Abstract
Covalent organic frameworks (COFs) with donor–acceptor (D–A) units are credible photocatalysts for their per-designed structure, inherent porosity, large surface area, splendid stability and so forth. Developing COFs with an excellent photocatalytic efficiency for hydrogen evolution is of a great significance in alleviating the energy crisis. Herein, a D–A type imine-linked crystalline Zn-Por-TT COF was fabricated successfully via the co-polymerization of electron-deficient Zinc (II) 5,10,15,20-tetrakis(para-aminophenyl) porphyrin (Zn-TAPP), and electron-rich thieno[3,2-b]thiophene-2,5-dicarbaldehyde (TT). Profiting from the D–A complex structure, the obtained Zn-Por-TT COF showcases an excellent photocatalytic activity with a hydrogen evolution rate of 8200 μmol/g/h, while the Zn-TAPP monomer presents practically no capacity for the generation of hydrogen under identical conditions. In addition, the counterparts Por-TT COF and COF-366-Zn were employed to illustrate the enhancement of the photocatalytic performance by metal catalytic sites and D–A structures. In addition, the counterparts Por-TT COF and COF-366-Zn were employed to illustrate the enhancement of metal catalytic sites and D–A structures for the photocatalytic performance.
1. Introduction
The pursuit of clean and sustainable energy sources is a desperate exploration to mitigate the consumption of fossil fuels and environmental pollution problems [1]. Hydrogen (H2) is a representative ideal green energy resource with a high energy density and free carbon emissions. A promising and sustainable path for hydrogen generation is photocatalytic water splitting under visible light, which is propelled by ubiquitous solar energy [2,3]. It has been demonstrated that photocatalysts play a decisive role in facilitating the kinetics of the H2 evaluation process. Consequently, the exploration of effective photocatalysts utilized to drive a H2 generation under visible light is of considerable significance [4].
Covalent organic frameworks (COFs) are an emerging kind of crystalline porous organic polymers, which possess properties such as a per-designed periodic structure, an inherent open channel, a large specific surface area, a satisfactory chemical/thermal stability, etc. [5,6,7,8,9,10,11]. These aforementioned characteristics enable COFs to function as potential desired photocatalysts for the generation of H2. However, the narrow absorption of visible light and the recombination of photon-generated charge carriers are key factors restricting the photocatalytic performance of COFs. The incorporation of donor–acceptor (D–A) combinations via a typical bottom-up design strategy is a reasonable strategy to broaden the absorption of visible light and promote the separation of photogenerated carriers, so that a series of D–A COFs have been designed and synthesized to function as photocatalysts for the evolution of H2 [12,13,14]. Among them, porphyrin, especially electron-deficient metalloporphyrin contained COFs, usually displayed a splendid catalytic performance thanks to the good visible light absorption ability and additional metal active sites [15,16,17,18,19,20,21]. Moreover, we noticed Zn-porphyrin-based COFs possess a longer-lasting state of the separation of electrons and holes than cobalt and nickel-coordinated porphyrin, which results from a suppressed ligand-to-metal charge transfer process caused by 3d10 configuration of the Zn2+ ion [22]. Therefore, Zn (II)-porphyrin-based COFs would be potential ideal photocatalysts for an efficient H2 evolution.
Herein, we select Zinc (II) 5,10,15,20-tetrakis(para-aminophenyl) porphyrin (Zn-TAPP) as the acceptor and thieno[3,2-b]thiophene-2,5-dicarbaldehyde (TT) as the donor to fabricate a metalloporphyrin-based D–A COF by the solvothermal method. The obtained Zn-Por-TT COF presented an excellent crystallinity, a large surface area, a good thermal/chemical stability, and a narrow band gap. As expected, the Zn-Por-TT COF demonstrated a good activity of photocatalytic hydrogen production and the hydrogen evolution rate of the Zn-Por-TT COF could attain to 8200 μmol/g/h in the presence of Pt co-catalyst and ascorbic acid sacrificial agent, which is above the average level among the reported COF photocatalysts. More importantly, a metal-free Por-TT COF and COF-366-Zn were synthesized and employed as counterparts to confirm that Zn2+ functions as the catalytic active site and the enhanced D–A interactions are beneficial for promoting the photocatalyst performance.
2. Results and Discussion
The Zn-Por-TT COF was synthesized in the mixed solvent system of o-dichlorobenzene/benzyl alcohol/6M AcOH at 120 °C for 3 days and what was obtained was a dark purple powder with a yield of 75% (Figure 1a). The successful co-condensation of Zn-TAPP and TT was unambiguously detected by Fourier transform infrared (FT-IR) spectroscopy (Figure 1b), the characteristic singles for -NH2 (~3352 and 3316 cm−1) and -CHO (~1660 cm−1) were dramatically decreased in Zn-Por-TT COF, and a new peak was able to be observed at ~1612 cm−1, which corresponded to the stretching vibration of C=N linkages. In addition, the single peak which appeared at ~150.4 ppm in the 13C solid-state NMR spectrum (Figure 1c) was able to ascribe to the carbon of the imine linkages, which further indicates the existence of C=N linkages. In addition, an X-ray photoelectron spectroscopy (XPS) measurement was carried out to evaluate the valence state of zinc in the prepared Zn-Por-TT COF (Figure 1d,e). The strong peaks which appeared at 1021.59 eV and 1044.60 eV should be attributed to Zn 2p2/3 and Zn 2p1/2, demonstrating that the valence state of Zn was +2. All these results indicate the efficient polymerization of Zn-TAPP and TT monomers.
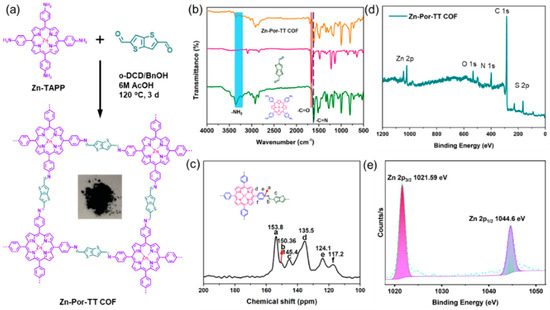
Figure 1.
(a) Synthetic diagram of Zn-Por-TT COF; (b) FT-IR spectra of Zn-Por-TT COF (orange), TT (pink), Zn-TAPP (green); (c) 13C CP-MAS NMR spectrum of Zn-Por-TT COF; (d) XPS of Zn-Por-TT-COF; and (e) high-resolution XPS spectrum of Zn.
The crystalline structure of the Zn-Por-TT COF was assessed by a powder X-ray diffraction measurement (PXRD) combined with the theoretical simulation (Figure 2a). There are at least six prominent diffraction peaks in the PXRD pattern, indicating the high crystallinity of Zn-Por-TT COF. The diffraction peaks appeared at 3.3°, 6.6°, 7.2°, 9.8°, 10.7°, and 22.2°, which can be assigned to (100), (200), (210), (300), (310), and (001) facts, respectively. The crystalline architecture of the Zn-Por-TT COF matched well with the simulated AA stacking model which provided a unit cell parameter of a = 27.23 Å, b = 27.34 Å, c = 4.29 Å, α = 76.3°, β = 100.9°, and γ = 95.8°. The calculated PXRD profile corresponds well to the experimental data and the deviations of the Pawley refinement are Rp = 2.76% and Rwp = 4.29%.
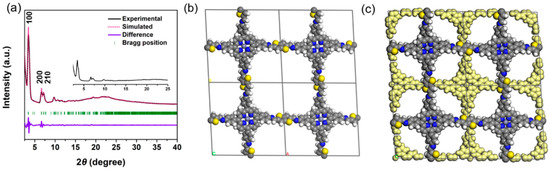
Figure 2.
(a) Experimental PXRD patterns of Zn-Por-TT COF (black), Pawley refinement (pink), their difference (violet), and Bragg positions (green); (b) AA-stacking; and (c) AB-stacking models of Zn-Por-TT COF.
To explore the permanent porosity of Zn-Por-TT COF, the N2 sorption experiment was proceeded at 77 K and the samples were degassed at 150 °C for 8 h under a vacuum (10−5 bar) before their analysis (Figure 3). As shown in Figure 3a, the Zn-Por-TT COF revealed characteristic type-IV sorption isotherms, which possessed an obvious step at P/P0 = 0.05–0.18, demonstrating the existence of mesoporous. The Brunauer–Emmett–Teller (BET) surface area of the Zn-Por-TT COF was 632 m2/g, calculated from the absorption curve. The pore size distribution was analyzed by the nonlocal density functional theory (NLDFT) method and afforded an average pore size of 2.6 nm, which is approximated with the theoretical value (2.5 nm). Moreover, the morphology feature of the Zn-Por-TT COF was assessed by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). As revealed by the SEM images (Figure 4a,b and Figures S1 and S2), the Zn-Por-TT COF presented random nanoflakes with an average size of ~200 nm. Additionally, clear lattice textures with an interval of 2.6 nm were readily observed in the TEM images, which attests the high crystalline feature and long-rang ordered structure of the Zn-Por-TT COF (Figure 4c,d and Figure S2). In addition, the energy-dispersive X-ray spectroscopy (EDS) elemental mappings revealed that C, N, S, and Zn were uniformly distributed in these nanoflakes (Figure 4e–h).
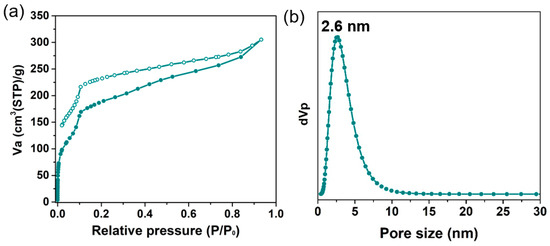
Figure 3.
(a) N2 absorption isotherms of Zn-Por-TT COF at 77 K; (b) pore size distribution profile of Zn-Por-TT COF.
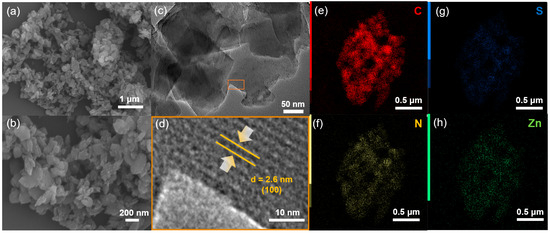
Figure 4.
(a,b) SEM and (c,d) TEM images of Zn-Por-TT COF; (e–h) elemental maps of Zn-Por-TT COF.
Additionally, the Zn-Por-TT COF showcased an excellent thermal stability which was confirmed by the thermogravimetric analysis (TGA) measurement under N2. The distinct weight loss appeared at ca. 500 °C, indicating that the skeleton of the Zn-Por-TT COF started to collapse (Figure 5a). The chemical stability of the Zn-Por-TT COF was detected by immersing the COF powder into the solution of hydrochloric acid (pH = 2) and 1 M of sodium hydroxide solution for 24 h. After these treatments, the crystallinity of the Zn-Por-TT COF remained well, indicating a good chemical stability of the Zn-Por-TT COF (Figure 5b). Furthermore, the UV-vis diffuse reflectance spectroscopy (UV-DRS) revealed that the Zn-Por-TT COF exhibited a broad visible light absorption capacity in the range of 250~700 nm and resulted in a narrow optical band gap of ~1.8 eV (Figure 6a). The Mott–Schottky measurement was further performed to determine the conduction band (CB) of Zn-Por-TT COF and it provided a value of −1.43 V vs. Ag/AgCl (−1.23 V vs. NHE) (Figure 6b). Subsequently, the corresponding valence band (VB) was estimated to be 0.57 V vs. NHE [23]. The band structure satisfies the photocatalytic water splitting property in thermodynamics.
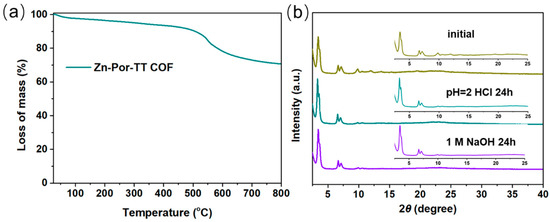
Figure 5.
(a) TGA profile of Zn-Por-TT COF in N2; (b) PXRD patterns of Zn-Por-TT COFs before (dark yellow) and after treatments in the solution of HCl (pH = 2, cyan) and NaOH (1 M, violet).
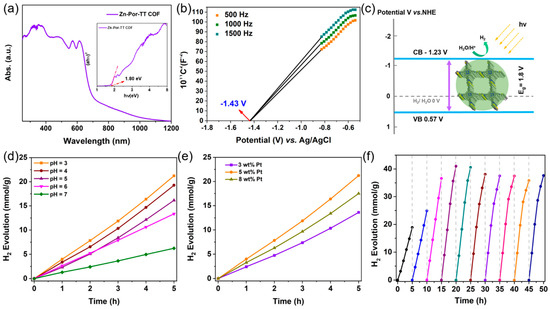
Figure 6.
(a) UV-Vis DRS spectrum of Zn-Por-TT COF, insert: (ahυ)2 versus hυ curve of Zn-Por-TT COF; (b) Mott–Schottky (M-S) plots of Zn-Por-TT COF at 500 Hz, 1000 Hz, 1500 Hz; (c) the CB and VB positions of Zn-Por-TT COF; (d) H2 evolution rate of Zn-Por-TT COF under different acidity; (e) H2 evolution rate of Zn-Por-TT COF with different Pt loading amounts; (f) time course of H2 productions under visible light (λ > 400 nm) irradiation of Zn-Por-TT COF.
A good chemical stability, an excellent visible light absorption capacity, an inherent porosity, and a suitable bond gap render the Zn-Por-TT COF an alternative photocatalyst for a H2 evolution under a visible light illumination. To demonstrate this hypothesis, the photocatalytic hydrogen production experiments were performed to evaluate the photocatalytic performance of a Zn-Por-TT COF, and the general H2 evaluation procedure is described as below. Primarily, 5 mg of the Zn-Por-TT COF powder was dispersed in 50 mL of ultrapure water, a few amounts of ascorbic acid (AA), and K2PtCl6 were added into the system which functions as an electron sacrificial agent and co-catalyst to produce hydrogen, respectively. The visible light (λ > 400 nm, xenon lamp) was employed as the optical source and the photocatalytic system should be kept at 4 °C during the process of a H2 evolution. In order to identify the optimal conditions for a H2 evolution catalyzed by Zn-Por-TT COF, the effects of the pH and co-catalyst ratio were investigated unambiguously. The optimal pH value was revealed to be three (Figure 6d) and the most appropriate amount of Pt co-catalyst was 5 wt% (Figure 6e), which both displayed higher H2 evolution rates compared with the control experiments. Intriguingly, the Zn-Por-TT COF displayed a moderate H2 evolution rate of 4100 μmol/g/h at the first 5 h while the rate could enhance steadily to attain 8200 μmol/g/h after the illumination for 10 h and was maintained for at least 40 h (Figure 6f), which is comparable and better than many other reported COF materials (Table 1). The enhancement of the hydrogen production rate in first 10 h could be attributed to the progressive deposition of Pt [24,25,26]. In addition, the chemical constitution, morphology, and crystalline structure of the Zn-Por-TT COF were able to remain well after photocatalysis, and the elements of C, N, S, Zn, and Pt were uniformly distributed or deposited in the Zn-Por-TT COF nanoparticle (Figure 7), which demonstrates the splendid cycle stability of Zn-Por-TT COF.

Table 1.
The photocatalytic performance comparison of Zn-Por-TT COF with other representative COF based photocatalysts.
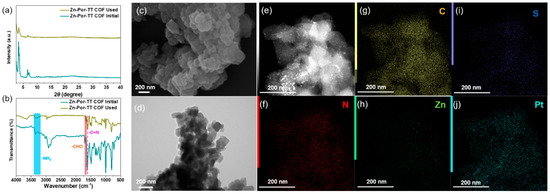
Figure 7.
(a) PXRD pattern of Zn-Por-TT COF before and after photocatalysis; (b) FT-IR spectra of Zn-Por-TT COF before and after photocatalysis; (c) SEM of Zn-Por-TT COF after photocatalysis; (d) TEM of Zn-Por-TT COF after photocatalysis; and (e–j) EDS Maps of Zn-Por-TT COF after photocatalysis.
To investigate the enhancement effect of the D–A structure and Zn (II) coordination center on the photocatalytic performance, COF-366-Zn without the TT donor units and a Por-TT COF free of Zn (II) coordination center were constructed and employed to function as contrasted photocatalysts for the H2 evolution. The chemical constitution, crystallinity, and morphology of COF-366-Zn and Por-TT COF were unequivocally characterized by PXRD, FT-IR, XPS, etc. (Figure 8a–f and Figures S3–S6). Both COF-366-Zn and Por-TT COF exhibited a similar broad absorption from approximately 250 to 700 nm and narrow band gaps which presumably caused by the incorporation of porphyrin (Figure 9a). However, the H2 evolution rates of the two contrasted COFs were significantly reduced (Figure 9b) and the photocurrents of COF-366-Zn and Por-TT COF were weaker than the Zn-Por-TT COF (Figure 9c), which suggests the faster charge transfer capacity of the Zn-Por-TT COF and indicates the D–A structure and the Zn (II) coordination center synergistically promoted the photocatalytic performance of Zn-Por-TT COF.
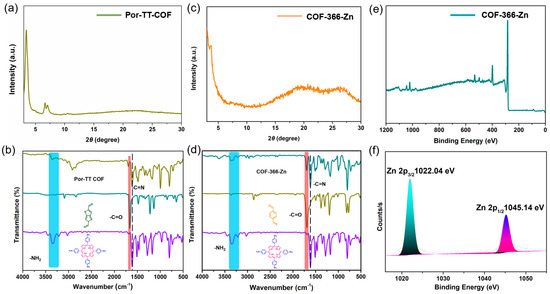
Figure 8.
(a) PXRD pattern of Por-TT COF; (b) FT-IR spectra of Por-TT-COF (dark yellow), TT (cyan), TAPP (violet); (c) PXRD pattern of COF-366-Zn; (d) FT-IR spectra of COF-366-Zn (cyan), BDT (dark yellow), TAPP (violet); (e) XPS of COF-366-Zn; and (f) high-resolution XPS spectra of Zn.
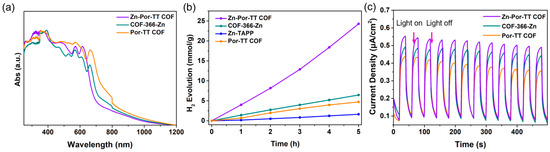
Figure 9.
(a) DRS-UV spectra of Zn-Por-TT COF (violet), COF-366-Zn (cyan), and Por-TT COF (orange); (b) H2 evolution rates of Zn-Por-TT COF (violet), COF-366-Zn (cyan), and Por-TT COF (orange); (c) photocurrent-time plots of Zn-Por-TT COF (violet), COF-366-Zn (cyan), and Por-TT COF (orange).
3. Conclusions
In summary, a Zn(II)-porphyrin built-in COF was successfully fabricated via a reasonable design and the obtained Zn-Por-TT COF exhibited a high crystallinity, a good stability, and a broad absorption capacity of visible light so that can be employed as an effective photocatalyst for a H2 generation. As our hypothesis, the Zn-Por-TT COF exhibits a satisfactory H2 evolution rate which can be stabilized at ~8200 μmol/g/h eventually. Moreover, the formed D–A structures and abundant metal catalytic active sites synergistically promote the H2 production performance of the Zn-Por-TT COF, as demonstrated by the controlled experiments. This work provides an available path for the construction of highly efficient photosensitizers for solar energy harvesting and conversion. We envision that COF photocatalysts with desirable properties will be constructed via incorporating stronger D–A complex structures, and related works are in progress in our laboratory.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym14224893/s1, Figure S1: (a–c) SEM images of Zn-Por-TT COF; Figure S2: (a,b) TEM images of Zn-Por-TT COF; Figure S3: (a,b) SEM and (c,d) TEM images of COF-366-Zn; Figure S4: (a–d) Elemental maps of COF-366-Zn; Figure S5: (a,b) SEM images of Por-TT COF; Figure S6: 1H NMR spectrum of TAPP in DMSO-d6; Figure S7: 1H NMR spectrum of Zn-TAPP in DMSO-d6; Table S1: Atomic coordinates of the AA-stacking mode of Zn-Por-TT COF using DFTB+ method. References [23,31,32] are listed in the Supplementary Materials.
Author Contributions
Conceptualization, Y.L. and F.B.; methodology, M.L., Z.C. and X.C.; software, X.R. and R.C.; validation, M.L., Y.L. and F.B.; formal analysis, M.L., Y.L. and F.B.; investigation, M.L.; resources, Y.L. and F.B.; data curation, M.L.; writing—original draft preparation, M.L.; writing—review and editing, Y.L. and F.B.; visualization, X.R.; supervision, Y.L. and F.B.; project administration, M.L., Y.L. and F.B.; funding acquisition, Y.L. and F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Postdoctoral Science Foundation of China (2021M701066), the National Natural Science Foundation of China (21771055, U21A2085), the Zhongyuan High Level Talents Special Support Plan (204200510010) and the Scientific and Technological Innovation Team in University of Henan Province (20IRTSTHN001) and Y.L. gratefully acknowledges financial support from Zhongyuan Youthful Postdoctoral Innovative Talent Program of Henan Province, China.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, S.; Pan, Y.; Lin, H.; Li, L.; Fu, X.; Long, J. Crystalline Covalent Organic Frameworks with Tailored Linkages for Photocatalytic H2 Evolution. ChemSusChem 2021, 14, 4958–4972. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Geng, K.; Jiang, D. Engineering Covalent Organic Frameworks for Light-Driven Hydrogen Production from Water. ACS Mater. Lett. 2019, 1, 203–208. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Chen, L.; Xu, H.; Xiong, Y. 2D Polymers as Emerging Materials for Photocatalytic Overall Water Splitting. Adv. Mater. 2018, 30, 1801955. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, X.; Chen, L.; Xiong, Y.; Xu, H. Van der Waals Heterostructures Comprised of Ultrathin Polymer Nanosheets for Efficient Z-Scheme Overall Water Splitting. Angew. Chem. Int. Ed. 2018, 57, 3454–3458. [Google Scholar] [CrossRef] [PubMed]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Q.; Xu, T.; Xie, Z.; Liu, J.; Yu, X.; Ma, S.; Qin, T.; Chen, L. De Novo Design and Facile Synthesis of 2D Covalent Organic Frameworks: A Two-in-One Strategy. J. Am. Chem. Soc. 2019, 141, 13822–13828. [Google Scholar] [CrossRef]
- Li, Y.; Guo, L.; Lv, Y.; Zhao, Z.; Ma, Y.; Chen, W.; Xing, G.; Jiang, D.; Chen, L. Polymorphism of 2D Imine Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2021, 60, 5363–5369. [Google Scholar] [CrossRef]
- Liang, R.-R.; Jiang, S.-Y.; Han, A.R.; Zhao, X. Two-dimensional covalent organic frameworks with hierarchical porosity. Chem. Soc. Rev. 2020, 49, 3920–3951. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Ding, X.; Yu, B.; Wu, H.; Zhou, W.; Liu, W.; Wei, C.; Chen, B.; Qi, D.; Wang, H.; et al. Two-Dimensional Covalent Organic Frameworks with Cobalt(II)-Phthalocyanine Sites for Efficient Electrocatalytic Carbon Dioxide Reduction. J. Am. Chem. Soc. 2021, 143, 7104–7113. [Google Scholar] [CrossRef]
- Pachfule, P.; Acharjya, A.; Roeser, J.; Sivasankaran, R.P.; Ye, M.-Y.; Brückner, A.; Schmidta, J.; Thomas, A. Donor–acceptor covalent organic frameworks for visible light induced free radical polymerization. Chem. Sci. 2019, 10, 8316–8322. [Google Scholar] [CrossRef]
- Vardhan, H.; Nafady, A.; Al-Enizi, A.; Ma, S. Pore surface engineering of covalent organic frameworks: Structural diversity and applications. Nanoscale 2019, 11, 21679–21708. [Google Scholar] [CrossRef] [PubMed]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; NTan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, J.; Yan, X.; Wu, C.; Zhu, X.; Li, B.; Li, T.; Guo, Q.; Gao, J.; Hu, M.; et al. Donor–Acceptor Covalent Organic Framework Hollow Submicrospheres with a Hierarchical Pore Structure for Visible-Light-Driven H2 Evolution. J. Mater. Chem. A 2022, 10, 11010–11018. [Google Scholar] [CrossRef]
- Zhao, Z.; Zheng, Y.; Wang, C.; Zhang, S.; Song, J.; Li, Y.; Ma, S.; Cheng, P.; Zhang, Z.; Chen, Y. Fabrication of Robust Covalent Organic Frameworks for Enhanced Visible-Light-Driven H2 Evolution. ACS Catal. 2021, 11, 2098–2107. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Zhang, S.; Zhang, Y.; Zhang, J.; Li, R.; Peng, T. Porphyrin-Based Conjugated Polymers as Intrinsic Semiconducting Photocatalysts for Robust H2 Generation under Visible Light. ACS Appl. Energy Mater. 2019, 2, 5665–5676. [Google Scholar] [CrossRef]
- Qian, Y.; Li, D.; Han, Y.; Jiang, H. Photocatalytic Molecular Oxygen Activation by Regulating Excitonic Effects in Covalent Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 20763–20771. [Google Scholar] [CrossRef]
- Jin, E.; Lan, Z.; Jiang, Q.; Geng, G.; Li, G.; Wang, X.; Jiang, D. 2D sp2 Carbon-Conjugated Covalent Organic Frameworks for Photocatalytic Hydrogen Production from Water. Chem 2019, 5, 1632–1647. [Google Scholar] [CrossRef]
- Cheung, P.L.; Lee, S.K.; Kubiak, C.P. Facile Solvent-Free Synthesis of Thin Iron Porphyrin COFs on Carbon Cloth Electrodes for CO2 Reduction. Chem. Mater. 2019, 31, 1908–1919. [Google Scholar] [CrossRef]
- Lin, S.; Diercks, C.S.; Zhang, Y.; Kornienko, N.; Nichols, E.M.; Zhao, Y.; Paris, A.R.; Kim, D.; Yang, P.; Yaghi, O.M.; et al. Covalent Organic Frameworks Comprising Cobalt Porphyrins for Catalytic CO2 Reduction in Water. Science 2015, 349, 1208–1213. [Google Scholar] [CrossRef]
- Wang, K.; Qi, D.; Li, Y.; Wang, T.; Liu, H.; Jiang, J. Tetrapyrrole Macrocycle Based Conjugated Two-Dimensional Mesoporous Polymers and Covalent Organic Frameworks: From Synthesis to Material Applications. Coordin. Chem. Rev. 2019, 378, 188–206. [Google Scholar] [CrossRef]
- Biswal, B.P.; Valligatla, S.; Wang, M.; Banerjee, T.; Saad, N.A.; Mariserla, B.M.K.; Chandrasekhar, N.; Becker, D.; Addicoat, M.; Senkovska, I.; et al. Nonlinear Optical Switching in Regioregular Porphyrin Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2019, 58, 6896–6900. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, Y.; Ma, Y.; Mal, A.; Gao, X.; Gao, L.; Qiao, L.; Li, X.; Wu, L.; Wang, C. Rational Design of Isostructural 2D Porphyrin-Based Covalent Organic Frameworks for Tunable Photocatalytic Hydrogen Evolution. Nat Commun. 2021, 12, 1354. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Liu, J.; Li, Q.; Zhang, M.; Liu, M.; Wang, J.; Yuan, D.; Lan, Y. Rational Design of Crystalline Covalent Organic Frameworks for Efficient CO2 Photoreduction with H2O. Angew. Chem. Int. Ed. 2019, 58, 12392–12397. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, L.; He, H.; Sun, L.; Wang, H.; Fang, X.; Zhao, Y.; Zheng, D.; Qi, Y.; Li, Z.; et al. In Situ Photodeposition of Platinum Clusters on A Covalent OrganicFramework forPhotocatalytic Hydrogen Production. Nat. Commun. 2022, 13, 1355. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Wang, J.; Li, Y.; Sun, J.; Bai, F. Morphology-Controlled Porphyrin Nanocrystals with Enhanced Photocatalytic Hydrogen Production. Nano Res. 2022, 15, 5719–5725. [Google Scholar]
- Liu, Y.; Wang, L.; Feng, H.; Ren, X.; Ji, J.; Bai, F.; Fan, H. Microemulsion-Assisted Self-Assembly and Synthesis of Size-Controlled Porphyrin Nanocrystals with Enhanced Photocatalytic Hydrogen Evolution. Nano Lett. 2019, 19, 2614–2619. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, W.; Liu, H. Facile Construction of Fully sp2-Carbon Conjugated Two-Dimensional Covalent Organic Frameworks Containing Benzobisthiazole Units. Nat. Commun. 2022, 13, 100. [Google Scholar] [CrossRef]
- Mao, C.; Hu, Y.; Yang, C.; Qin, C.; Dong, G.; Zhou, Y.; Zhang, Y. Well-Designed Spherical Covalent Organic Frameworks with an Electron-Deficient and Conjugate System for Efficient Photocatalytic Hydrogen Evolution. ACS Appl. Energy Mater. 2021, 4, 14111–14120. [Google Scholar] [CrossRef]
- Chen, W.; Wang, L.; Mo, D.; He, F.; Wen, Z.; Wu, X.; Xu, H.; Chen, L. Modulating Benzothiadiazole-Based Covalent Organic Frameworks via Halogenation for Enhanced Photocatalytic Water Splitting. Angew. Chem. Int. Ed. 2020, 59, 16902–16909. [Google Scholar]
- Zhang, W.; Chen, L.; Dai, S. Reconstructed Covalent Organic Frameworks. Nature 2022, 604, 72–79. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, E.; Gao, Y.; Yang, W.; Huang, C.; Liu, F. A Conjugated Copper Porphyrin Polymer for Catalytic Coupling of Acetonitrile and Alcohols. Chem. Sel. 2019, 4, 10653. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Y.; Huang, X.; Dong, L.; Lu, M.; Guo, C.; Yuan, D.; Chen, Y.; Xu, G.; Li, S.; et al. Porphyrin-Based COF 2D Materials: Variable Modification of Sensing Performances by Post-Metallization. Angew. Chem. Int. Ed. 2022, 61, e202115308. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).