A Gen. 2001, 222, 183. [Google Scholar] [CrossRef]
- Kartohardjono, S.; Merry, C.; Rizky, M.S.; Pratita, C.C. Nitrogen oxide reduction through absorbent solutions containing nitric acid and hydrogen peroxide in hollow fiber membrane modules. Heliyon 2019, 5, e02987. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Nanayakkara, C.E.; Grassian, V.H. Titanium Dioxide Photocatalysis in Atmospheric Chemistry. Chem. Rev. 2012, 112, 5919–5948. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Gao, L.; Zhang, Q. Synthesizing and Comparing the Photocatalytic Properties of High Surface Area Rutile and Anatase Titania Nanoparticles. J. Am. Ceram. Soc. 2003, 86, 1677. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Fabrication of Titania Nanofibers by Electrospinning. Nano Lett. 2003, 3, 555–560. [Google Scholar] [CrossRef]
- Rivaldo-Gómez, C.M.; Ferreira, F.F.; Landi, G.T.; Souza, J.A. New route for hollow materials. Sci. Rep. 2016, 6, 32107. [Google Scholar] [CrossRef]
- Chen, H.; Wang, N.; Di, J.; Zhao, Y.; Song, Y.; Jiang, L. Nanowire-in-Microtube Structured Core/Shell Fibers via Multifluidic Coaxial Electrospinning. Langmuir 2010, 26, 11291–11296. [Google Scholar] [CrossRef]
- Hou, H.; Shang, M.; Wang, L.; Li, W.; Tang, B.; Yang, W. Efficient Photocatalytic Activities of TiO2 Hollow Fibers with Mixed Phases and Mesoporous Walls. Sci. Rep. 2015, 5, 15228. [Google Scholar] [CrossRef]
- Xiong, S.; Wang, Q.; Xia, H. Template synthesis of polyaniline/TiO2 bilayer microtubes. Synth. Met. 2004, 146, 37–42. [Google Scholar] [CrossRef]
- Yu, L.-Y.; Shen, H.-M.; Xu, Z.-L. PVDF–TiO2 composite hollow fiber ultrafiltration membranes prepared by TiO2 sol–gel method and blending method. J. Appl. Polym. Sci. 2009, 113, 1763–1772. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Y.; Lee, M.; Malde, C.; Wang, R. Development of low mass-transfer-resistance fluorinated TiO2-SiO2/PVDF composite hollow fiber membrane used for biogas upgrading in gas-liquid membrane contactor. J. Membr. Sci. 2018, 552, 253–264. [Google Scholar] [CrossRef]
- Majidi, R.; Parhizkar, J.; Karamian, E. Photocatalytic Removal of NOx Gas from Air by TiO2/Polymer Composite Nanofibers. Nanochem. Res. 2018, 3, 212–218. [Google Scholar] [CrossRef]
- López-Herrera, J.M.; Barreroa, A.; López, A.; Loscertales, I.G.; Márquez, M.J. Coaxial jets generated from electrified Taylor cones. Scaling laws. Aerosol. Sci. 2003, 34, 535. [Google Scholar] [CrossRef]
- Di, J.; Chen, H.; Wang, X.; Zhao, Y.; Jiang, L.; Yu, J.; Xu, R. Fabrication of Zeolite Hollow Fibers by Coaxial Electrospinning. Chem. Mater. 2008, 20, 3543–3545. [Google Scholar] [CrossRef]
- Anka, F.H.; Balkus, K.J., Jr. Novel Nanofiltration Hollow Fiber Membrane Produced via Electrospinning. Ind. Eng. Chem. Res. 2013, 52, 3473–3480. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Z.; Wang, Z. Photocatalytic reduction of carbon dioxide using sol–gel derived titania-supported CoPc catalysts. Photochem. Photobiol. Sci. 2007, 6, 695–700. [Google Scholar] [CrossRef]
- Hua, G.; Zhang, L.; Fei, G.; Fang, M. Enhanced catalytic activity induced by defects in mesoporous ceria nanotubes. J. Mater. Chem. 2012, 22, 6851–6855. [Google Scholar] [CrossRef]
- Chuangchote, S.; Jitputti, J.; Sagawa, T.; Yoshikawa, S. Photocatalytic activity for hydrogen evolution of electrospun TiO2 nanofibers. ACS Appl. Mater. Interfaces 2009, 1, 1140–1143. [Google Scholar] [CrossRef]
- Yu, J.; Wang, W.; Cheng, B. Synthesis and Enhanced Photocatalytic Activity of a Hierarchical Porous Flowerlike p–n Junction NiO/TiO2 Photocatalyst. Chem. Asian J. 2010, 5, 2499–2506. [Google Scholar] [CrossRef]
- Phromma, S.; Wutikhun, T.; Kasamechonchung, P.; Eksangsri, T.; Sapcharoenkun, C. Effect of calcination temperature on photocatalytic activity of synthesized TiO2 nanoparticles via wet ball milling sol-gel method. Appl. Sci. 2020, 10, 993. [Google Scholar] [CrossRef]
- Ananpattarachai, J.; Kajitvichyanukul, P.; Seraphin, S. Visible light absorption ability and photocatalytic oxidation activity of various interstitial N-doped TiO2 prepared from different nitrogen dopants. J. Hazard. Mater. 2009, 168, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Augustynski, J. The role of the surface intermediates in the photoelectrochemical behaviour of anatase and rutile TiO2. Electrochim. Acta 1993, 38, 43–46. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Robben, L.; Alkaima, A.; Bahnemann, D. Brookite versus anatase TiO2 photocatalysts: Phase transformations and photocatalytic activities. Photochem. Photobiol. Sci. 2013, 12, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the enhanced photocatalytic activity of Degussa P25 mixed-phase TiO2 using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, D.; Jing, D.; Liu, H.; Liu, L.; Jia, Y.; Gao, M.; Guo, L.; Huo, Z. Enhanced photodynamic therapy of mixed phase TiO2 (B)/anatase nanofibers for killing of HeLa cells. Nano Res. 2014, 7, 1659–1669. [Google Scholar] [CrossRef]
- Amano, F.; Nogami, K.; Tanaka, M.; Ohtani, B. Correlation between Surface Area and Photocatalytic Activity for Acetaldehyde Decomposition over Bismuth Tungstate Particles with a Hierarchical Structure. Langmuir 2010, 26, 7174–7180. [Google Scholar] [CrossRef]



| Flow Rate (mL h−1) | ||||
|---|---|---|---|---|
| Outer | 8 | |||
| Core | 1 | 2 | 3 | 0 |
| Sample # | TiO2@C1 | TiO2@C2 | TiO2@C3 | TiO2@C0 |
| TiO2@C1 | TiO2@C2 | TiO2@C3 | TiO2@C0 | |
|---|---|---|---|---|
| Average sheath diameter (µm) | 1.81 (±0.8) | 2.09 (±0.88) | 3.94 (±1.43) | 3.95 (±1.44) |
| Average core diameter (µm) | 1.51 (±0.37) | 1.80 (±1.01) | 2.83 (±1.28) | - |
| Wall thickness (µm) | 0.53 (±0.30) | 0.38 (±0.10) | 0.26 (±0.10) | - |
| BET surface area (m2 g−1) | 33.04 | 40.73 | 51.28 | 16.01 |
| Average pore diameter (nm) | 18.8 | 11.3 | 11.1 | 28.9 |
| TiO2@C1 | TiO2@C2 | TiO2@C3 | TiO2@C0 | |
|---|---|---|---|---|
| Anatase (%) | 53.5 | 15.3 | 25 | 60.1 |
| Rutile (%) | 7.2 | 11.8 | 24.3 | 9.5 |
| Brookite (%) | 39.3 | 72.9 | 50.7 | 30.3 |
| NO removal (%) | 33.5 | 56.8 | 66.2 | 31.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J. Hollow TiO2/Poly (Vinyl Pyrrolidone) Fibers Obtained via Coaxial Electrospinning as Easy-to-Handle Photocatalysts for Effective Nitrogen Oxide Removal. Polymers 2022, 14, 4942. https://doi.org/10.3390/polym14224942
Kim J. Hollow TiO2/Poly (Vinyl Pyrrolidone) Fibers Obtained via Coaxial Electrospinning as Easy-to-Handle Photocatalysts for Effective Nitrogen Oxide Removal. Polymers. 2022; 14(22):4942. https://doi.org/10.3390/polym14224942
Chicago/Turabian StyleKim, Juran. 2022. "Hollow TiO2/Poly (Vinyl Pyrrolidone) Fibers Obtained via Coaxial Electrospinning as Easy-to-Handle Photocatalysts for Effective Nitrogen Oxide Removal" Polymers 14, no. 22: 4942. https://doi.org/10.3390/polym14224942
APA StyleKim, J. (2022). Hollow TiO2/Poly (Vinyl Pyrrolidone) Fibers Obtained via Coaxial Electrospinning as Easy-to-Handle Photocatalysts for Effective Nitrogen Oxide Removal. Polymers, 14(22), 4942. https://doi.org/10.3390/polym14224942






