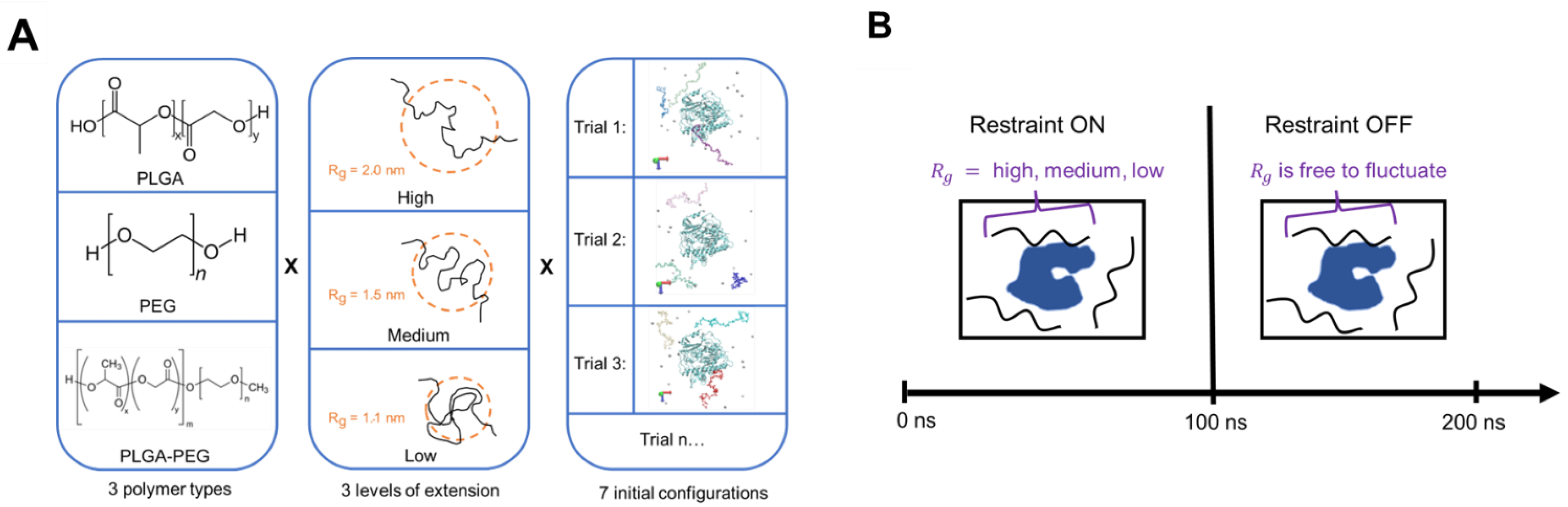
3.1. Copolymerization and Extension Effects on Protein–Polymer Interactions
Protein stability across both restraint phases was evaluated by extracting ID2S backbone RMSD from each simulation trial as a function of time and comparing that time series to ID2S in water only (
Figure A6). Across the three polymer types tested, RMSD fluctuations during all simulation trials were lower or similar to ID2S in water alone, showing that interactions between the protein and oligomers did not result in protein unfolding and that the simulated polymers primarily sample the ID2S surface. We visualized three of the four classes of interactions in
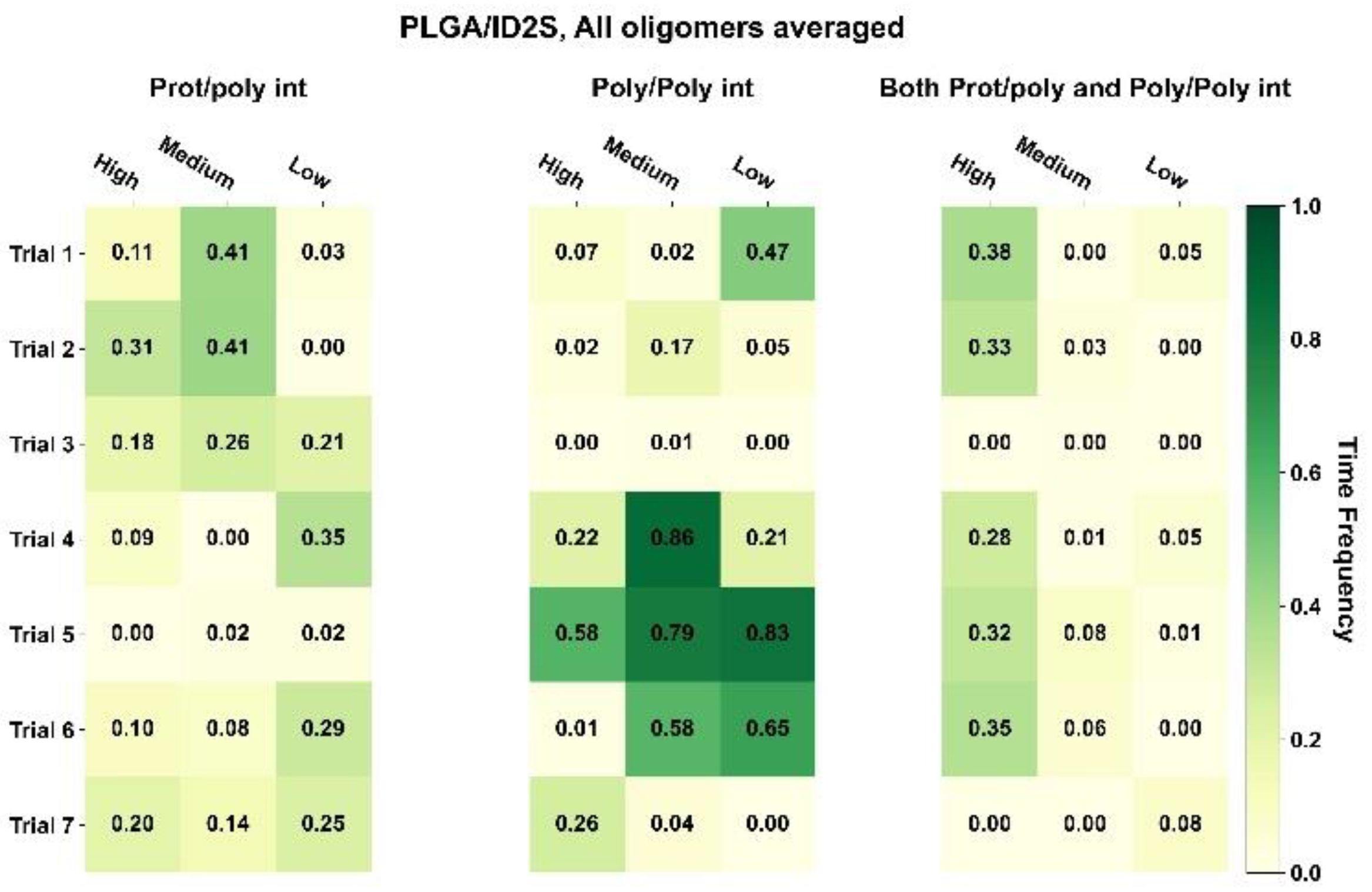
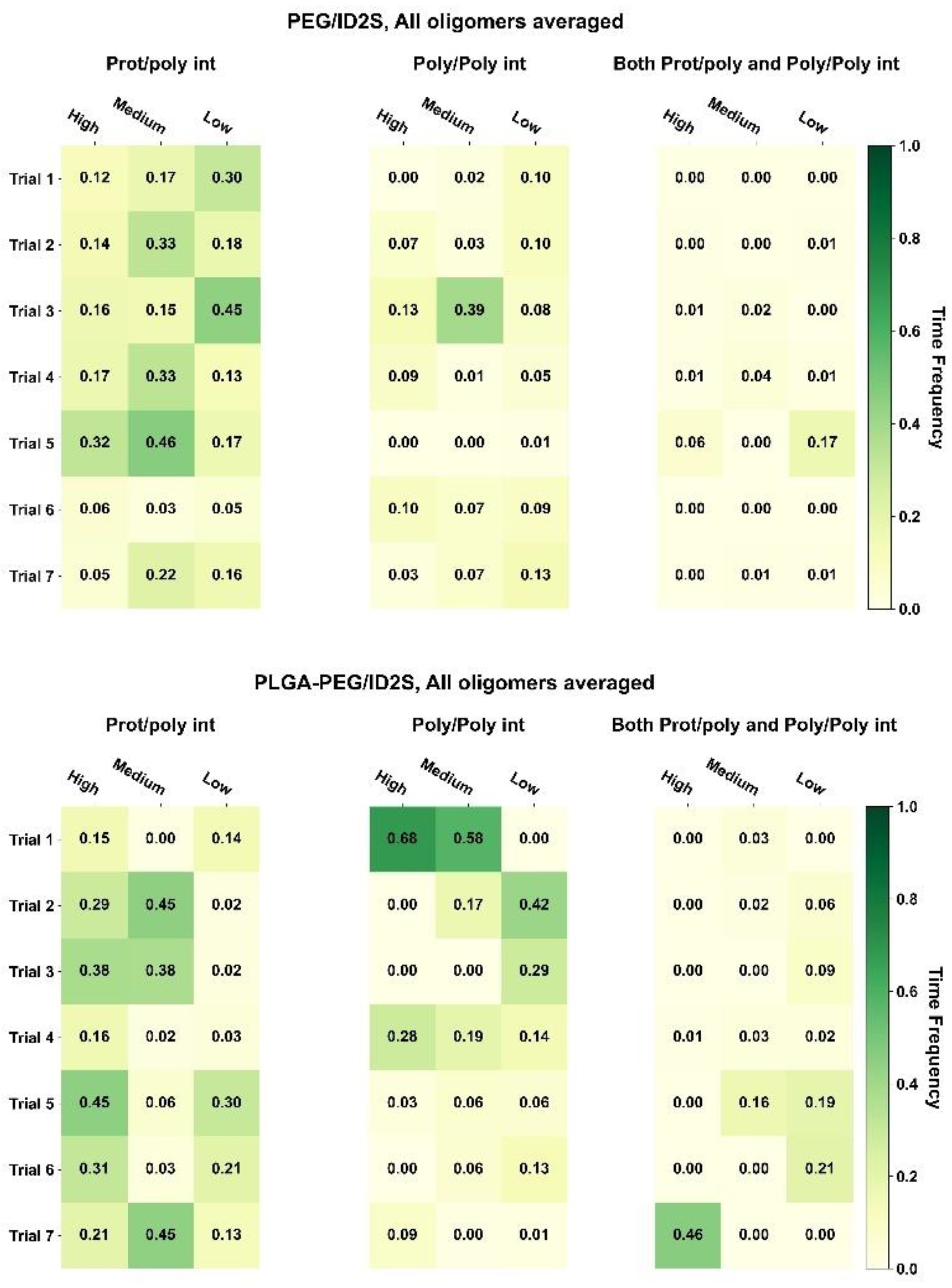
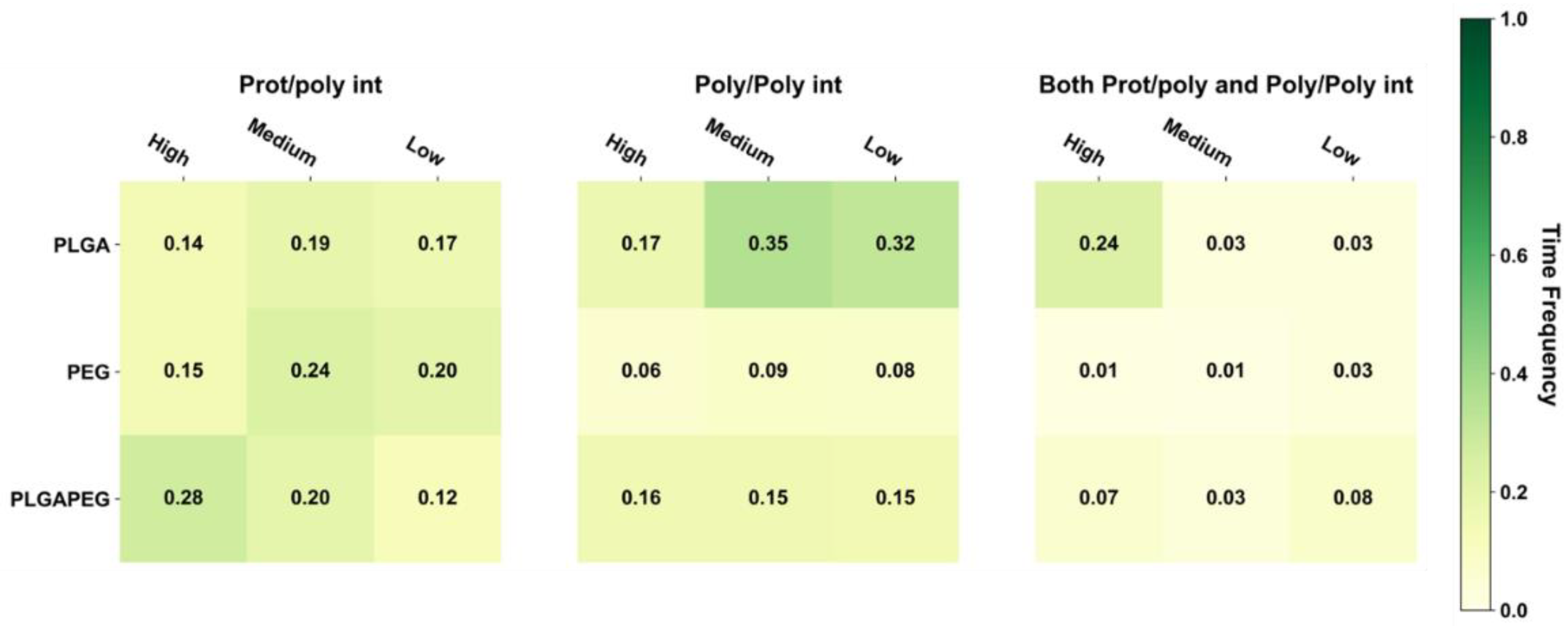
Figure 3, whereby the oligomer-averaged and time-averaged time frequency (
) for those three interaction classes can be seen for PLGA-PEG and its homopolymer constituents.
For PLGA/ID2S systems, poly–poly interactions occurred more often than prot–poly interactions for oligomers at medium and low levels of extension, whereas both prot–poly and poly–poly interactions were most frequent for the high extension systems. Moreover, prot–poly interactions only were still present for all levels of extension, showing that, in all system configurations, PLGA oligomers sampled the ID2S surface. An examination of TF across various configurations (
Figure A7) at high and medium levels of extension shows that three of the seven trials (trials 4–6) underwent significant poly–poly interactions, indicating that polymer oligomers primarily aggregated and were free in solution at medium extension levels. This is due to PLGA oligomers existing in poor solvent conditions, resulting in more dominant LGA-LGA interactions. At low extension levels, trials 4 and 6 showed the highest frequency of prot–poly interactions only when compared to other trials, with little to no frequency observed for both prot–poly and poly–poly interactions across the seven trials. This suggests that, most times, collapsed PLGA oligomers would either individually contact the protein or be aggregated together in solution at low extension levels. At medium extension levels, trials 1–3 had a higher frequency of prot–poly interactions when compared to the other two classes, despite the driving force of PLGA oligomer aggregation; the effect of multiple trials is seen for PLGA/ID2S systems since four of the seven simulations were still able to undergo prot–poly binding without the oligomers rapidly aggregating.
As for the PEG/ID2S trials, poly–poly and both prot–poly and poly–poly interactions were minimal, where trials 3 and 5 were responsible for the TF values in these interaction classes. A medium level of extension was found to have the largest averaged TF value for the prot–poly interaction class, resulting from four trials experiencing favorable binding. This observation may be explained by the PEG oligomers being in good solvent conditions. At high extension levels, polymer–solvent interactions can occur more easily, and are likely to be more favorable than prot–poly interactions. The ease in which polymer–solvent interactions can occur, however, is reduced at medium and low levels of extension since opportunities for hydrogen bonding is limited; collapsed PEG oligomers still interact with ID2S, but TF values across trials were below 0.3 (6 of 7 trials), indicating that, for PEG/ID2S systems, the solvent had an appreciable impact on prot–poly interactions. Lastly, PLGA-PEG/ID2S systems showed that copolymerization reduces the extent of poly–poly interactions; furthermore, trial systems with oligomers at high extension levels resulted in more frequent prot–poly interactions (
Figure A7), when compared to medium and low extension systems. Trials 5–7 had higher TF values for both prot–poly and poly–poly interactions, while trials 1–4 encountered more poly–poly interactions. Overall, increasing PLGA extension promoted more prot–poly interactions. When PLGA was copolymerized with PEG, poly–poly interactions were reduced, allowing favorable binding modes to be stabilized. During nanoparticle formulation, PLGA-PEG chains will have differing polymer blocks in their interaction with the solvent. This means that oligomers with collapsed LGA and extended EG domains may undergo less frequent protein contact, whereas extended LGA and EG domains result in more frequent protein contact.
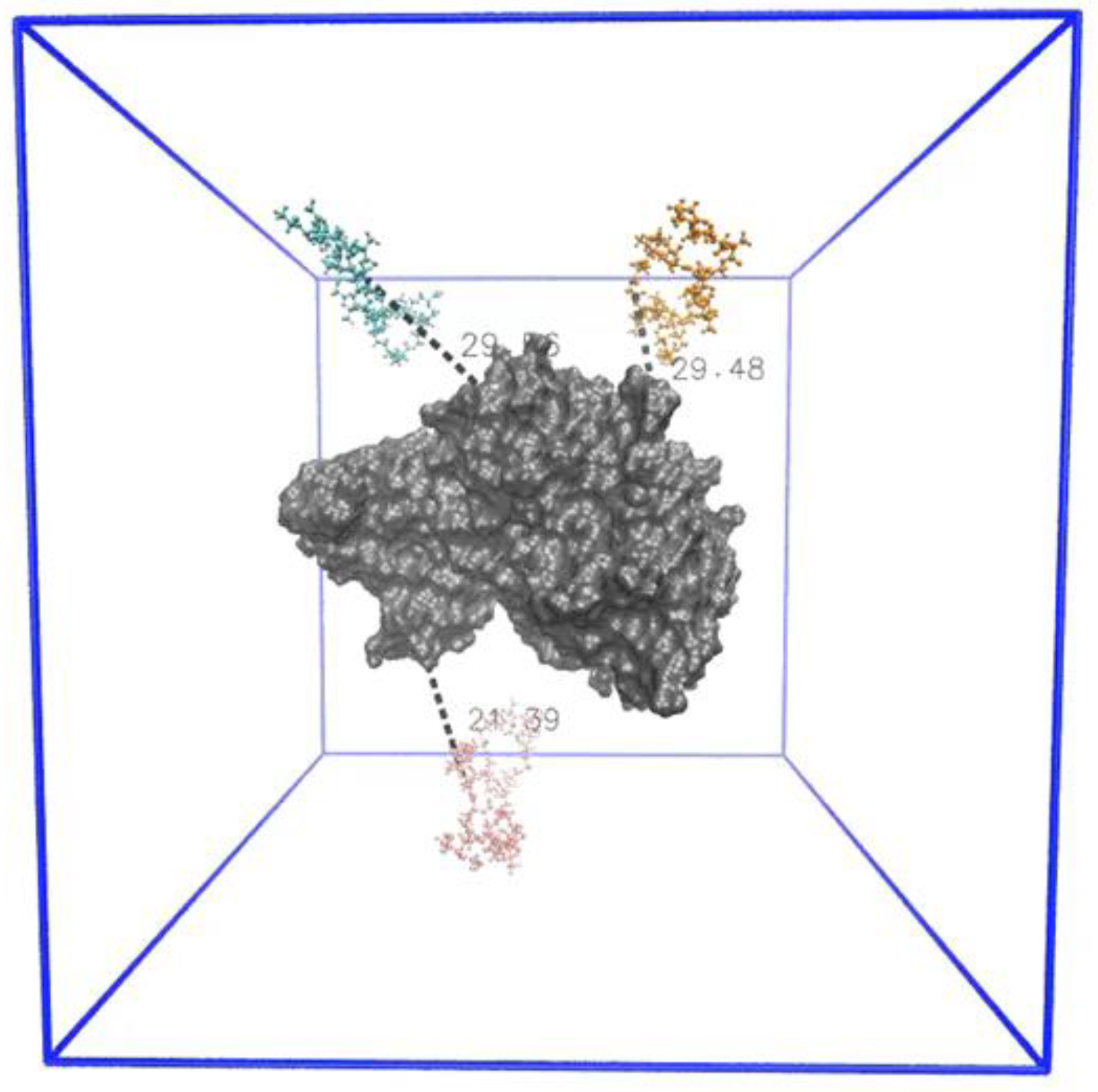
In order to understand how different levels of extension affects oligomer binding and extent of its interaction with ID2S, R
g time series and probability density data were extracted for each oligomer, as shown in
Figure 4 for select simulation trials. Furthermore, the four interaction classes were overlaid over the R
g time series data to examine the transience of protein–polymer binding when the restraint was on or off. For the high extension case, oligomers in PLGA/ID2S trial 2 were initially free in solution, and then two oligomers encountered the ID2S surface separately when the restraint was on. Once the restraint was off, poly–poly interactions started to occur between the oligomers, while they were in contact with ID2S. One oligomer underwent rapid collapse, due to being in poor contact with ID2S, while the other two took 40–60 ns to collapse to 1 nm in size. This slow collapse can also be seen in a broader distribution of the probability density for those oligomers. This behavior was also observed in other trials whereby favorable PLGA oligomer binding with ID2S in the restrained phase resulted in the slow decay of R
g when the restraint was turned off. In addition, poly–poly interactions in early simulation times impacted the frequency of prot–poly interactions since oligomer aggregation persisted in some trials during the restrained phase and subsequently collapsed together while contacting the protein or being free in solution.
PEG/ID2S trial 1 oligomers at high extension levels showed reversible protein binding in both restrained and unrestrained phases. Probability densities of the oligomers across all trials were broader when compared to PLGA/ID2S trials after the restraint was turned off, indicating their return back to ideal-chain conformations due to being in good solvent condition. Across PEG/ID2S trials, regardless of the extension level, polymer–polymer interactions were infrequent, and oligomers were mostly free in solution in both phases; TF values extracted across trials also confirmed this observation of PEG/ID2S binding (
Figure A7). Trial 5 at medium extension levels and trial 3 at low extension levels, however, exhibited irreversible binding between ID2S and one oligomer, showing that favorable PEG-ID2S interactions occur despite the strength of PEG–water interactions. Two oligomers in PLGA-PEG/ID2S trial 7 at high extension levels were mostly free and experienced transient prot–poly and poly–poly interactions in the restraint-ON phase, whereas one oligomer underwent irreversible binding. In the unrestrained phase, poly–poly interactions increased, while all three copolymer oligomers contacted the ID2S surface, resulting in a broader range of Rg values similar to the PEG/ID2S systems. Four trials showed irreversible binding between ID2S and at least one oligomer in the restrained phase and persisted in the unrestrained phase, with poly–poly interactions occurring less frequently than PLGA/ID2S systems. Overall, reducing polymer–polymer interactions between PLGA domains during the early stages of nanoparticle formation will likely be critical to promoting protein–polymer contact; however, strong polymer–solvent interactions would also not desired, as seen with the PEG/ID2S systems, because this leads to more reversible polymer binding events. Through PLGA copolymerization to PEG, poly–poly interactions were reduced, and more polymer–solvent interactions were able to occur, resulting in an increase in protein–polymer interactions.
As for the low extension case, PLGA oligomers stayed collapsed across all trials after the restraint was turned off, whereby the frequency of both prot–poly and poly–poly interactions occurring together was much lower when compared to other polymer/ID2S systems. Oligomer aggregation was persistent for some trials, but it did not prevent PLGA/ID2S systems from experiencing irreversible protein–polymer binding (trial 6 at low extension levels), as seen in three out of the seven trials. Moreover, collapsed oligomers did not always aggregate together and stayed in solution. On the contrary, PEG oligomers expanded their structure in the unrestrained phase across simulation trials, regardless of whether they underwent protein contact. Only trials 1 and 3 at low extension levels resulted in irreversible ID2S binding when the restraint was on, likely due to PEG oligomers being free in solution with minimal polymer–polymer interactions occurring. Most protein–polymer binding occurred in the unrestrained phase, since the oligomers exhibit more expanded conformations, as seen with the broader Rg probability density distribution. PLGA-PEG/ID2S systems at low extension levels encountered the lowest frequency of protein–polymer interactions across trials, whereby trials 5 and 6 contained oligomers that underwent irreversible binding events. Most oligomers were free in solution and underwent transient prot–poly and poly–poly interactions but, in the unrestrained phase, they did not expand to a great extent and stayed relatively collapsed, similar to PLGA/ID2S systems.
In the medium extension case, oligomers in three PLGA/ID2S trials experienced protein binding but, in other trials, they were mostly dominated by poly–poly interactions or were free in solution. Across all trials, oligomers’ Rg decreased quickly to values representative of a collapsed state in the unrestrained phase, even if irreversible binding occurred. In trial 2, however, one oligomer irreversibly bound to ID2S and stayed at the same Rg value even after the restraint was turned off, confirming that favorable protein–polymer binding modes are dependent on a polymeric chain’s level of expansion. PEG/ID2S systems at medium extension levels showed oligomers with more frequent prot–poly interactions only in both the restrained and unrestrained phases across trials, in addition to a slight drop in Rg and broader Rg distribution in the last 100 ns. A low frequency of prot–poly interactions was observed across four PLGA-PEG/ID2S trials, with oligomers being free in solution or undergoing poly–poly interactions. Interestingly, oligomer binding, similar to PLGA/ID2S trial 2, was also observed with PLGA-PEG/ID2S trial 2, whereby an oligomer stayed near the same Rg as it was in the restrained phase. Furthermore, some copolymer oligomers in the unrestrained phase did undergo collapse but this was not always the case across trials, unlike the PLGA/ID2S systems; some oligomer Rg probability density distributions were similar to those of PEG oligomers at medium extension levels. At the simulated contour lengths used for this study, a medium level of extension, for PEG/ID2S systems, seemed to promote the most frequent prot–poly interactions when the restraint was on; for PLGA/ID2S and PLGA-PEG/ID2S systems, binding events without a drastic collapse in the oligomer structure were observed, indicating that these interactions at a medium level of extension are highly favorable and can overcome dominant LGA self-interactions in the unrestrained phase.
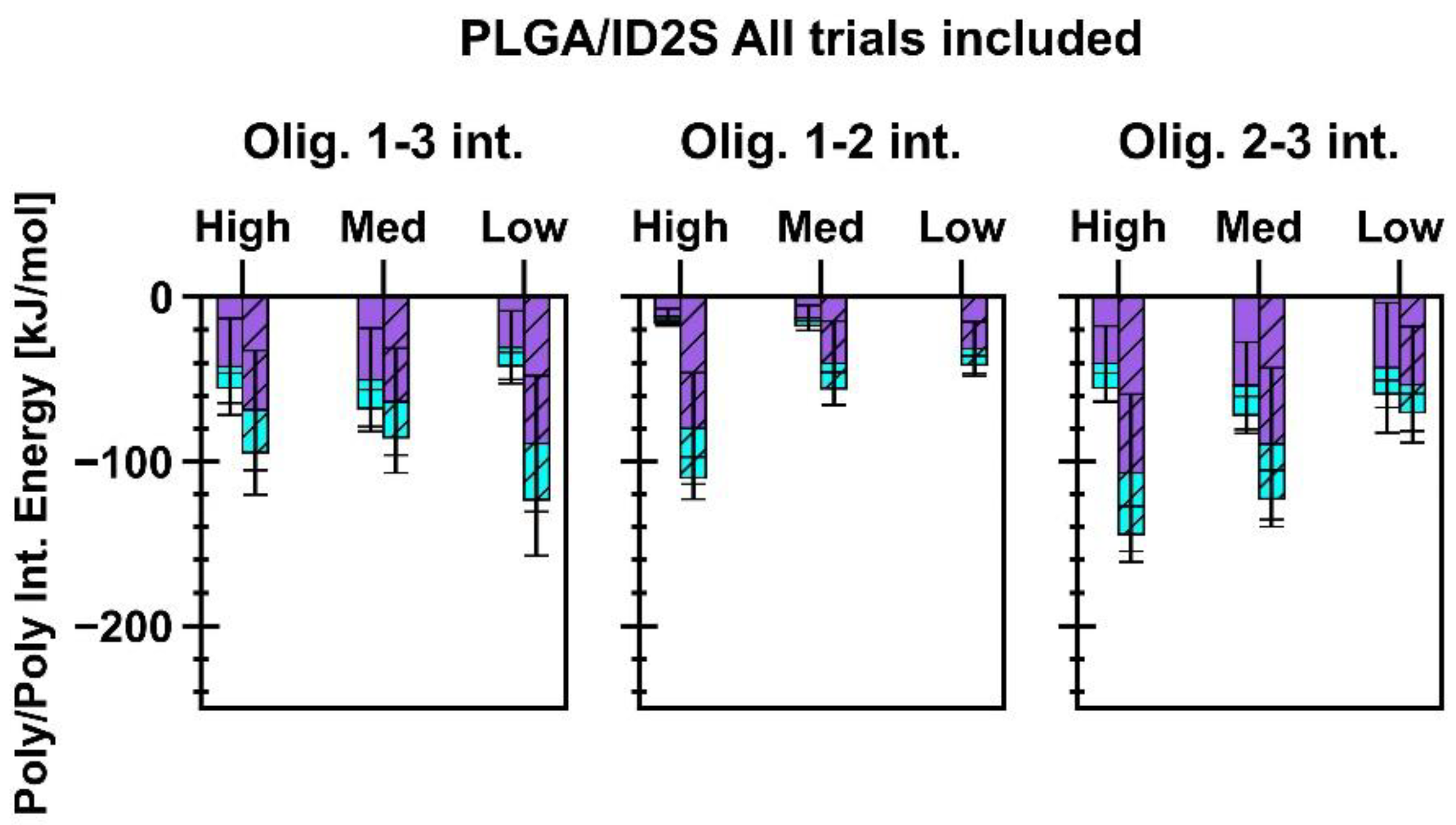
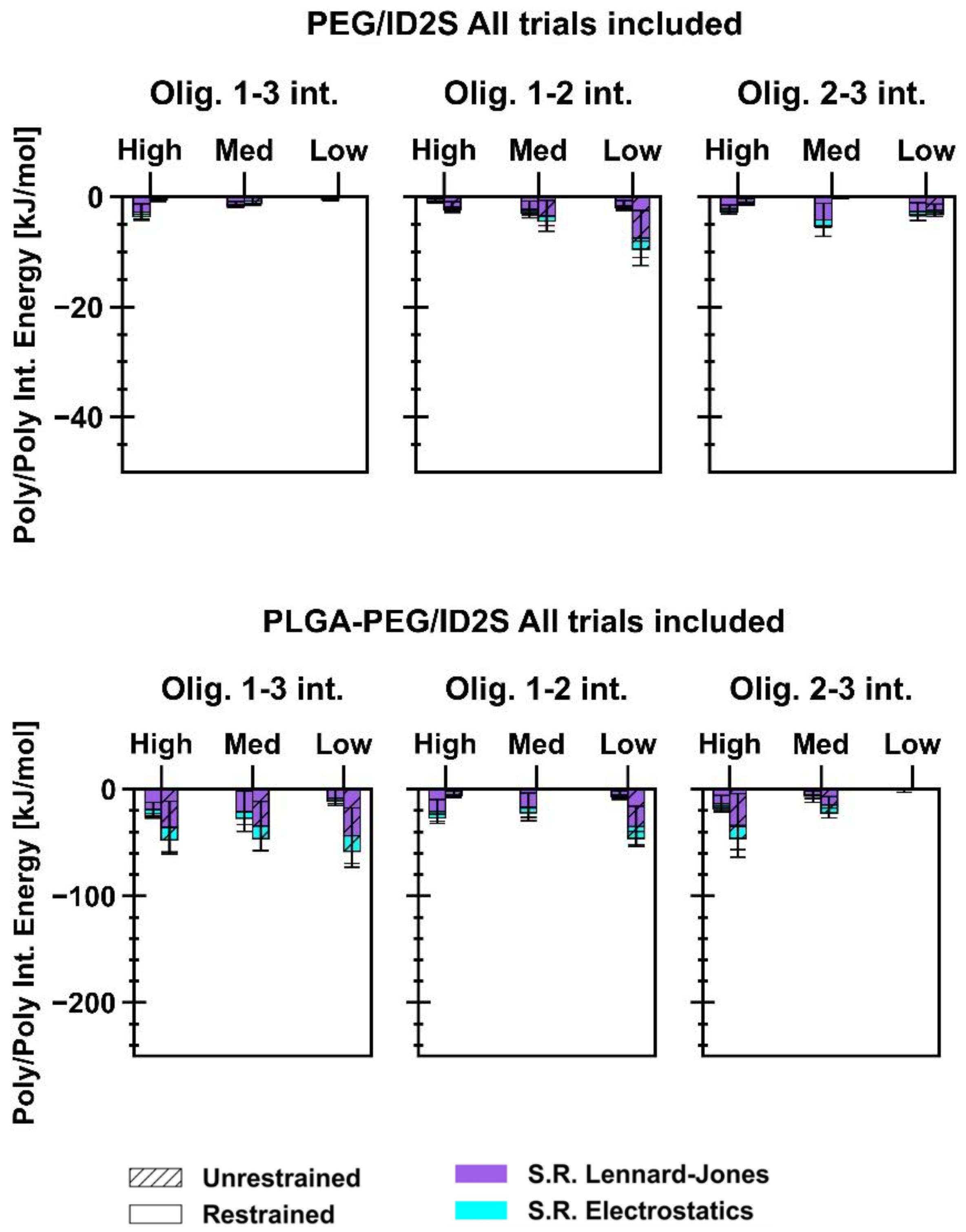
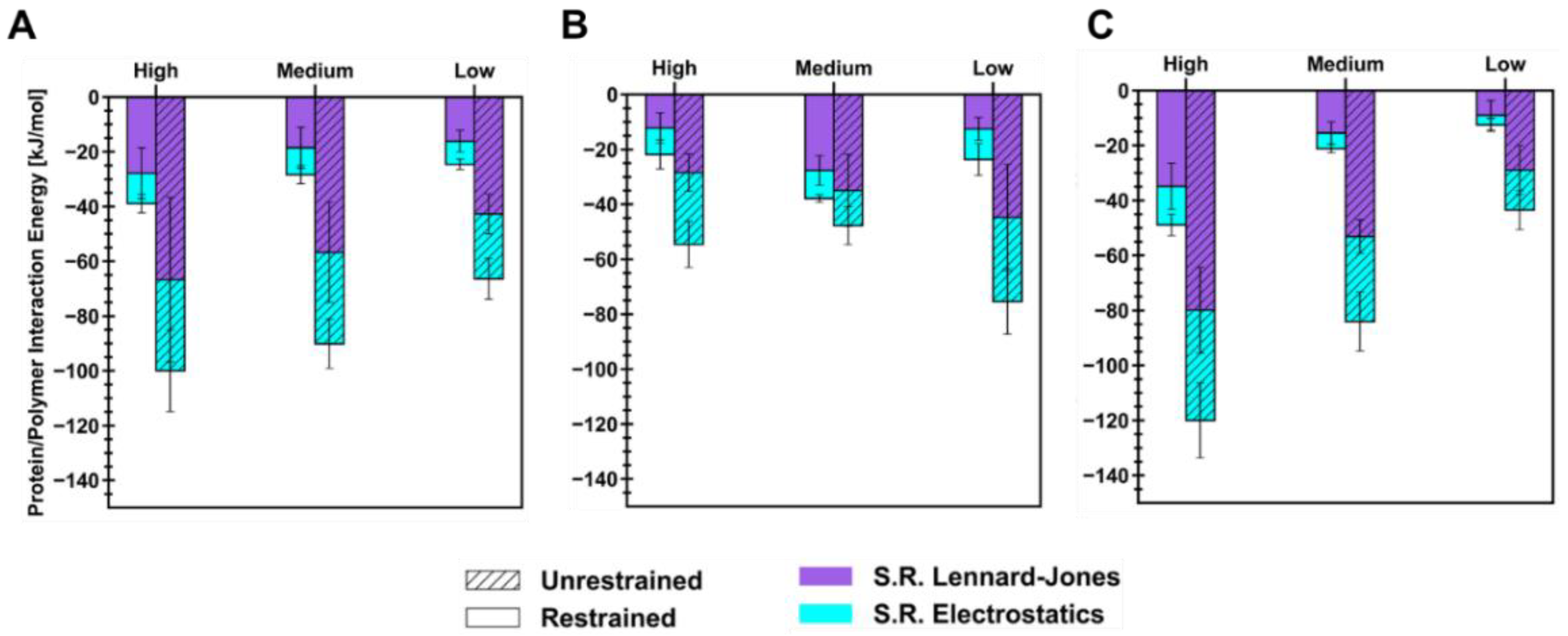
Energetics of short-range (SR) non-bonded interactions were extracted from polymer/ID2S simulations to examine the extent of favorable binding between the protein and oligomers, in addition to evaluating oligomer–oligomer behavior. As seen in
Figure 5, the total interaction energy (E
int) across different levels of extension for each polymer type increased after the restraint was turned off, but the magnitude of its increase was seen to be dependent on the extension of the polymer in the restrained phase. This increase can be attributed to the polymer oligomers which interact with more ID2S surface residues, while attempting to reach their equilibrium conformations when dissolved in pure water. For the PLGA/ID2S simulations, a linear decrease in the mean E
int was observed as the set oligomer conformation shifted from high to low, with the high extension case showing the most favorable protein–polymer binding. SR Lennard–Jones interaction energies were larger than SR electrostatics across the different levels of extension in both phases, showing that dispersion forces were primarily responsible for PLGA binding. Polymer–polymer interaction energies (
Figure A8) were similar in magnitude to protein–polymer interaction energies at high and medium extension levels, showing that expanded structures were important in aiding protein–polymer binding despite the strong attraction between PLGA oligomers. As for the PEG/ID2S systems, a significant increase in E
int was seen at high and low extension levels in the unrestrained phase, when compared to the medium level of the extension system with only a slight increase in E
int. Furthermore, a change in E
int in the unrestrained phase was observed to be the largest at the low level of extension, whereas for the high level of extension, E
int was similar in magnitude to the medium extension case. This suggests that collapsed PEG oligomers’ return to good solvent conditions, leading to an expansion of chain structure, and resulting in more favorable protein–polymer interactions, when compared to the other level of extension. Polymer–polymer interactions were minimally attractive in nature (
Figure A8), further confirming the prevalence of polymer–solvent interactions that drive the increase in R
g in the unrestrained phase for PEG oligomers in the low extension case, leading to an increase in more favorable protein–polymer interactions. For highly extended PEG chains, their conformations shifted to lower R
g values matching those seen for PEG oligomers in water at the given contour length, allowing them to interact with the ID2S surface at a similar magnitude as the medium level of extension.
The trend in E
int, observed for the PLGA/ID2S simulations, was the same for the PLGA-PEG/ID2S system. At the highest extension, protein–polymer interactions were most favorable in the restrained and unrestrained phases when compared to medium and low extension levels. The added effect of copolymerization was likely responsible for the observed differences since an expanded PLGA structure in the restrained phase, in addition to PEG domain being in good solvent conditions and PLGA relaxation around the ID2S surface in the unrestrained phase, resulted in overall stronger binding. Moreover, strong polymer–polymer interaction between copolymer oligomers were significantly reduced when compared to PLGA/ID2S systems (
Figure A8), further supporting the idea that the PEG domain mediates the extent of LGA interactions and allows for more favorable PLGA–protein binding. Such insights confirm the importance of choosing the right solvent when formulating protein-loaded PLGA-PEG nanoparticles because protein–polymer interactions are likely to be more favorable when PLGA and copolymer domains are highly extended prior to NP formation and growth. Furthermore, neutron scattering measurements of BSA-PEG aqueous mixtures by Abbott et al., showed that PEG had a slightly attractive interaction with BSA, despite having a net repulsive interaction [
43]. PEG oligomers have been observed to undergo transient binding with ID2S which is generally less favorable when compared to the other polymer types, corroborating their experimental results.
3.2. Characteristics of the Protein–Polymer Interface
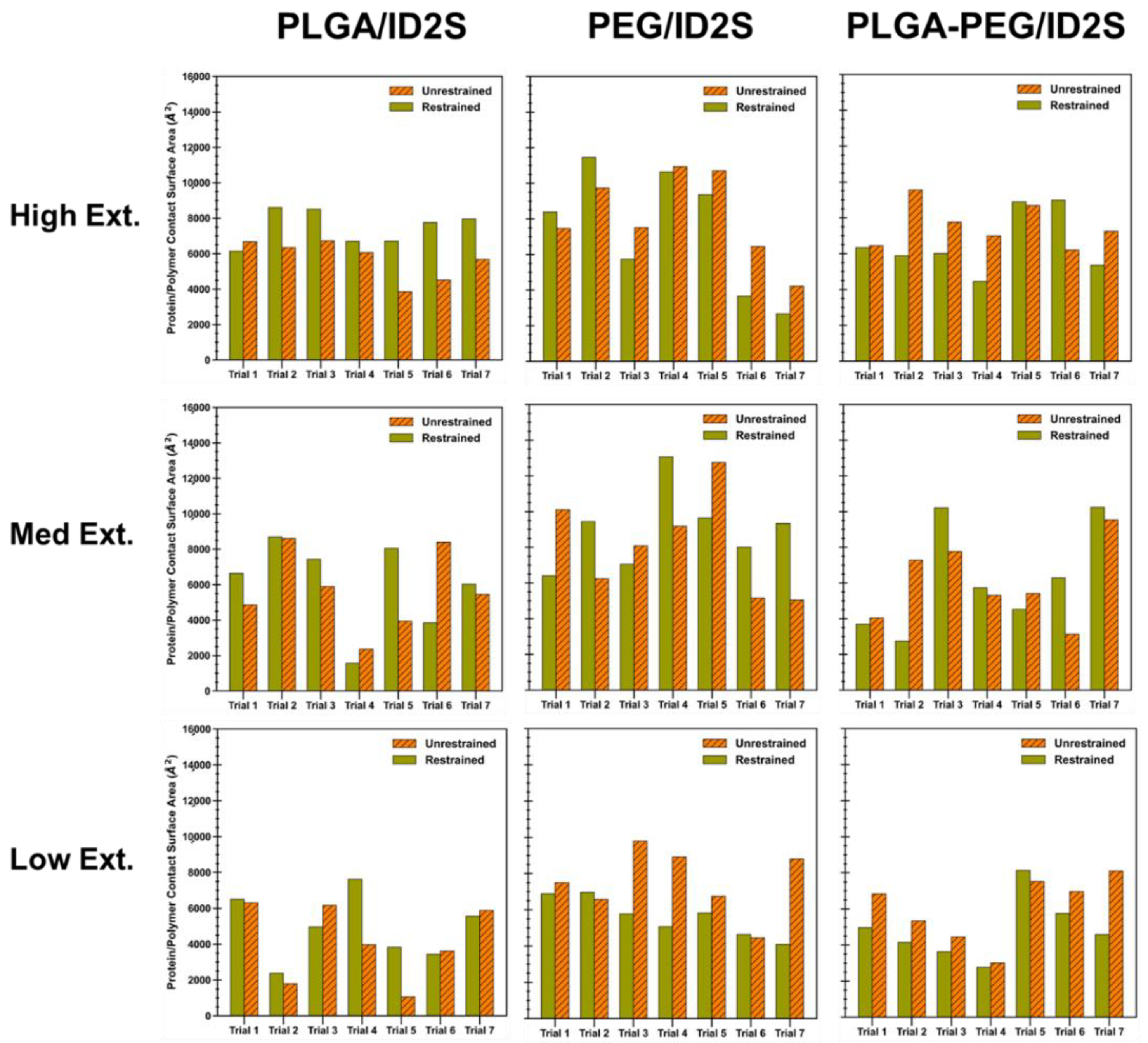
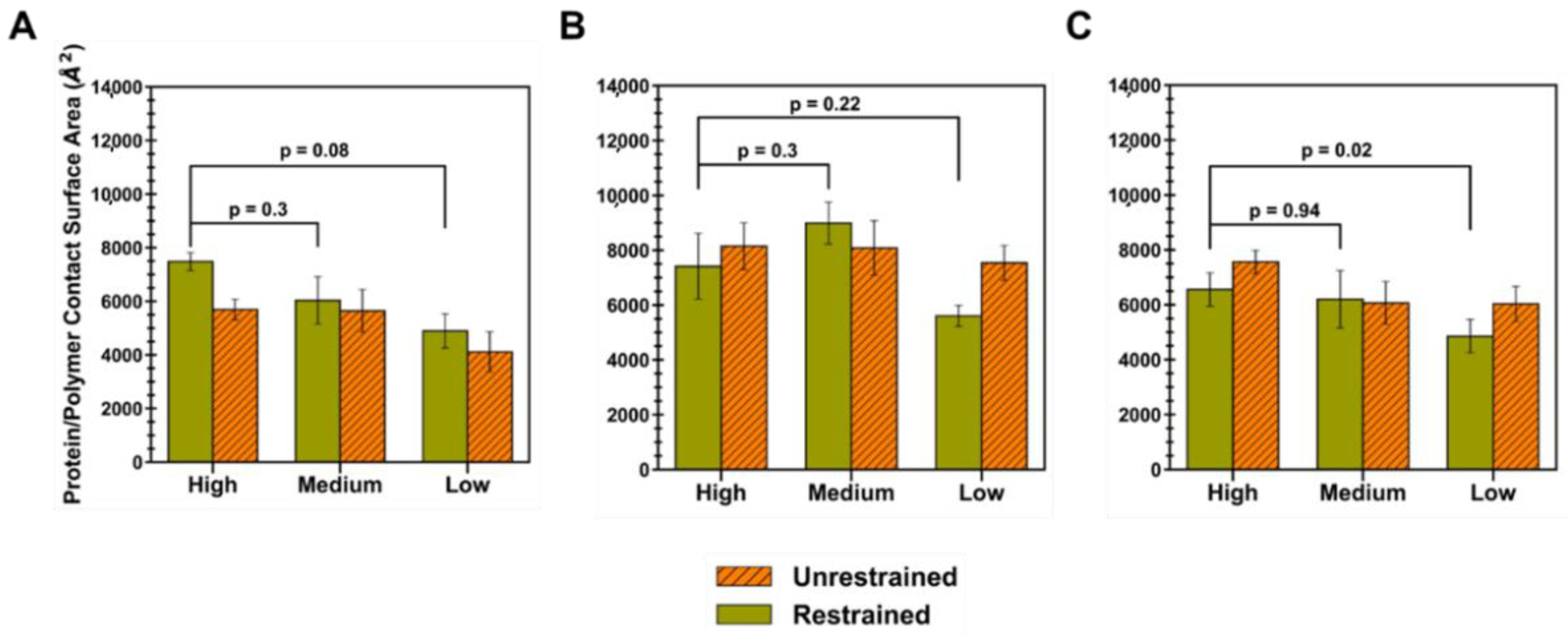
In order to understand how the mean interaction surface area varied across polymer types and levels of extension, the protein–polymer CSA was calculated for each simulation trial and averaged, for both the restraint-on and -off phases (
Figure 6). Briefly, the CSA is given by Equation (7), where the interface residues are defined by whether a surface residue has a non-zero occupancy value. Out of a total ID2S surface area of 20,438 Å
2, oligomers at high extension levels, for PLGA/ID2S systems, had the largest average CSA in the restraint-on phase, followed by medium and low extension systems; however, a drop in CSA was observed for the high extension case in the restraint-off phase and across simulation trials, likely due to dominant LGA interactions causing oligomers to exhibit a more semi-collapsed conformation on the ID2S surface. For the restrained phases, the CSA for the high extension case was nearly statistically significant (
p = 0.08) when compared to the low extension case. A negligible change in CSA was observed for the medium extension case, while a slight drop in CSA was seen for the low extension in the restraint-off phase. The number of trials with CSA values greater than 8000 Å
2 decreased from three in the medium extension case to zero in the low extension case (
Figure A9), showing that expanded PLGA chain conformation results in a larger protein–polymer contact surface area. As for the PEG/ID2S systems, a medium extension of PEG oligomers led to the largest average CSA, when compared to other extension cases. As the restraint is turned off, PEG oligomers contact more of the ID2S surface, as seen with the slight rise in CSA for the high extension case and a greater CSA increase for the low extension case; this was also observed across multiple trials, with the medium extension case in the restraint-on phase resulting in trials with the largest CSA values (>8000 Å
2) compared to the other polymer types.
For the PLGA-PEG/ID2S systems, high and medium extension cases showed similar CSA values (p = 0.94), while the low extension case had a statistically significant lower CSA (p = 0.02) when compared to the high extension case in the restrained phase. A majority of trials at high and medium extension levels caused CSA increases in protein–polymer interfaces in the unrestrained phase, but the overall magnitude of the CSA at low extension levels was much lower in both phases when compared to high extension levels. Furthermore, less contact was observed as copolymer oligomers went from medium to low extension levels, similar to the PLGA/ID2S systems. This shows that PLGA-PEG chain expansion in good solvent conditions should allow for greater and more favorable contact with the protein. Moreover, PEG chains moving from a collapsed to semi-extended state resulted in larger protein–polymer contact that was less favorable when compared to PLGA domains. In the context of protein release from a PLGA-PEG nanoparticle in an aqueous environment, PLGA domain collapse in nanoparticle regions where water absorption was significant and LGA interactions were dominant. In addition, PEG–protein interactions were likely not favorable enough to prevent the protein drug from diffusing out of the nanoparticle, reducing the therapeutic efficacy of the delivery vehicle. In addition, protein interactions with collapsed LGA domains would be weak and occur less frequently with a small CSA, causing further drug leakage from the nanocarrier as water diffuses into its matrix. Future drug release studies should consider the relaxation time of the PLGA-PEG polymer at different molecular weights in solvent mediums of interest since a fast transition from a highly extended to collapsed conformation during nanoparticle formation or during drug release could have a critical impact on protein–polymer interactions, which in turn influences protein loading and in vivo release profiles.
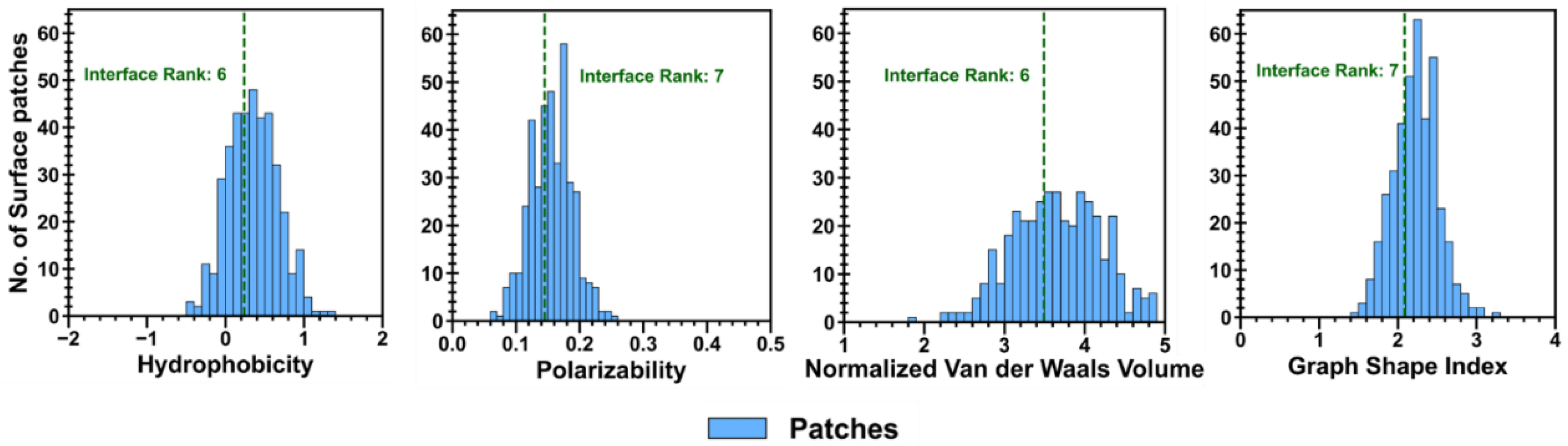
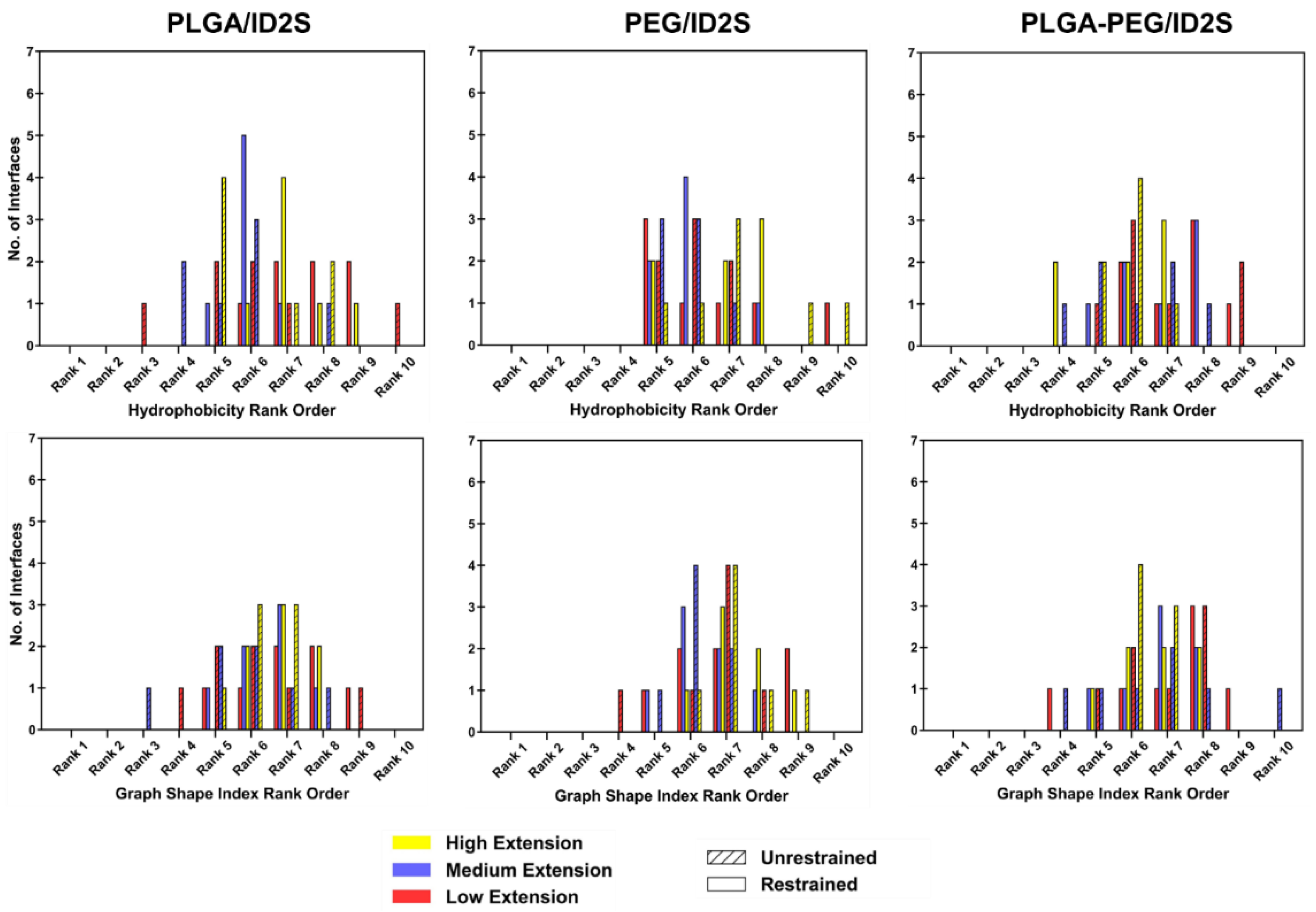
Physiochemical descriptors of the protein–polymer interfaces, used to calculate CSA for each simulation trial, were ranked relative to those properties for all surface patches to examine how their chemical nature shifts across polymer types. Hydrophobicity, polarizability, normalized van der Waals volume, and the graph shape index of all surface patches were distributed between −0.8–1.4, 0–0.26, 2–5, and 1.4–3.4, respectively, as shown in
Figure A5. The graph shape index interface ranks across polymer types and levels of extension were between ranks 4 and 10, with no major discernable trends (
Figure A10). As for the hydrophobicity interface rank distribution, most PLGA/ID2S interfaces from the restrained phase at high extension levels had a rank of 7 (hydrophobicity ~0.10), which then shifted to rank 5 (hydrophobicity ~0.16) in the unrestrained phase. This shows that PLGA collapse around the ID2S surface results in the interaction with hydrophobic residues, resulting in a more hydrophobic interface. For the low extension case for both restraint-on and -off phases, a broad interface rank distribution (ranks 3–10) was observed, while a slightly narrower spread (ranks 4–7) was seen for the medium extension case. PEG/ID2S (ranks 5–8) and PLGA-PEG/ID2S (ranks 4–9) interfaces also showed the same widespread expression and did not show any major differences across the levels of extension.
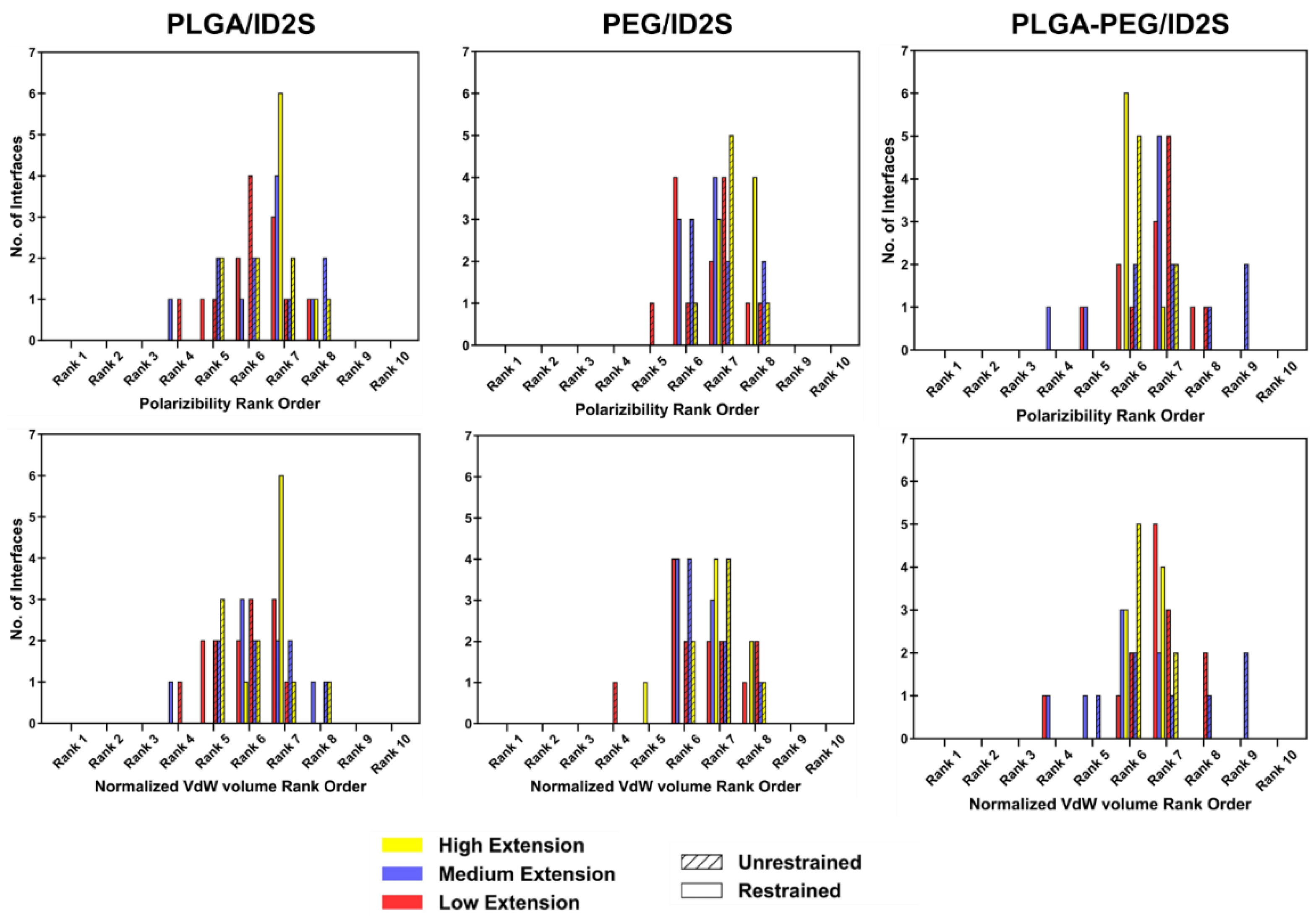
Polarizability and normalized van der Waals volume (
Figure A11), on the other hand, were more valuable in understanding variations in the physiochemical properties of the protein–polymer interface. At high extension levels, the PLGA/ID2S interface ranks for polarizability were centered at ranks 7–8 (polarizability ~0.14) in the restrained phase but shifted to a broader rank spread (ranks 4–8) in the unrestrained phase, indicating that oligomers also interacted with more polarizable residues during their contraction around ID2S. At low and medium levels of extension, polarizability ranks were between 4 and 8, with a peak at ranks 6–7. For PEG/ID2S interfaces, polarizability ranks were centered between ranks 6 and 8 across different extension levels, whereas for the PLGA-PEG/ID2S interfaces, polarizability ranks at high extension fell mostly on rank 6 in the restrained phase but shifted to ranks 3–10 in the unrestrained phase, similar in trend to PLGA/ID2S interface ranks; this could be due to PEG domains interacting with more ID2S surface residues in some simulation trials after the restraint is off. Lastly, normalized van der Waals volume parameter ranking for PLGA/ID2S at high extension levels showed six out of seven interfaces, from the restrained phase, which had a rank of 7 (a normalized van der Waals volume of ~3.3), whereas some interfaces from the unrestrained phases changed to ranks 5–6; such differences indicate that the PLGA oligomers contact more residues with a slightly larger normalized van der Waals volume when released from the restraint. At low and medium extension levels, normalized van der Waals volume ranks were distributed between 4 and 8, regardless of restraint-on or -off phases. PEG/ID2S (ranks 4–8) and PLGA-PEG (ranks 4–9) interfaces shows a similar spread in the normalized van der Waals volume ranking across levels of extension and in both phases, with peaks observed between ranks 6 and 7. Overall, shifts in parameter ranking distribution for the protein–polymer interfaces were useful in the identification of unique interface characteristics, in addition to understanding how the unrestrained phase results in PLGA/ID2S interfaces are more polarizable, larger in their van der Waals volume, and slightly more hydrophobic.
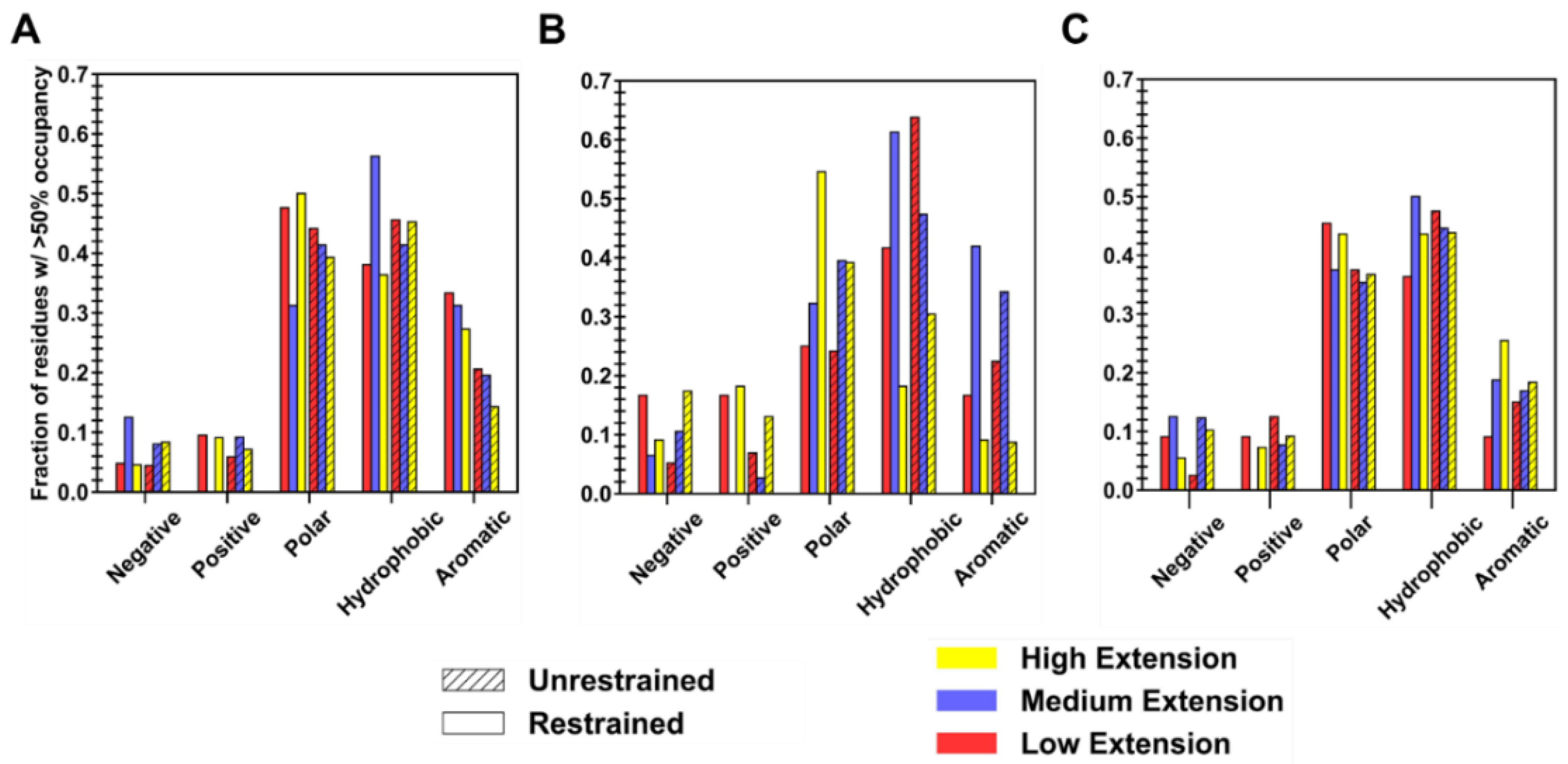
Surface residues that are responsible for favorable binding for each polymer type were extracted by first generating a collapsed protein–polymer interaction interface from all simulation trials in both restraint-on and -off phases (two averaged interfaces per level of extension), containing amino acids with a non-zero percent occupancy. A cutoff of >50% was then applied to each collapsed interface to sub-select high-residency residues, whose amino acid composition can be seen in
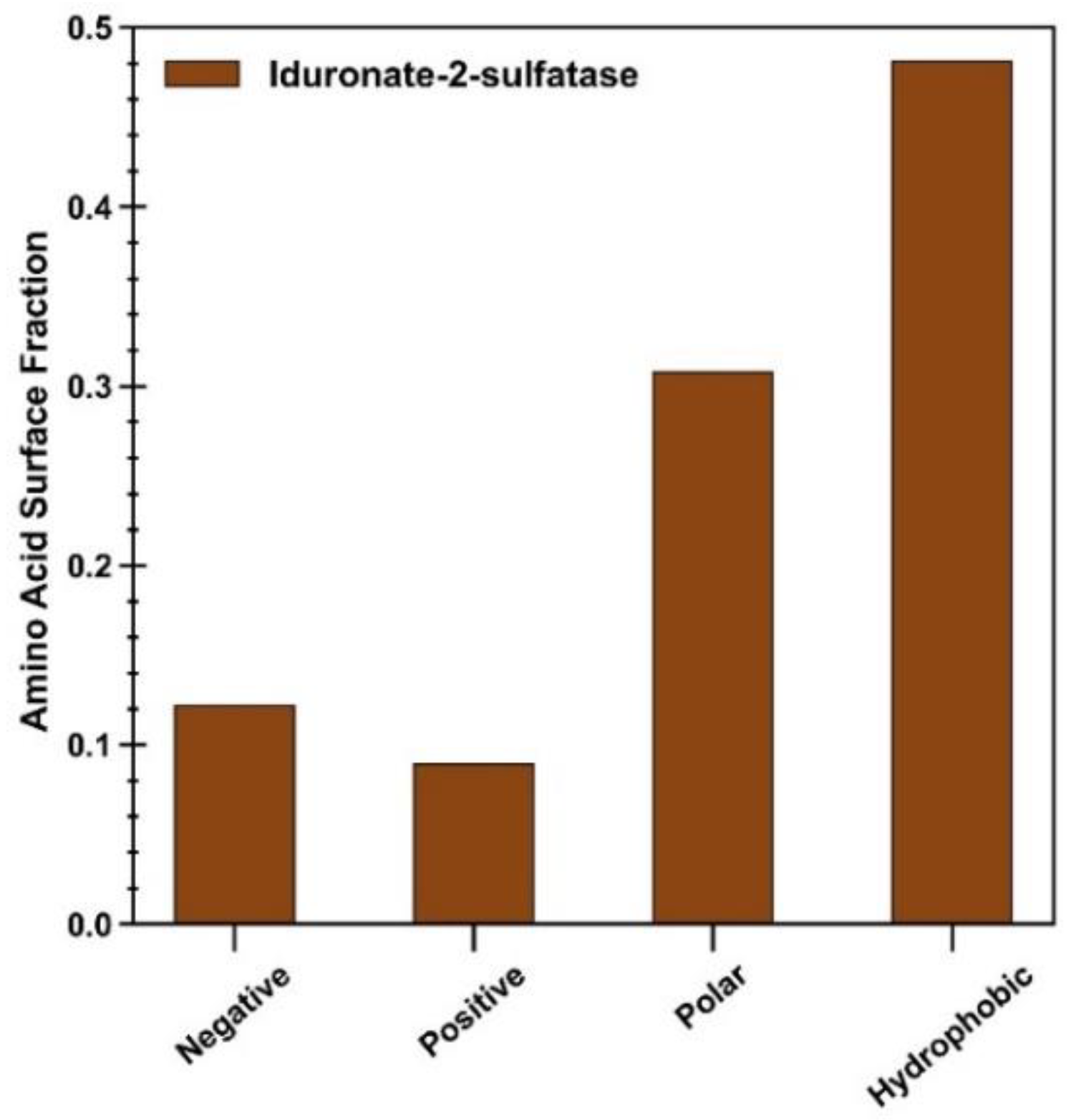
Figure 7. For clarity, high-residency residues are amino acids with >90% occupancy, while residues with occupancies between 50 and 90% are considered to be important in constructing the protein–polymer interface. Given that ID2S surface residues are composed of ~30% polar, ~48% hydrophobic, ~12% negatively, and ~9% positively charged residues at neutral pH in its native state (
Figure A12), it is not surprising that a large fraction of residues are primarily polar and hydrophobic, with fractional values being less than 0.15 for charged surface residues across polymer types. The ID2S-PLGA filtered interface, in the restrained phase at high and low extension levels, was primarily composed of mostly polar and hydrophobic residues, where aromatic groups contribute a significant proportion to the fraction of nonpolar residues that drive binding. In the unrestrained phase, the fraction of hydrophobic residues increases while that of polar and aromatic residues decreases, suggesting that PLGA oligomers increased their interactions with non-aromatic hydrophobic residues as they collapsed around the ID2S surface. As for the medium extension case when the restraint was on, the hydrophobic fraction of residues was the largest (~0.6), while polar and aromatic fractions were similar in value, indicating that this intermediate R
g value was highly favorable for hydrophobic interactions to occur between PLGA and ID2S. In the restraint-off phase, an increase in the polar fraction was observed along with a decrease in hydrophobic and aromatic fraction, showing that both polar and hydrophobic residues drive PLGA oligomer stabilization on the ID2S surface; the presence of methyl and carboxyl groups within polymer chain likely mediate favorable contact with ID2S, resulting in large occupancy values and favorable polymer binding.
As for PEG/ID2S collapsed interface in the restrained phase at high extension levels, polar residues made up a large portion, followed by positively charged and hydrophobic residues; in the unrestrained phase, the fraction of polar and positively charged residues dropped while an increase in the fraction of negatively charged and hydrophobic residues was observed. This could be due to the shift in PEG oligomers toward ideal-chain conformations, resulting in a greater contact with non-aromatic hydrophobic residues. At low extension cases in the restrained phase, charged and aromatic residues made up a similar fraction (~0.15), with the rest of the interface being composed of hydrophobic residues. The polar fraction of residues remained constant in the unrestrained phase, while a drastic increase in the hydrophobic residues fraction and decrease in the charged residue fraction were observed. Such trends indicate an increase in ID2S/PEG CSA in the unrestrained phase is due to expanded PEG oligomers contacting a greater number of hydrophobic residues. At medium extension levels, polar and hydrophobic residues composed a large fraction of the collapsed interface in both restraint-on and -off phases, with a slight decrease and increase in the polar fraction being seen in the unrestrained phase; this can be explained by the strength of polymer–solvent interactions that pulls PEG oligomers away from the ID2S surface and allows them to explore other polar residues.
At high extension levels for the PLGA-PEG/ID2S interfaces, polar and hydrophobic residue fractions combined to be ~80%, for the restrained phase, but polar and aromatic residue fraction decreased, while the hydrophobic fraction stayed constant for the unrestrained phase. This could be due to persistent PLGA-ID2S interactions and PEG’s slight attraction to ID2S, across the various simulation trials. Polar and negatively charged fraction of residues decreased when compared to interfaces from both phases in the low extension case, while polar and hydrophobic fraction increased. For the medium extension case, a slight decrease for the hydrophobic and polar categories was observed, as well as an increase in positively charged fraction, indicating that irreversible copolymer-ID2S binding could be attributed to polar residues, in addition to a mixture of aromatic and non-aromatic hydrophobic AAs. Overall, the interplay of chemical moieties on a polymer chain and its time-averaged conformation in solution have a critical impact on whether strong protein–polymer binding events occur during nanoparticle formulation or degradation.
3.3. Surface Patch Characteristics at High and Low Extension Levels
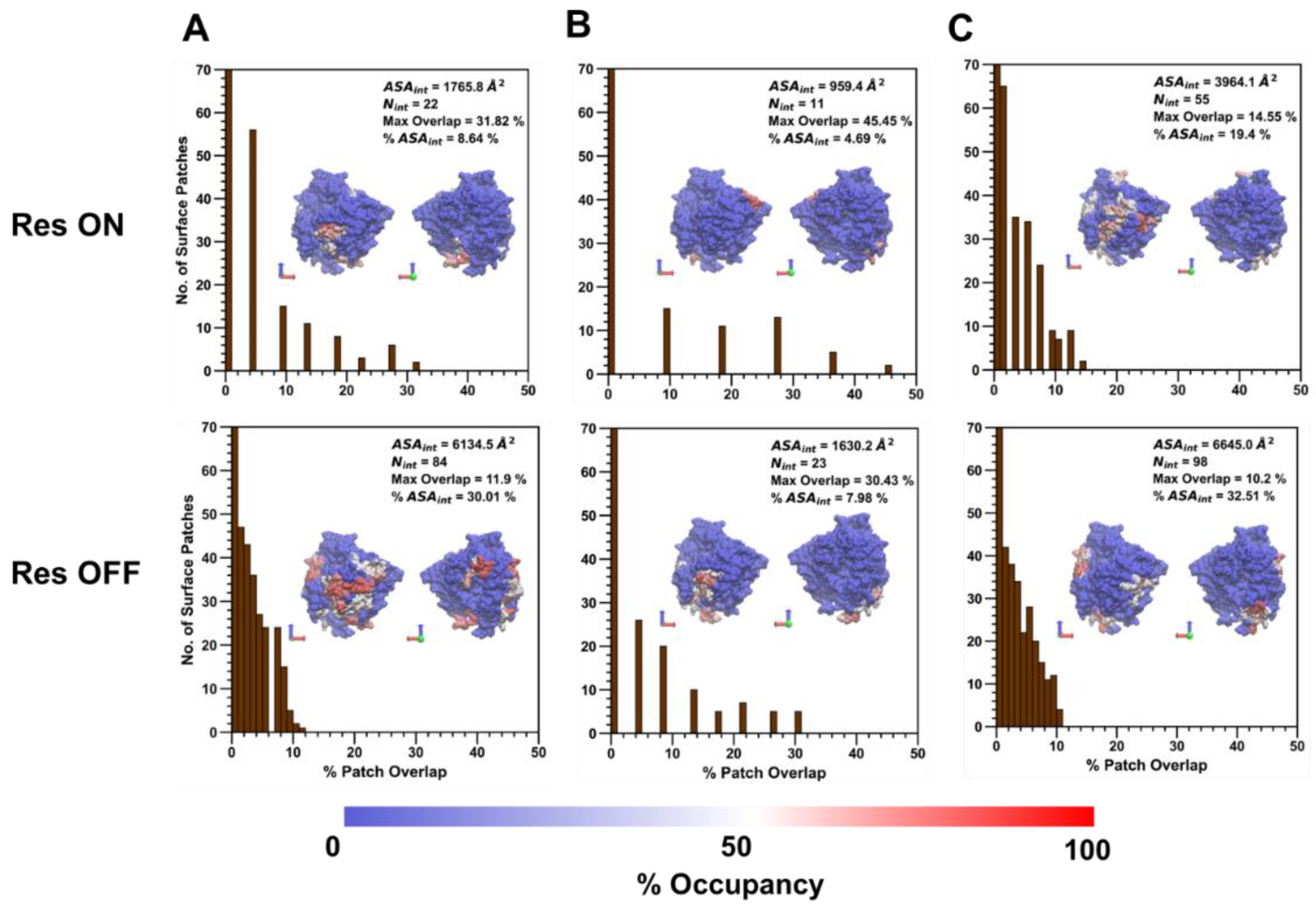
Following the analysis of the amino acid composition of the collapsed protein–polymer interface containing residues with >50% occupancy across polymer types, the percent patch overlap of generated surface patches with the polymer/ID2S interface was calculated for the high (
Figure 8) and low (Figure 10) extension cases to show how residue clusters containing high-residency surface AAs can be identified using this patch analysis method proposed by Jones et al. [
39,
44]. Across all polymer/ID2S systems, most surface patches had an overlap with the collapsed interface between 0 and 1%, with ~8–15% of patches having an overlap greater than 5%; moreover, N
int was smaller in the restraint-on phase, when compared to the restraint-off phase, across all polymer types. This is likely due to the presence of the restraint ensuring that the polymer oligomers maintain the set R
g; thus, monomers that are weakly bound to the ID2S surface will be pulled off, resulting in a large majority of interacting surface residues with low occupancy values. This idea is also supported by an analysis of non-bonded interaction energetics and the smaller ASA
int of the filtered interface, when compared to the average CSA for polymer/ID2S systems at high extension levels for the restrained phase. As N
int increases for the unrestrained phases, a general drop in the percent max overlap for certain surface patches was observed. For the PLGA/ID2S filtered interface, the decrease in the percent max overlap from 31.8% to 11.9% signifies that the increase in the interface area results in a fewer amount of contiguous surface patches that overlap with a high percentage of the protein–polymer interface. Regardless, most surface patches with the largest percent overlap typically contained high-residency residues. As for the PEG/ID2S interfaces, ASA
int and N
int were smaller in magnitude than the other polymer/ID2S interfaces, providing more evidence that PEG–water interactions were dominant and PEG oligomers preferred to be in solution; furthermore, % max overlap only dropped to 30.4%. The effect of high extension and copolymerization can also be seen in the PLGA-PEG/ID2S interface, whereby ASA
int and N
int were largest and % max overlap values were the smallest in both restraint-on and -off phases when comparing to the homopolymer/ID2S systems.
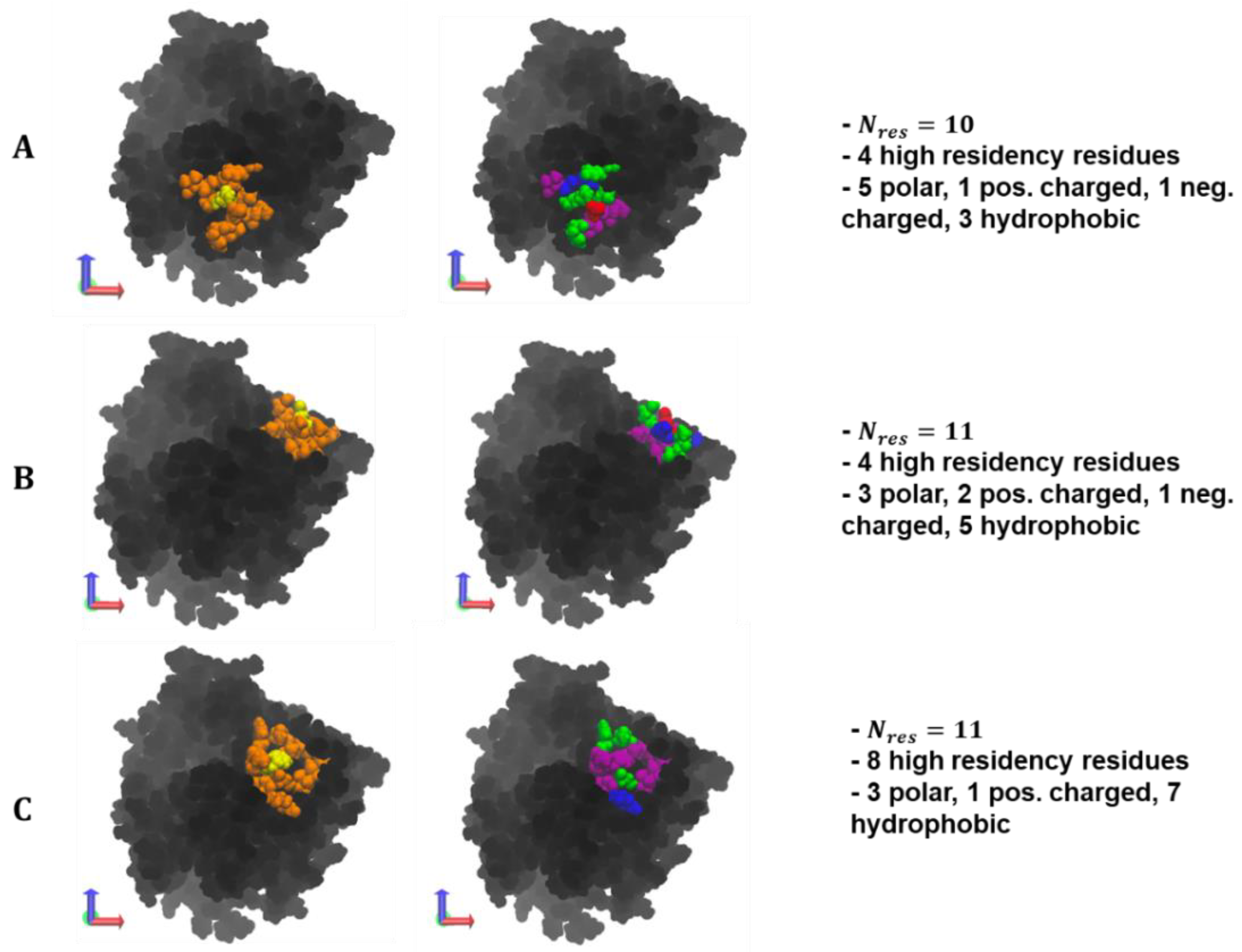
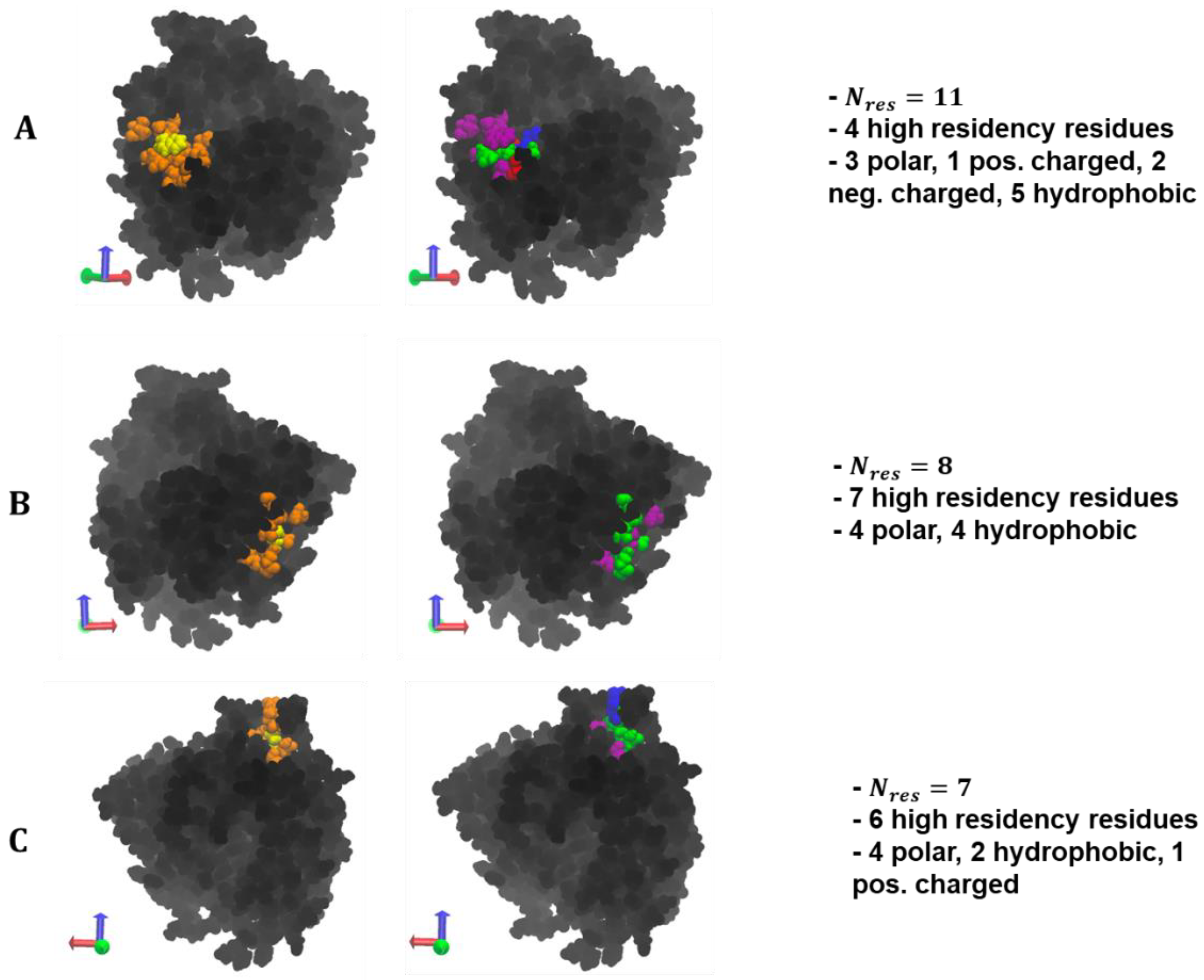
A visualization of the max overlap patches and the amino acid composition can be found in
Figure 9. Across the polymer types, selected residue clusters consisted of 10–11 residues, with a majority of them being polar and hydrophobic and varying in shape and morphology. The selected surface patches, across the polymer/ID2S systems, contained four and eight high-residency residues, showing that this method is capable of identifying residue clusters containing polymer binding hotspots. For the PLGA/ID2S patch, the distance between the three hydrophobic residues, filled with the five polar residues, could result in a binding mode where carboxyl groups on the oligomer chain first undergo dipole–dipole interactions then as the oligomer settles on the ID2S surface, methyl groups on either side of the chain experience hydrophobic interactions, leading to irreversible binding. The PEG patch had three charged and three polar residues, suggesting that this residue cluster would be relatively hydrophilic and would undergo hydrogen bonding or dipole–dipole interactions with PEG oligomers; hydrophobic residues were interspersed within the patch and provided some hydrophobic character. Seven hydrophobic residues covered most of the PLGA-PEG patch, along with the three polar residues and one positively charged residue. The morphology of this residue cluster is likely to promote binding of the PLGA domain and allow for oligomer collapse, since multiple methyl groups can interact with the hydrophobic strip as the chain changes conformation. Overall, selected patches at the highest percent overlap with the prot–poly interaction interface allows for the systematic identification of binding zones that can be evaluated for their uniqueness and be engineered to increase nanoparticle drug loading or tune drug release profiles.
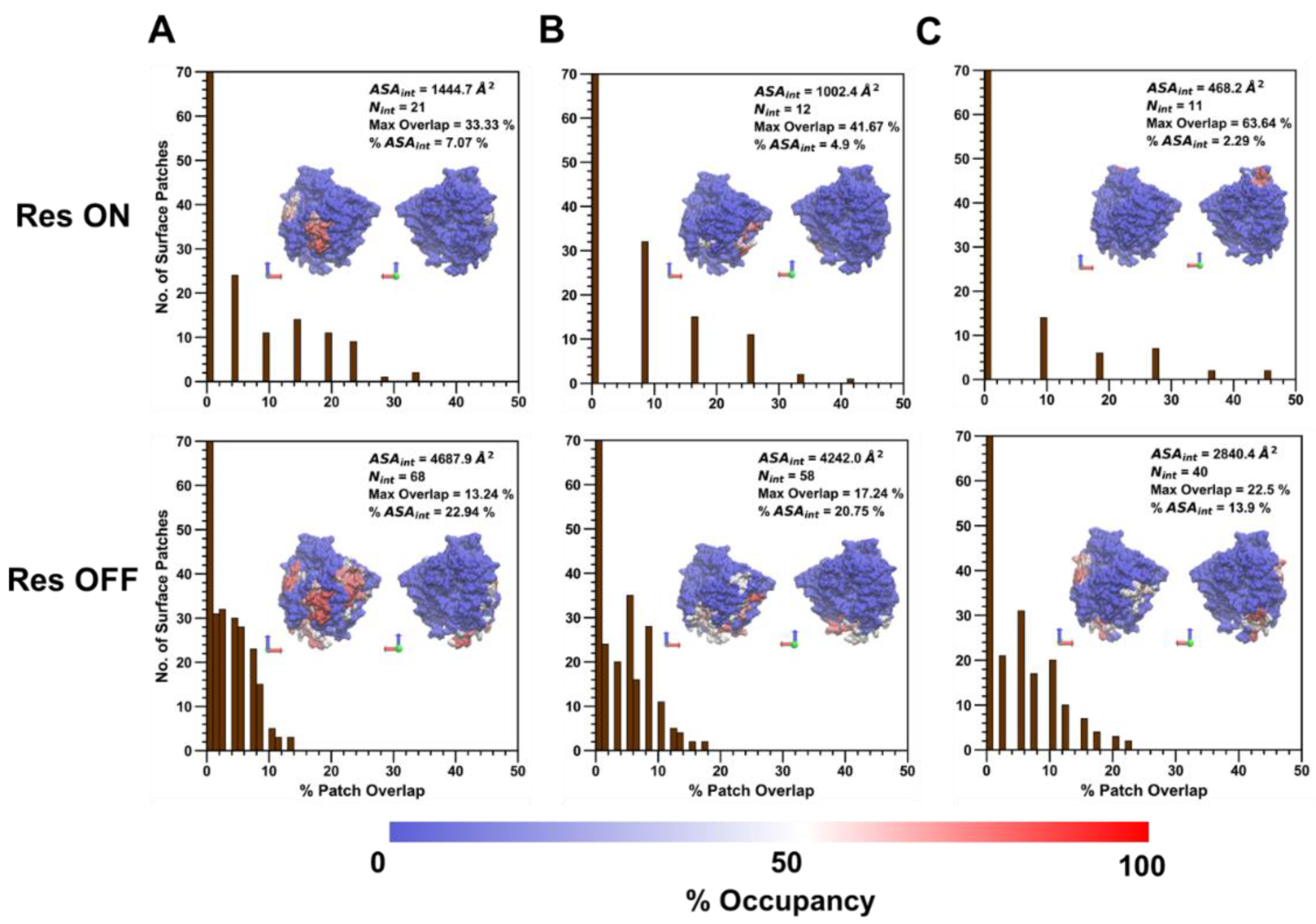
PEG/ID2S and PLGA-PEG/ID2S filtered interfaces in the restrained phase had smaller ASA
int and N
int values when compared to the PLGA interface (
Figure 10). Moreover, the percent max overlap of certain surface patches increased as the interface became smaller, when going from PLGA, PEG, and then the copolymer. This shows that the PEG and copolymer oligomers at low extension levels undergo mostly reversible binding with ID2S and tend to be free in solution or interact with another oligomer in solution. PLGA oligomers in both the restrained and unrestrained phase, however, were still able to undergo favorable and irreversible binding with 7% and 22.9% of the protein surface area, even in the presence of strong polymer–polymer interactions. Comparing to the PLGA interface surface area at high extension levels from the restraint-off phase, ASA
int and N
int were smaller, showing that expanded PLGA oligomers lead to interactions with more surface residues. The PLGA/ID2S interface percent max overlap was the lowest among the polymer types for interfaces from the restraint-off phase. When the restraint was turned off, the large increase observed in ASA
int and N
int, in addition to the drop in percent max overlap for the PEG/ID2S interface, further confirms the oligomers’ return to good solvent conditions from a collapsed conformation results in an increased interaction with ID2S surface residues. A similar trend was also seen for PLGA and PLGA-PEG interfaces. In this case, the collapsed PLGA domain mostly interacted with hydrophobic and polar residues, as does the PEG domain in the scenario of ideal-chain conditions. These observations help explain why there was an increase in CSA from the unrestrained phase.
For the PLGA 11-residue patch at low extension levels (
Figure 11), hydrophobic residues surround polar residues and charged residues seemed to be slightly less exposed to the surface. Collapsed oligomers with methyl groups on their surface were likely able to favorably contact the front-facing hydrophobic residues, leading to irreversible binding. As for the PEG patch, four polar and four hydrophobic residues primarily make up this eight-residue cluster, whereby seven surface residues had high occupancy values. This may be due to the spread of polar residues that allow for multiple opportunities for hydrogen bonding, while hydrophobic residues interact with methylene groups on the PEG chain. The seven-residue patch for the copolymer interface was made up of four polar and two hydrophobic AAs, with one positively charged residue. Comparing to the location of high-residency residues for PLGA-PEG/ID2S interface in the restraint-on phase, it is reasonable to conclude that this patch and its arrangement of polar and hydrophobic residues resulted in favorable copolymer binding, driven by the presence of methyl, carboxyl, and/or ester groups. It should be noted that these selected surface patches also promoted strong oligomer binding across the polymer types at both levels of extension. A stricter occupancy cutoff can be employed to generate a smaller protein–polymer interface, leading to larger % max overlap values for certain surface patches. Ultimately, an examination of the local chemical environment of the protein surface with strong protein–polymer binding is a nontrivial task, and this patch analysis provides a way to gain such information.