Collagen Obtained from Leather Production Waste Provides Suitable Gels for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Collagen Extraction
2.3. The Content of Total Nitrogen
2.4. Determination of Moisture Content
2.5. Mineral Content
2.6. Calcium Hydroxide Content
2.7. Measurement of the Total Protein Content by the Biuret Method
2.8. Measurement of the Total Protein Content by the Bradford Method
2.9. Ion Exchange Liquid Column Chromatography of Amino Acids
2.10. Cell Adhesion on Different Collagen Coatings
2.11. Statistical Data Analysis
3. Results and Discussion
3.1. General Composition of the Starting Material
3.2. Stages of Collagen Extraction from Wastes Samples
3.3. Identification and Amino Acid Composition of the Collagen Extracts
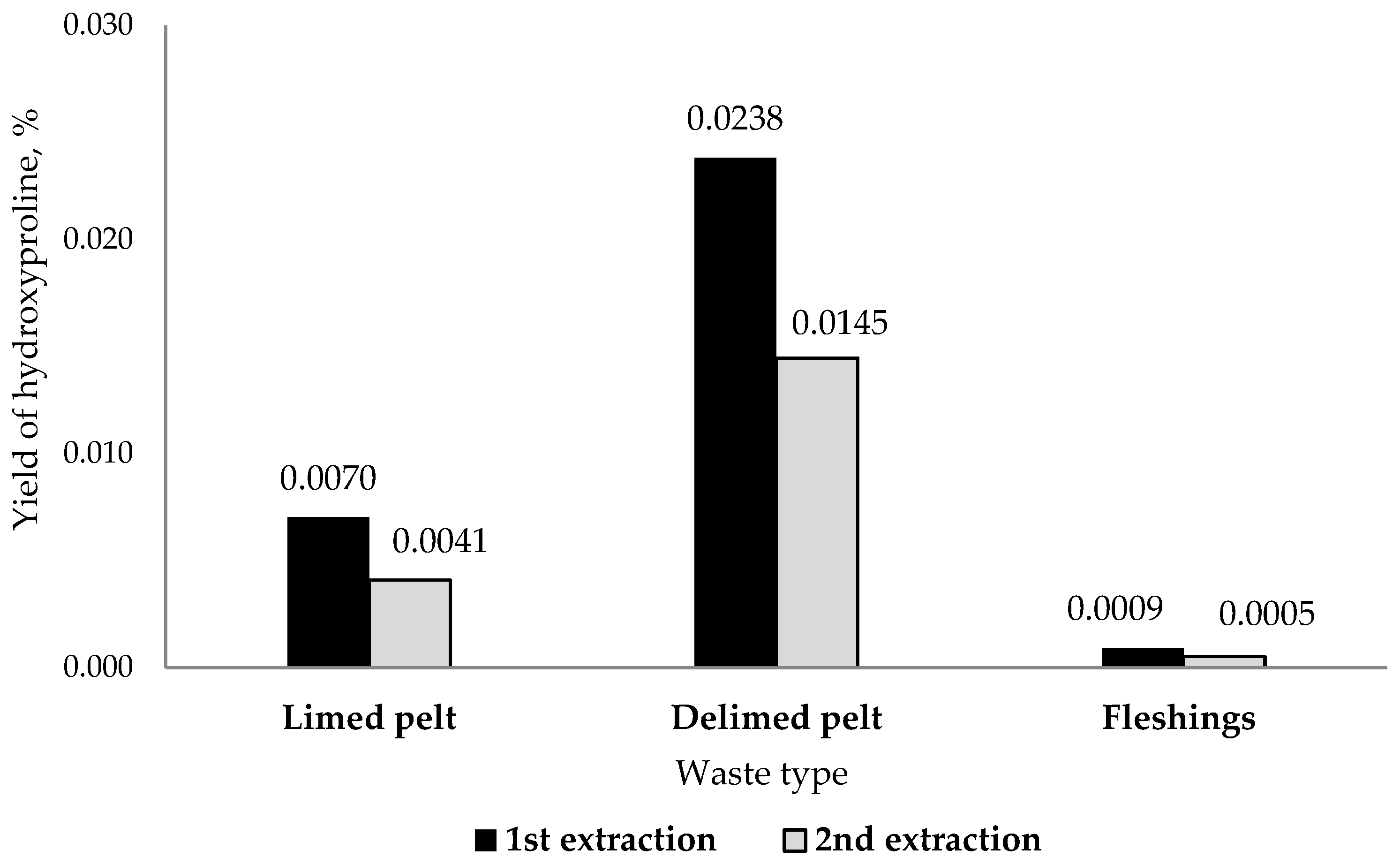
3.4. Collagen Extraction Efficiency and Yield Estimates
3.5. Collagen Gel Formation
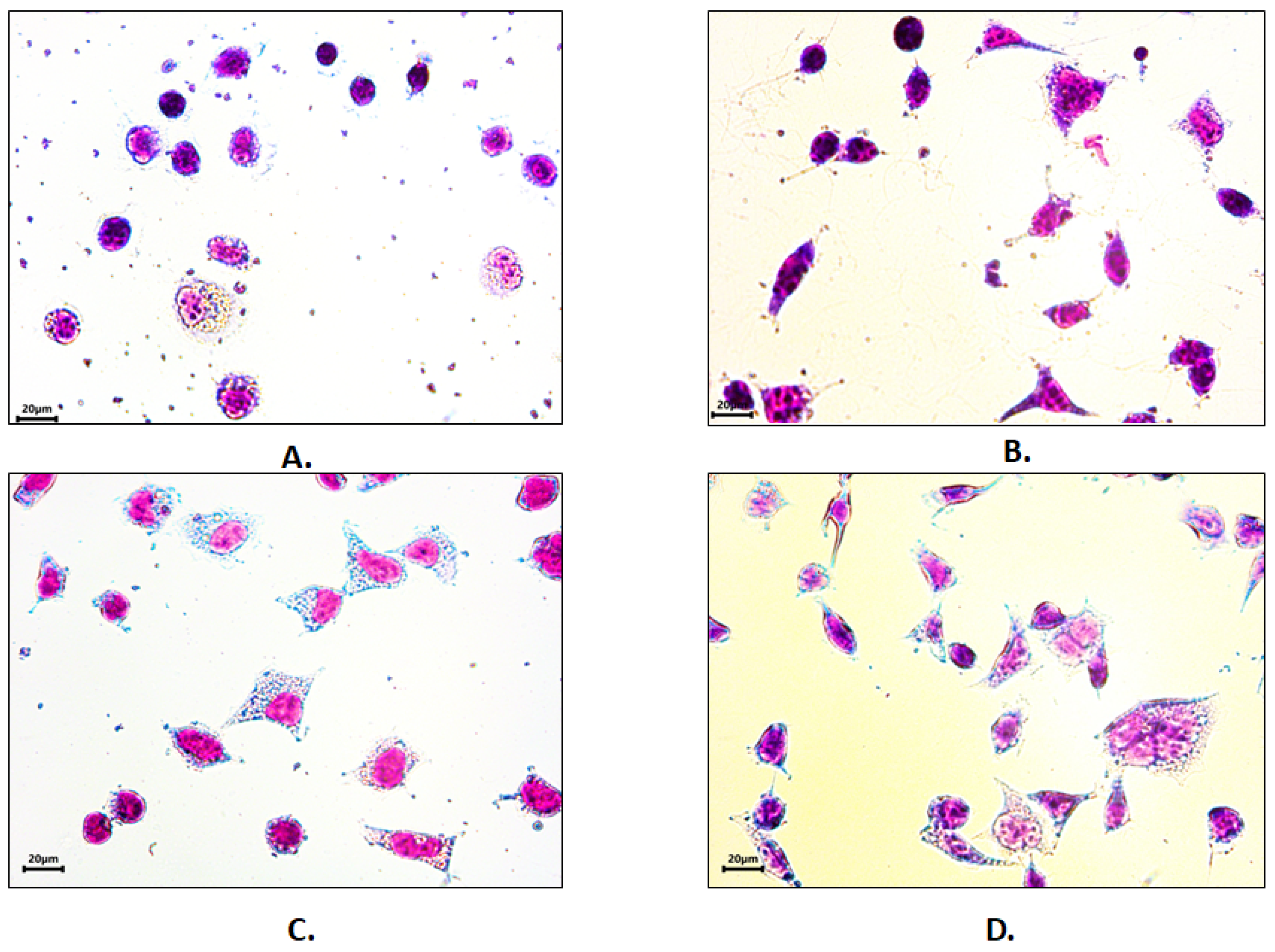
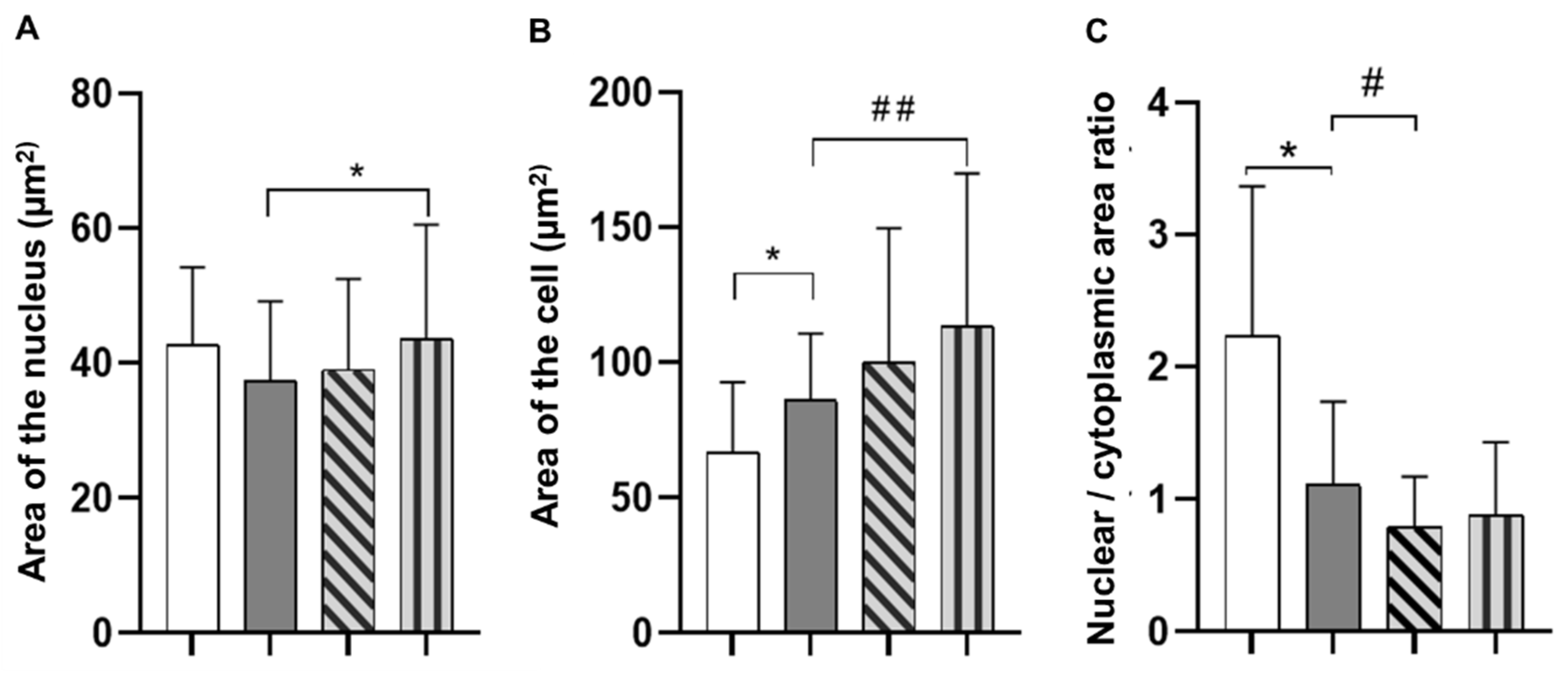
3.6. Cell Adhesion
3.7. Advantages and Limitations of the Proposed Extraction Method
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bailey, A.J.; Paul, R.G. Collagen: A not so simple protein. J. Soc. Leather Technol. Chem. 1998, 82, 104–110. [Google Scholar]
- Birk, D.E.; Bruckner, P. Collagen suprastructures. Top. Curr. Chem. 2005, 24, 185–205. [Google Scholar]
- Sarmad, N.; Sikarwar, A. Collagen: New dimension in cosmetic and healthcare. Int. J. Biochem. Res. Rev. 2016, 14, 1–8. [Google Scholar] [CrossRef]
- Reilly, D.M.; Lozano, J. Skin collagen through the lifestages: Importance for skin health and beauty. Plast. Aesthetic Res. 2021, 8, 2. [Google Scholar] [CrossRef]
- Elango, J.; Hou, C.; Bao, B.; Wang, S.; Maté Sánchez de Val, J.E.; Wenhui, W. The Molecular Interaction of Collagen with Cell Receptors for Biological Function. Polymers 2022, 14, 876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Elango, J.; Wang, S.; Hou, C.; Miao, M.; Li, J.; Na, L.; Wu, W. Characterization of Immunogenicity Associated with the Biocompatibility of Type I Collagen from Tilapia Fish Skin. Polymers 2022, 14, 2300. [Google Scholar] [CrossRef]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish collagen: Extraction, characterization, and applications for biomaterials engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef]
- Ahmed, M.; Verma, A.K.; Patel, R. Collagen extraction and recent biological activities of collagen peptides derived from sea-food waste: A review. Sustain. Chem. Pharm. 2020, 18, 100315. [Google Scholar] [CrossRef]
- Ehrlich, H.P.; Gabbiani, G.; Meda, P. Cell coupling modulates the contraction of fibroblast-populated collagen lattices. J. Cell. Physiol. 2000, 184, 86–92. [Google Scholar] [CrossRef]
- Bell, E. Organotypic and Histiotypic Models of Engineered Tissues. In Principles of Tissue Engineering, 2nd ed.; Lanza, R., Langer, R., Vacanti, J., Eds.; Academic Press: San Diego, CA, USA, 2000; pp. 181–193. [Google Scholar]
- Kanagaraj, J.; Velappan, K.C.; Chandra Babuand, N.K.; Sadulla, S. Solid wastes generation in the leather industry and its utilization for cleaner environment. J. Sci. Ind. Res. 2006, 65, 541–548. [Google Scholar] [CrossRef]
- Waller, J.M.; Maibach, H.I. Age and skin structure and function, a quantitative approach (II): Protein, glycosaminoglycan, water, and lipid content and structure. Skin Res. Technol. 2006, 12, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Savchuk, O.; Raksha, N.; Ostapchenko, L.; Mokrousova, O.; Andreyeva, O. Extraction and Characterization of Collagen Obtained from Collagen-Containing Wastes of the Leather Industry. Solid State Phenom. 2017, 267, 172–176. [Google Scholar] [CrossRef]
- Li, G.Y.; Fukunaga, S.; Takenouchi, K.; Nakamura, F. Physiological and cell biological properties in vitro of collagen isolated from calf limed splits. J. Soc. Leather Technol. Chem. 2004, 88, 66–71. [Google Scholar]
- Kirk, P.L. Kjeldahl method for total nitrogen. Anal. Chem. 1950, 22, 354–358. [Google Scholar] [CrossRef]
- Sáez-Plaza, P.; Michałowski, T.; Navas, M.J.; Asuero, A.G.; Wybraniec, S. An overview of the Kjeldahl method of nitrogen determination. Part I. Early history, chemistry of the procedure, and titrimetric finish. Crit. Rev. Anal. Chem. 2013, 43, 178–223. [Google Scholar] [CrossRef]
- Zhou, P.; Regenstein, J.M. Determination of total protein content in gelatin solutions with the Lowry or Biuret assay. J. Food Sci. 2006, 71, C474–C479. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford method for protein quantitation. In The Protein Protocols Handbook, 2nd ed.; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 15–21. [Google Scholar]
- Bahadir Acikara, Ö. Ion-Exchange Chromatography and Its Applications. In Column Chromatography; Martin, D.F., Martin, B.B., Eds.; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Begemann, H.; Rastetter, J. Staining methods. In Atlas of Clinical Hematology; Begemann, H., Rastetter, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1979; pp. 9–23. [Google Scholar]
- Layfield, L.J.; Esebua, M.; Frazier, S.R.; Hammer, R.D.; Bivin, W.W.; Nguyen, V.; Ersoy, I.; Schmidt, R.L. Accuracy and Reproducibility of Nuclear/Cytoplasmic Ratio Assessments in Urinary Cytology Specimens. J. Diagn. Cytopathol. 2017, 45, 107–112. [Google Scholar] [CrossRef]
- Brown, A.M. A new software for carrying out one-way ANOVA post hoc tests. Comput. Methods Programs Biomed. 2005, 79, 89–95. [Google Scholar] [CrossRef]
- Maistrenko, L.; Iungin, O.; Savchuk, O.; Okhmat, O. Collagen matrices from leather industry wastes for biomedical application. In Proceedings of the 8th International Conference on Advanced Materials and Systems (ICAMS 2020), INCDTP-ICPI, Bucharest, Romania, 1–3 October 2020; pp. 195–200. [Google Scholar]
- Ignat’eva, N.Y.; Danilov, N.A.; Averkiev, S.V.; Obrezkova, M.V.; Lunin, V.V.; Sobol, E.N. Determination of hydroxyproline in tissues and the evaluation of the collagen content of the tissues. J. Anal. Chem. 2007, 62, 51–57. [Google Scholar] [CrossRef]
- Zoia, L.; Binda, A.; Cipolla, L.; Rivolta, I.; La Ferla, B. Binary Biocompatible CNC–Gelatine Hydrogel as 3D Scaffolds Suitable for Cell Culture Adhesion and Growth. Appl. Nano 2021, 2, 118–127. [Google Scholar] [CrossRef]
- Pulix, M.; Lukashchuk, V.; Smith, D.C.; Dickson, A.J. Molecular characterization of HEK293 cells as emerging versatile cell factories. Curr. Opin. Biotechnol. 2021, 71, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Zeltz, C.; Gullberg, D. The integrin–collagen connection—A glue for tissue repair? J. Cell Sci. 2016, 129, 653–664. [Google Scholar] [CrossRef] [PubMed]
| Step | Stage | Extraction | ||
|---|---|---|---|---|
| 1st | 2nd | 3rd | ||
| 1 | Deliming with ammonium sulphate 3% by weight of the samples, duration 1 h, temperature 38–40 ° C (only to obtain samples of delimed pelt) | + | – | – |
| 2 | Grinding samples to a size of 3 × 3 mm | + | – | – |
| 3 | Weighing | + | – | – |
| 4 | Rinsing in water at a temperature of 20 °C for 45 min with water change each 15 min | + | – | – |
| 5 | Extraction of non-collagen proteins with a 10% sodium chloride solution, 1 h of rotation on a shaker at 20 °C, then 22 h of rest at 4 °C and again 1 h of rotation on a shaker at 20 °C | + | – | – |
| 6 | Rinsing of the samples with distilled water at pH = 6.5 | + | – | – |
| 7 | Extraction of collagen with 0.5 M acetic acid solution in the presence of 5 mm EDTA in a ratio of 1:10 (weight: volume), 2 h of rotation on a shaker at 20 °C, then 20 h of rest at 4 °C and again 2 h of rotation at 20 °C | + | + * | + ** |
| 8 | Filtering through a paper filter. The crushed samples were used for further extraction steps. | + | + | + |
| 9 | Precipitation of collagen from the filtrate with dry sodium chloride for 24 h at 4 °C | + | + | + |
| 10 | Centrifugation for 30 min at 3000 rpm | + | + | + |
| 11 | Dissolution of precipitated collagen in minimal volume of 0.5 M acetic acid | + | + | + |
| 12 | Re-precipitation of collagen with dry sodium chloride (to a concentration of the latter in a solution of 0.9 M) for 24 h at 4 °C | + | + | + |
| 13 | Centrifugation for 30 min at 3000 rpm | + | + | + |
| 14 | Dissolution of precipitated collagen in minimal volume of 0.1 M acetic acid | + | + | + |
| Mass Fraction, % | ||||
|---|---|---|---|---|
| Waste Type | Moisture | Minerals * | Total Nitrogen * | Calcium Hydroxide * |
| Limed pelt | 82.1 ± 0.2 | 10.7 ± 0.2 | 14.3 ± 0.4 | 2.6 ± 0.2 |
| Delimed pelt | 80.4 ± 0.2 | 3.3 ± 0.2 | 15.0 ± 0.4 | 0.4 ± 0.2 |
| Fleshings | 84.2 ± 0.2 | 31.7 ± 0.2 | 5.4 ± 0.4 | 6.7 ± 0.2 |
| Group | Waste Type | Extraction | Volume of Collagen Solution, mL | Total Nitrogen, mg | Protein Amount (Biuret Method), mg |
|---|---|---|---|---|---|
| I | Limed pelt | 1st | 18.0 ± 0.90 | 7.6 ± 0.04 | 10,1 ± 0.10 |
| II | 2nd | 70.0 ± 3.50 | 39.2 ± 0.20 | 5.0 ± 0.05 | |
| III | 3rd | 16.0 ± 0.80 | 3.3 ± 0.02 | 2.2 ± 0.02 | |
| IV | Delimed pelt | 1st | 48.0 ± 2.40 | 50.4 ± 0.25 | 25.3 ± 0.03 |
| V | 2nd | 85.0 ± 4.25 | 71.4 ± 0.36 | 11.4 ± 0.11 | |
| VI | 3rd | 15.0 ± 0.75 | 1.0 ± 0.01 | 4.7 ± 0.05 | |
| VII | Fleshings | 1st | 12.5 ± 0.63 | 3.3 ± 0.02 | 3.0 ± 0.03 |
| VIII | 2nd | 5.0 ± 0.25 | 11.5 ± 0.06 | 1.4 ± 0.01 | |
| IX | 3rd | 2.5 ± 0.13 | 0.5 ± 0.01 | 0.6 ± 0.01 |
| Protein Concentration (mg/100 mL) | ||
|---|---|---|
| Group | Biuret Method | Bradford Method |
| I | 56.11 ± 0.56 | 66.97 ± 0.67 |
| II | 7.14 ± 0.07 | 13.66 ± 0.14 |
| IV | 52.71 ± 0.53 | 63.51 ± 0.64 |
| V | 13.41 ± 0.13 | 34.23 ± 0.34 |
| Amino Acid | Amount, mg | |||||
|---|---|---|---|---|---|---|
| I | II | IV | V | VII | VIII | |
| Hydroxylysine | 3.214 | 1.350 | 3.780 | 1.350 | 1.746 | 1.350 |
| Lysine | 12.746 | 10.950 | 19.467 | 12.167 | 11.536 | 12.167 |
| Histidine | 2.627 | 1.788 | 4.769 | 3.726 | 3.212 | 3.726 |
| Arginine | 14.500 | 8.286 | 33.143 | 10.357 | 7.142 | 10.357 |
| Hydroxyproline | 27.292 | 5.955 | 23.818 | 11.909 | 5.133 | 11.909 |
| Aspartic acid | 13.793 | 6.650 | 24.106 | 8.728 | 4.299 | 8.728 |
| Threonine | 5.785 | 1.436 | 8.207 | 5.129 | 1.769 | 5.129 |
| Serine | 10.244 | 3.134 | 14.104 | 6.269 | 2.026 | 6.269 |
| Glutamic acid | 30.531 | 10.608 | 48.495 | 13.639 | 5.225 | 13.639 |
| Proline | 32.583 | 3.594 | 43.125 | 21.563 | 6.196 | 21.563 |
| Glycine | 70.724 | 36.694 | 90.726 | 44.355 | 21.202 | 44.355 |
| Alanine | 29.000 | 11.908 | 48.261 | 18.803 | 5.403 | 18.803 |
| Cysteine | 3.333 | 1.429 | 7.143 | 2.857 | 1.231 | 2.857 |
| Valine | 5.408 | 0.975 | 3.900 | 1.950 | 0.924 | 1.950 |
| Methionine | 0.876 | 1.014 | 0.507 | 1.014 | 0.699 | 1.014 |
| Isoleucine | 3.388 | 2.113 | 5.634 | 2.817 | 1.214 | 2.817 |
| Leucine | 6.550 | 4.010 | 10.694 | 4.010 | 1.728 | 4.010 |
| Tyrosine | 1.885 | 2.105 | 4.209 | 2.105 | 0.907 | 2.105 |
| Phenylalanine | 5.030 | 2.324 | 5.810 | 2.905 | 1.502 | 2.905 |
| Total | 279.510 | 116.322 | 399.898 | 175.652 | 83.096 | 175.652 |
| Amino Acid | Amount, mg | |||||
| I | II | IV | V | VII | VIII | |
| Hydroxyproline | 27.292 | 5.955 | 23.818 | 11.909 | 5.133 | 11.909 |
| Hydroxylysine | 3.214 | 1.350 | 3.780 | 1.350 | 1.746 | 1.350 |
| Molarity, µM | ||||||
| Hydroxyproline | 208.1 | 45.41 | 181.6 | 90.82 | 39.14 | 11.909 |
| Hydroxylysine | 19.82 | 8.324 | 23.31 | 8.324 | 10.77 | 8.324 |
| Hyp/Hyl molar ratio | 10.49 | 5.45 | 7.79 | 10.91 | 3.63 | 1.43 |
| By-Product | Coarse Raw Materials of Cattle | Small Raw Materials of Cattle | ||
|---|---|---|---|---|
| % | kg/ton | % | kg/ton | |
| fleshings | 18.5–23.0 | 185–230 | 15–17 | 150–170 |
| pelt trimmings | 1.8–2.0 | 18–20 | 2–4 | 20–40 |
| pelt split trimmings | 13.5–14.0 | 135–140 | – | – |
| pelt split | 10–14 | 100–140 | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maistrenko, L.; Iungin, O.; Pikus, P.; Pokholenko, I.; Gorbatiuk, O.; Moshynets, O.; Okhmat, O.; Kolesnyk, T.; Potters, G.; Mokrousova, O. Collagen Obtained from Leather Production Waste Provides Suitable Gels for Biomedical Applications. Polymers 2022, 14, 4749. https://doi.org/10.3390/polym14214749
Maistrenko L, Iungin O, Pikus P, Pokholenko I, Gorbatiuk O, Moshynets O, Okhmat O, Kolesnyk T, Potters G, Mokrousova O. Collagen Obtained from Leather Production Waste Provides Suitable Gels for Biomedical Applications. Polymers. 2022; 14(21):4749. https://doi.org/10.3390/polym14214749
Chicago/Turabian StyleMaistrenko, Lesia, Olga Iungin, Polina Pikus, Ianina Pokholenko, Oksana Gorbatiuk, Olena Moshynets, Olena Okhmat, Tetiana Kolesnyk, Geert Potters, and Olena Mokrousova. 2022. "Collagen Obtained from Leather Production Waste Provides Suitable Gels for Biomedical Applications" Polymers 14, no. 21: 4749. https://doi.org/10.3390/polym14214749
APA StyleMaistrenko, L., Iungin, O., Pikus, P., Pokholenko, I., Gorbatiuk, O., Moshynets, O., Okhmat, O., Kolesnyk, T., Potters, G., & Mokrousova, O. (2022). Collagen Obtained from Leather Production Waste Provides Suitable Gels for Biomedical Applications. Polymers, 14(21), 4749. https://doi.org/10.3390/polym14214749







