Reusable Macroporous Oil Sorbent Films from Plastic Wastes
Abstract
1. Introduction
2. Experimental Section
2.1. Method and Materials
2.2. Characterizations
3. Results and Discussion
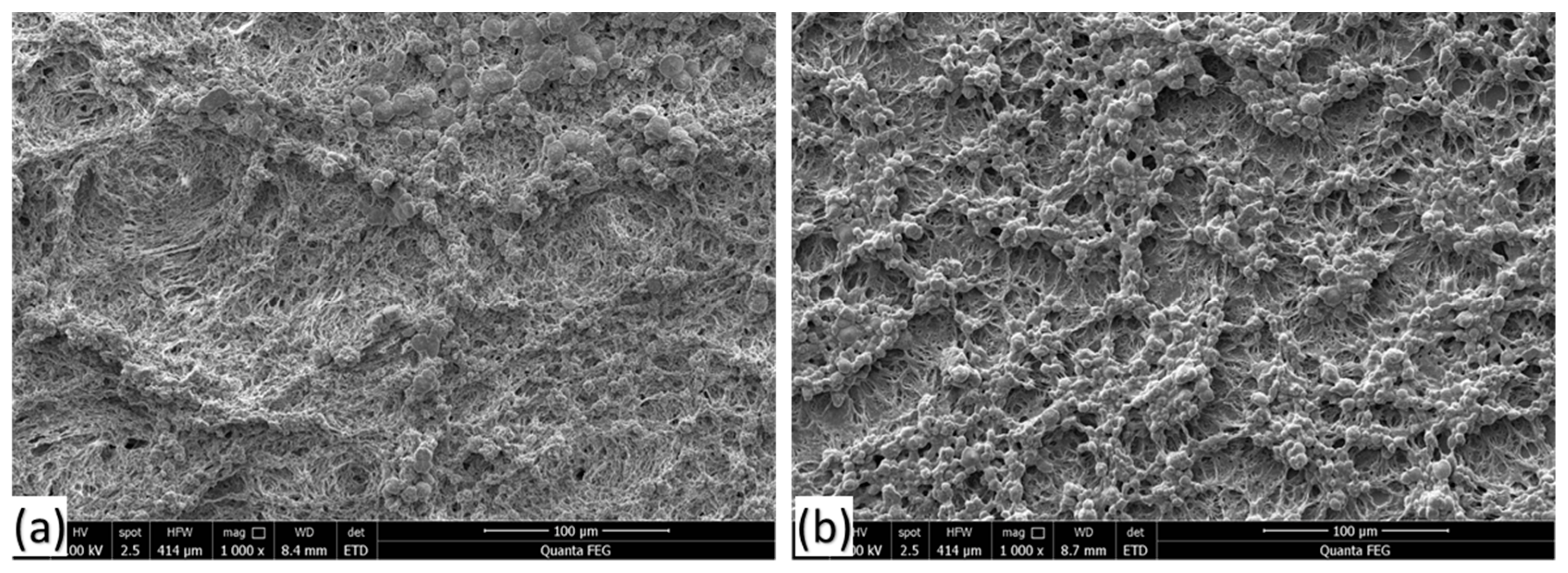
3.1. SEM
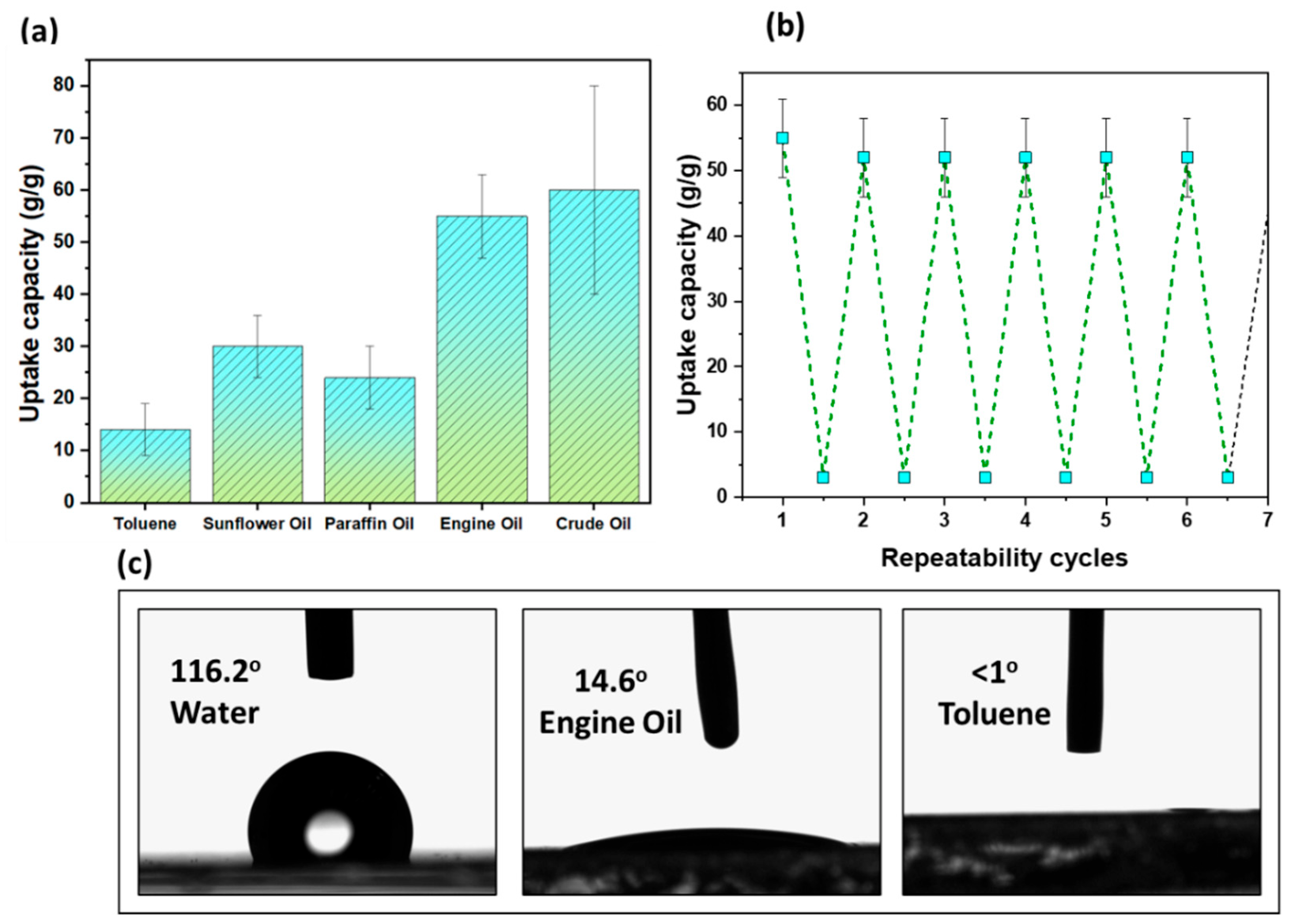
3.2. Oil Study

3.3. Recyclability and Contact Angle
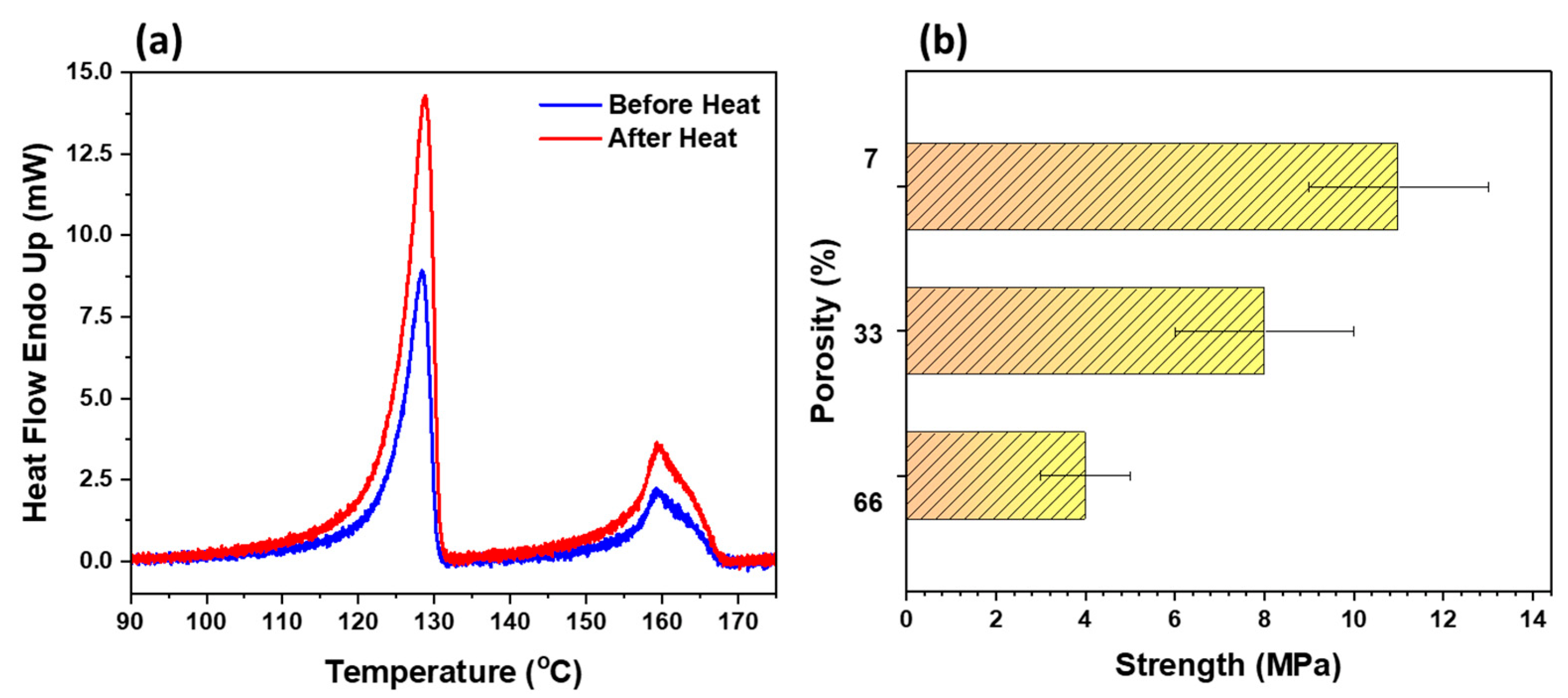
3.4. DSC and Tensile Strength
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Saleem, J.; Adil Riaz, M.; Gordon, M. Oil Sorbents from Plastic Wastes and Polymers: A Review. J. Hazard. Mater. 2018, 341, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Bazargan, A.; Hui, C.; McKay, G. Porous Carbons from Plastic Waste. Adv. Polym. Sci. Springer 2013, 266, 1–25. [Google Scholar] [CrossRef]
- Baroulaki, I.; Karakasi, O.; Pappa, G.; Tarantili, P.A.; Economides, D.; Magoulas, K. Preparation and Study of Plastic Compounds Containing Polyolefins and Post Used Newspaper Fibers. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1613–1625. [Google Scholar] [CrossRef]
- Wilcox, C.; Van Sebille, E.; Hardesty, B.D. Threat of Plastic Pollution to Seabirds Is Global, Pervasive, and Increasing. Proc. Natl. Acad. Sci. USA 2015, 112, 11899–11904. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M. Plastic Waste Inputs from Land into the Ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Ellen Macarthur Foundation. The New Plastic Economy: Rethinking the Future of Plastics; The World Economic Forum: Geneva, Switzerland, 2016; pp. 1–117. [Google Scholar]
- Sandra, L.; Matthew, T. A Million Bottles a Minute: World’s Plastic Binge “as Dangerous as Climate Change”. 2017. Available online: https://www.theguardian.com/environment/2017/jun/28/a-million-a-minute-worlds-plastic-bottle-binge-as-dangerous-as-climate-change (accessed on 28 June 2017).
- Rahimi, A.; García, J.M. Chemical Recycling of Waste Plastics for New Materials Production. Nat. Rev. Chem. 2017, 1, 0046. [Google Scholar] [CrossRef]
- Čolnik, M.; Kotnik, P.; Knez, Ž.; Škerget, M. Chemical Recycling of Polyolefins Waste Materials Using Supercritical Water. Polymers 2022, 14, 4415. [Google Scholar] [CrossRef]
- Alsabri, A.; Tahir, F.; Al-Ghamdi, S.G. Life-Cycle Assessment of Polypropylene Production in the Gulf Cooperation Council (GCC) Region. Polymers 2021, 13, 3793. [Google Scholar] [CrossRef]
- Kahlen, I.S.; Braun, H.; Liu, Y.; Hubner, G. Compatibilization of Recycled Polyethylene-Polypropylene Blends. CA3135074C, 08 March 2020. [Google Scholar]
- Xiao, C.; Allen, L.; Biddle, M.; Fisher, M.M. Electrostatic Separation and Recovery of Mixed Plastics; Annual Recycling Conference (ARC): Dearborn, MI, USA, 1999. [Google Scholar]
- Tilmatine, A.; Medles, K.; Bendimerad, S.-E.; Boukholda, F.; Dascalescu, L. Electrostatic Separators of Particles: Application to Plastic/Metal, Metal/Metal and Plastic/Plastic Mixtures. Waste Manag. 2009, 29, 228–232. [Google Scholar] [CrossRef]
- Ying, H.; Cheng, J. Hydrolyzable Polyureas Bearing Hindered Urea Bonds. J. Am. Chem. Soc. 2014, 136, 16974–16977. [Google Scholar] [CrossRef]
- Maio, F.D.; Rem, P.; Hu, B.; Serranti, S.; Bonifazi, G. The W2Plastics Project: Exploring the Limits of Polymer Separation. Open Waste Manag. 2010, 3, 90–98. [Google Scholar] [CrossRef]
- Gondal, M.A.; Siddiqui, M.N. Identification of Different Kinds of Plastics Using Laser-Induced Breakdown Spectroscopy for Waste Management. J. Environ. Sci. Health Part A 2007, 42, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Serranti, S.; Fraunholcz, N.; Di Maio, F.; Bonifazi, G. Recycling-Oriented Characterization of Polyolefin Packaging Waste. Waste Manag. 2013, 33, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Kahlen, S. Polypropylene-Polyethylene Composition with Improved Flowability. U.S. Patent No. 10,059,785, 28 August 2018. [Google Scholar]
- Kahlen, S. Polypropylene Polyethylene Mixture Upgrading. EP3,936,565A1, 13 January 2021. [Google Scholar]
- Kahlen, S. Upgraded Recycled Polypropylene Rich Polyolefin Material. TWI730435B 2, 20 April 2021. [Google Scholar]
- Kahlen, S. Upgraded Recycled Polyethylene Polypropylene Blend. US2022/0127439A1, 03 February 2022. [Google Scholar]
- Kahlen, S. Upgraded Recycled Relatively Polyethyleen Rich Polyolefin Materials. US2021/0347970A1, 27 April 2021. [Google Scholar]
- Kahlen, S. Recycled Polyethylene-Polypropylene Blend with Compatibilizer KR20210137576A. KR20210137576A, 30 August 2020. [Google Scholar]
- Yuan, J.; Liu, X.; Akbulut, O.; Hu, J.; Suib, S.L.; Kong, J.; Stellacci, F. Superwetting Nanowire Membranes for Selective Absorption. Nat. Nanotechnol. 2008, 3, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Bayat, A.; Aghamiri, S.F.; Moheb, A.; Vakili-Nezhaad, G.R. Oil Spill Cleanup from Sea Water by Sorbent Materials. Chem. Eng. Technol. 2005, 28, 1525–1528. [Google Scholar] [CrossRef]
- Herkenberg, W. Method for the Removal of Oil from Oil Spills. U.S. Patent 5,451,325, 19 September 1995. [Google Scholar]
- Saleem, J.; Bazargan, A.; Barford, J.; McKay, G. Application of Strong Porous Polymer Sheets for Superior Oil Spill Recovery. Chem. Eng. Technol. 2015, 38, 482–488. [Google Scholar] [CrossRef]
- Saleem, J.; Ning, C.; Barford, J.; McKay, G. Combating Oil Spill Problem Using Plastic Waste. Waste Manag. 2015, 44, 34–38. [Google Scholar] [CrossRef]
- Kaimai, N.; Takita, K.; Kono, K.; Funaoka, H. Method of Producing Highly Permeable Microporous Polyolefin Membrane. U.S. Patent No. 6,153,133, 28 November 2000. [Google Scholar]
- Saleem, J.; McKay, G. Waste HDPE Bottles for Selective Oil Sorption. Asia-Pacific J. Chem. Eng. 2016, 11, 642–645. [Google Scholar] [CrossRef]
- Topuz, F.; Abdulhamid, M.A.; Szekely, G. Superoleophilic Oil-Adsorbing Membranes Based on Porous and Nonporous Fluorinated Polyimides for the Rapid Remediation of Oil Spills. Chem. Eng. J. 2022, 449, 137821. [Google Scholar] [CrossRef]
- Guselnikova, O.; Semyonov, O.; Kirgina, M.; Ivanov, A.; Zinoviev, A.; Postnikov, P. Polymer Waste Surgical Masks Decorated by Superhydrophobic Metal-Organic Frameworks towards Oil Spills Clean-Up. J. Environ. Chem. Eng. 2022, 10, 107105. [Google Scholar] [CrossRef]
- Topuz, F.; Oldal, D.G.; Szekely, G. Valorization of Polyethylene Terephthalate (PET) Plastic Wastes as Nanofibrous Membranes for Oil Removal: Sustainable Solution for Plastic Waste and Oil Pollution. Ind. Eng. Chem. Res. 2022, 61, 9077–9086. [Google Scholar] [CrossRef]
- Wunderlich, B. Thermal Analysis. Academic Press: Cambridge, MA, USA, 1990; pp. 417–431. [Google Scholar]
- Blaine, R.L. Thermal Applications Note: Polymer Heats of Fusion; TA Instruments: New Castle, DE, USA.
| Sorbate | Density (kg/m3) |
|---|---|
| Toluene | 867.0 |
| Sunflower oil | 918.8 |
| Paraffin oil | 800.0 |
| Crude oil | 890.0 |
| Engine oil | 890.0 |
| SN | Porosity (%) | Temperature (°C) | Time (min) | Strength (MPa) |
|---|---|---|---|---|
| 1 | 78 | 25 | 0 | 1 ND |
| 2 | 73 | 150 | 5 | ND |
| 3 | 67 | 150 | 10 | 1 |
| 4 | 61 | 150 | 15 | 4 |
| 5 | 33 | 150 | 20 | 8 |
| 6 | 7 | 150 | 25 | 11 |
| 7 | <1 | 165 | 5 | 12 |
| Polymer | Enthalpy Change (J/g) | |
|---|---|---|
| Before Heat | After Heat | |
| HDPE | 83.8 | 91.4 |
| PP | 28.5 | 47.7 |
| HDPE-PP | 112.3 | 139.1 |
| Polymer | % Relative Crystallinity | % Change in Relative Crystallinity | |
|---|---|---|---|
| Before Heat | After Heat | ||
| HDPE | 57.2 | 62.4 | 5.2 |
| PP | 27.5 | 46.1 | 18.6 |
| HDPE-PP | 44.9 | 55.6 | 11.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, J.; Baig, M.Z.K.; Luyt, A.S.; Shakoor, R.A.; Mansour, S.; McKay, G. Reusable Macroporous Oil Sorbent Films from Plastic Wastes. Polymers 2022, 14, 4867. https://doi.org/10.3390/polym14224867
Saleem J, Baig MZK, Luyt AS, Shakoor RA, Mansour S, McKay G. Reusable Macroporous Oil Sorbent Films from Plastic Wastes. Polymers. 2022; 14(22):4867. https://doi.org/10.3390/polym14224867
Chicago/Turabian StyleSaleem, Junaid, Moghal Zubair Khalid Baig, Adriaan Stephanus Luyt, Rana Abdul Shakoor, Said Mansour, and Gordon McKay. 2022. "Reusable Macroporous Oil Sorbent Films from Plastic Wastes" Polymers 14, no. 22: 4867. https://doi.org/10.3390/polym14224867
APA StyleSaleem, J., Baig, M. Z. K., Luyt, A. S., Shakoor, R. A., Mansour, S., & McKay, G. (2022). Reusable Macroporous Oil Sorbent Films from Plastic Wastes. Polymers, 14(22), 4867. https://doi.org/10.3390/polym14224867








