Preparation Methods and Functional Characteristics of Regenerated Keratin-Based Biofilms
Abstract
1. Introduction
2. Novel Extraction Methods of Keratin
2.1. Thermal Hydrolysis
2.2. Ultrasonic Technology
2.3. Eco-Friendly Solvent System
2.4. Microbial Decomposition
3. Preparation Method of Keratin-Based Biofilms
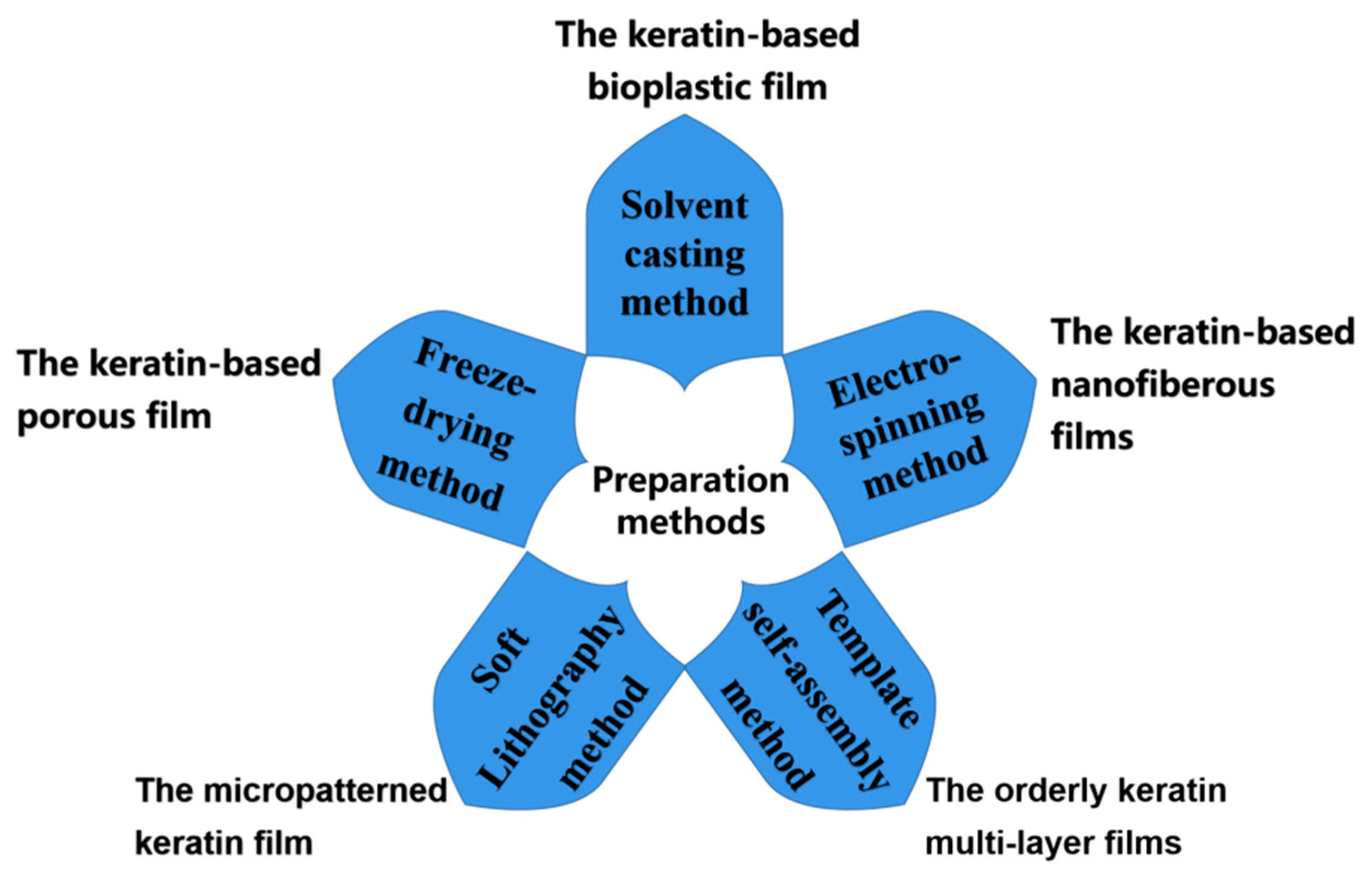
3.1. Solvent Casting Method
3.2. Electrospinning Method
3.3. Template Self-Assembly Method
3.4. Freeze-Drying Method
3.5. Soft Lithography Method
4. Functional Properties of Keratin-Based Films

4.1. Biocompatibility
4.2. Biodegradability
4.3. Hygroscopic Nature
4.4. Adsorption
5. New Development Direction of Keratin-Based Biofilm
- (1)
- Due to the different sources and complex structures, keratin is difficult to extract and purify, which increases the costs related to producing keratin-based biofilms. In the future, we should continue to develop cost-effective, time-efficient, and eco-friendly keratin extraction methods, in order to achieve efficient large-scale production of keratin-based biofilms. Only in this way can the waste and environmental pollution related to fur and hair be solved;
- (2)
- The structure–activity relationship between the structural and functional properties during the preparation of keratin-based biofilms should be further investigated. Precise regulation of the degradation rate of natural keratin-based biofilms to achieve the controlled release of drugs comprises a key difficulty to be resolved in the future;
- (3)
- The unique properties of keratin-based biofilms should be fully exploited in order to further expand their application range.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tasaki, K. A novel thermal hydrolysis process for extraction of keratin from hog hair for commercial applications. Waste Manag. 2020, 104, 33–41. [Google Scholar] [CrossRef]
- Feroz, S.; Muhammad, N.; Ratnayake, J.; Dias, G. Keratin-based materials for biomedical applications. Bioact. Mater. 2020, 5, 496–509. [Google Scholar] [CrossRef]
- Jin, E.; Reddy, N.; Zhu, Z.; Yang, Y. Graft polymerization of native chicken feathers for thermoplastic applications. J. Agric. Food Chem. 2011, 59, 1729–1738. [Google Scholar] [CrossRef]
- Dou, Y.; Huang, X.; Zhang, B.; He, M.; Yin, G.; Cui, Y. Preparation and characterization of a dialdehyde starch crosslinked feather keratin film for food packaging application. RSC Adv. 2015, 5, 27168–27174. [Google Scholar] [CrossRef]
- Oluba, O.M.; Osayam, E.; Shoyombo, A.O. Production and characterization of keratin-starch bio-composite film from chicken feather waste and turmeric starch. Biocatal. Agric. Biotechnol. 2021, 33, 101996–102002. [Google Scholar] [CrossRef]
- Mukherjee, A.; Kabutare, Y.H.; Ghosh, P. Dual crosslinked keratin-alginate fibers formed via ionic complexation of amide networks with improved toughness for assembling into braids. Polym. Test. 2019, 81, 106286–106295. [Google Scholar] [CrossRef]
- Li, B.; Sun, Y.; Yao, J.; Wu, H.; Shen, Y.; Zhi, C.; Li, J. An environment-friendly chemical modification method for thiol groups on polypeptide macromolecules to improve the performance of regenerated keratin materials. Mater. Des. 2022, 217, 110611–110622. [Google Scholar] [CrossRef]
- Sanchez, R.D.O.; Cruz-Maya, I.; Vineis, C.; Claudia, V.; Cinzia, T.; Alessio, V.; Vincenzo, G. Design of asymmetric nanofibers-membranes based on polyvinyl alcohol and wool-keratin for wound healing applications. J. Funct. Biomater. 2021, 12, 76. [Google Scholar] [CrossRef]
- Khumalo, M.; Sithole, B.; Tesfaye, T. Valorisation of waste chicken feathers: Optimisation of keratin extraction from waste chicken feathers by sodium bisulphite, sodium dodecyl sulphate and urea. J. Environ. Manag. 2020, 262, 110329. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Z.; Zhao, Q.; Yun, Y. Study on the structure and properties of biofunctional keratin from rabbit hair. Adv. Catal. Mater. 2021, 14, 379. [Google Scholar] [CrossRef]
- Zhang, N.; Lai, H.Y.; Gautam, A.; Kwek, D.Y.D.; Dong, Y.; Wang, Q.; Ng, K.W. An Enzymatic Method for Harvesting Functional Melanosomes after Keratin Extraction: Maximizing Resource Recovery from Human Hair. J. Polym. Environ. 2022, 30, 1045–1054. [Google Scholar] [CrossRef]
- Raja, S.T.K.; Thiruselvi, T.; Sailakshmi, G.; Ganesh, S.; Gnanamani, A. Rejoining of cut wounds by engineered gelatin-keratin glue. Biochim. Biophys. Acta. 2013, 1830, 4030–4039. [Google Scholar] [CrossRef]
- Pan, F.; Lu, Z.; Tucker, I.; Sarah, H.; Jordan, P.; Lu, J. Surface active complexes formed between keratin polypeptides and ionic surfactants. J. Colloid Interface Sci. 2016, 484, 125–134. [Google Scholar] [CrossRef]
- Ye, W.; Qin, M.; Qiu, R.; Li, J. Keratin-based wound dressings: From waste to wealth. Int. J. Biol. Macromol. 2022, 211, 183–197. [Google Scholar] [CrossRef]
- Jaiswar, G.; Modak, S.; Singh, R.; Dabas, N. Functionalization of biopolymer keratin-based biomaterials and their absorption properties for healthcare application. Woodhead Publ. Ser. Polym. Biomater. 2022, 2022, 257–270. [Google Scholar]
- Esparza, Y.; Bandara, N.; Ullah, A.; Wu, J. Hydrogels from feather keratin show higher viscoelastic properties and cell proliferation than those from hair and wool keratins. Mater. Sci. Eng. C 2018, 90, 446–453. [Google Scholar] [CrossRef]
- Rajabi, M.; Ali, A.; Mcconnell, M.; Cabral, J. Keratinous materials: Structures and functions in biomedical applications. Mater. Sci. Eng. C 2020, 110, 110612–110634. [Google Scholar] [CrossRef]
- Bertini, F.; Canetti, M.; Patroccu, A.; Zoccola, M. Wool keratin-polypropylene composites: Properties and thermal degradation. Polym. Degrad. Stab. 2013, 98, 980–987. [Google Scholar] [CrossRef]
- Gaidau, C.; Epure, D.G.; Enascuta, C.E.; Carsote, C.; Sendrea, C.; Proietti, N.; Chen, W.; Gu, H. Wool keratin total solubilisation for recovery and reintegration-an ecological approach. J. Cleaner Prod. 2019, 236, 117586–117598. [Google Scholar] [CrossRef]
- Wu, W.; Ma, S.; Chen, R.; Huang, Y.; Deng, Y. Genome-wide analysis of keratinibaculum paraultunense strain KD-1 T and its key genes and metabolic pathways involved in the anaerobic degradation of feather keratin. Arch. Microbiol. 2022, 204, 634–648. [Google Scholar] [CrossRef]
- Wang, B.; Yang, W.; McKittrick, J.; Meyers, M. Keratin: Structure, mechanical properties, occurrence in biological organisms, and efforts at bioinspiration. Prog. Mater. Sci. 2016, 76, 229–318. [Google Scholar] [CrossRef]
- Ramya, K.R.; Thangam, R.; Madhan, B. Comparative analysis of the chemical treatments used in keratin extraction from red sheep’s hair and the cell viability evaluations of this keratin for tissue engineering applications. Process Biochem. 2020, 90, 223–232. [Google Scholar] [CrossRef]
- Ye, J.P.; Gong, J.S.; Su, C.; Liu, Y.; Jiang, M.; Pan, H.; Li, R.; Geng, Y.; Xu, Z.; Shi, J. Fabrication and characterization of high molecular keratin based nanofibrous membranes for wound healing. Colloids Surf. B 2020, 194, 111158–111197. [Google Scholar] [CrossRef] [PubMed]
- Posati, T.; Giuri, D.; Nocchetti, M.; Sagnella, A.; Gariboldi, M.; Ferroni, C.; Sotgiu, G.; Varchi, G.; Zamboni, R.; Aluigi, A. Keratin-hydrotalcites hybrid films for drug delivery applications. Eur. Polym. J. 2018, 105, 177–185. [Google Scholar] [CrossRef]
- Zuniga, K.; Isaac, A.; Christy, S.; Wrice, N.; Mangum, L.; Natesan, S.; Burnett, L.; Kowalczewski, C. Characterization of a human platelet lysate-loaded keratin hydrogel for wound healing applications in vitro. Int. J. Mol. Sci. 2022, 23, 4100. [Google Scholar] [CrossRef]
- Oluba, O.M.; Obokare, O.; Bayo-Olorunmeke, O.A.; Ojeaburu, S.I.; Ogunlowo, O.M.; Irokanulo, E.O.; Akpor, O.B. Fabrication, characterization and antifungal evaluation of polyphenolic extract activated keratin starch coating on infected tomato fruits. Sci. Rep. 2022, 12, 4340–4351. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, L.; Wang, X.; Li, Y.; Shen, R. Intelligent drug delivery system based on silk fibroin/wool keratin. Math. Probl. Eng. 2022, 2022, 6748645. [Google Scholar]
- Mori, H.; Hara, M. Transparent biocompatible wool keratin film prepared by mechanical compression of porous keratin hydrogel. Mater. Sci. Eng. C 2018, 91, 19–25. [Google Scholar] [CrossRef]
- Suarato, G.; Contardi, M.; Perotto, G.; Heredia-Guerrero; Fiorentini, F.; Ceseracciu, L.; Pignatelli, C.; Debellis, D.; Bertorelli, R.; Athanassiou, A. From fabric to tissue: Recovered wool keratin/polyvinylpyrrolidone biocomposite fibers as artificial scaffold platform. Mater. Sci. Eng. C 2020, 116, 111151–111163. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Li, J.; Zhao, Z.; Liu, X.; Li, Z.; Han, Y.; Hu, J.; Chen, A. Isolation and characterization of biofunctional keratin particles extracted from wool wastes. Powder Technol. 2013, 246, 356–362. [Google Scholar] [CrossRef]
- Silva, O.A.; Pellá, M.G.; Popat, K.C.; Kipper, M.J.; Rubira, A.F.; Martins, A.F.; Follmann, H.D.M.; Silva, R. Rod-shaped keratin nanoparticles extracted from human hair by acid hydrolysis as photothermally triggered berberine delivery system. Adv. Powder Technol. 2022, 33, 103353–103363. [Google Scholar] [CrossRef]
- Gong, X.Y.; Dang, G.Y.; Guo, J.; Liu, Y.; Gong, Y. A sodium alginate/feather keratin composite fiber with skin-core structure as the carrier for sustained drug release. Int. J. Biol. Macromol. 2020, 155, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Song, N.B.; Lee, J.H.; Mijan, M.A.; Song, K.B. Development of a chicken feather protein film containing clove oil and its application in smoked salmon packaging. LWT-Food Sci. Technol. 2014, 57, 453–460. [Google Scholar] [CrossRef]
- Hammouche, H.; Achour, H.; Makhlouf, S.; Chaouchi, A.; Laghrouche, M. A comparative study of capacitive humidity sensor based on keratin film, keratin/graphene oxide, and keratin/carbon fifibers. Sens. Actuators B 2021, 329, 112805–112822. [Google Scholar] [CrossRef]
- Garrido, T.; Penalba, M.; Caba, K.D.L.; Guerrero, P. A more effificient process to develop protein films derived from agro-industrial by-products. Food Hydrocoll. 2019, 86, 11–17. [Google Scholar] [CrossRef]
- Dąbrowska, M.; Sommer, A.; Sinkiewicz, I.; Taraszkiewicz, A.; Staroszczyk, H. An optimal designed experiment for the alkaline hydrolysis of feather keratin. Environ. Sci. Pollut. Res. 2022, 29, 24145–24154. [Google Scholar] [CrossRef]
- Sillapawattana, P.; Wongputtisin, P. Primary quality assessment of keratin extracted from chicken feather waste as feed component. Food Appl. Biosci. J. 2021, 9, 51–60. [Google Scholar]
- Sadeghi, A.; Nourmohammadi, J.; Ghaee, A.; Soleimani, N. Carboxymethyl cellulose-human hair keratin hydrogel with controlled clindamycin release as antibacterial wound dressing. Int. J. Biol. Macromol. 2020, 147, 1239–1247. [Google Scholar] [CrossRef]
- Alahyaribeik, S.; Ullah, A. Methods of keratin extraction from poultry feathers and their effects on antioxidant activity of extracted keratin. Biomacromol. Mass Spectrom. 2020, 148, 449–456. [Google Scholar] [CrossRef]
- Garrido, T.; Leceta, I.; Caba, K.; Guerrero, P. Chicken feathers as a natural source of sulphur to develop sustainable protein fifilms with enhanced properties. Biomacromol. Mass Spectrom. 2017, 106, 8035–8064. [Google Scholar]
- Zhang, H.; Liu, P. Bio-inspired keratin-based core-crosslinked micelles for pH and reduction dual-responsive triggered DOX delivery. Biomacromol. Mass Spectrom. 2018, 123, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, P.; Madhan, B. Fabrication of keratin-silica hydrogel for biomedical applications. Mater. Sci. Eng. C 2016, 66, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Ravi, V.; Muhamed, J.; Chatterjee, K.; Nagalingam, R.; Sundaresan, N.R. A simplified protocol for culture of murine neonatal cardiomyocytes on nanoscale keratin coated surfaces. Int. J. Cardiol. 2017, 232, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Lusiana; Reichl, S.; Müller-Goymann, C.C. Keratin film made of human hair as a nail plate model for studying drug permeation. Eur. J. Pharm. Biopharm. 2011, 78, 432–440. [Google Scholar] [CrossRef]
- Mykhaliuk, V.V.; Havryliak, V.V. Obtaining human hair keratin-based films and their characteristics. Biol. Stud. 2021, 15, 27–36. [Google Scholar] [CrossRef]
- Schwab, R.; Reichl, S. Dexamethasone-loaded keratin films for ocular surface reconstruction. J. Mater. Sci. Mater. Med. 2022, 33, 29. [Google Scholar] [CrossRef]
- Nakata, R.; Osumi, Y.; Miyagawa, S.; Tachibana, A.; Tanabe, T. Preparation of keratin and chemically modified keratin hydrogels and their evaluation as cell substrate with drug releasing ability. J. Biosci. Bioeng. 2015, 120, 111–116. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Arab-Bafrani, Z.; Karimi, F.; Javid, N. Designing hybrid nanofibers based on keratin-poly (vinyl alcohol) and poly (Ɛ-caprolactone) for application as wound dressing. J. Ind. Text. 2022, 51, 1729–1949. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Posati, T.; Sotgiu, G.; Tiboni, M.; Barbalinardo, M.; Valle, F.; Casettari, L.; Zamboni, R.; Lotti, N.; et al. Regenerated wool keratin-polybutylene succinate nanofibrous mats for drug delivery and cells culture. Polym. Degrad. Stab. 2020, 179, 109272–109304. [Google Scholar] [CrossRef]
- Ganesan, P. Natural and biopolymer curative films for wound dressing medical applications. Wound Med. 2017, 18, 33–40. [Google Scholar] [CrossRef]
- Selmin, F.; Cilurzo, F.; Aluigi, A.; Franzè, S.; Minghetti, P. Regenerated keratin membrane to match the in vitro drug diffusion through human epidermis. Results Pharma Sci. 2012, 2, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Koleva, M.; Zheleva, D. Methods for obtaining of keratin hydrolysates from sheep wool. J. Chem. Technol. Metall. 2022, 57, 76–83. [Google Scholar]
- Isarankura, N.A.S.; Tanpichai, S.; Wootthikanokkhan, J. Keratin extracted from chicken feather waste: Extraction, preparation, and structural characterization of the keratin and keratin/biopolymer films and electrospuns. J. Polym. Environ. 2015, 23, 506–516. [Google Scholar] [CrossRef]
- Veerasubramanian, P.K.; Thangavel, P.; Kannan, R.; Chakraborty, S.; Ramachandran, B.; Suguna, L.; Muthuvijayan, V. An investigation of konjac glucomannan-keratin hydrogel scaffold loaded with Avena sativa extracts for diabetic wound healing. Colloids Surf. B 2018, 165, 92–102. [Google Scholar] [CrossRef]
- Poole, A.J.; Church, J.S. The effects of physical and chemical treatments on Na2S produced feather keratin films. Int. J. Biol. Macromol. 2014, 73, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, L.; Wang, Q.; Liu, Y.; Zhu, P. Study on dissolution and recovery of waste wool by sodium sulfide system. Ferroelectrics 2020, 562, 114–124. [Google Scholar] [CrossRef]
- Nogja, G.N.; Adhav, M.P. Extraction of keratin from chicken feather and spectral characterization of protein by FTIR. Int. J. Emerg. Technol. Innov. Res. 2019, 6, 1698–1702. [Google Scholar]
- Sen, P.; Arun, C.; Divvyapriya, J. A pilot-scale study on the extraction & optimization of keratin from human hair-an adapted strategy for the control of environmental menace. J. Environ. Treat. Technol. 2021, 9, 342–348. [Google Scholar]
- Brenner, M.; Weichold, O. Autogenous cross-linking of recycled keratin from poultry-feather waste to hydrogels for plant-growth media. Polymers 2021, 13, 3581. [Google Scholar] [CrossRef]
- Pourjavaheri, F.; Pour, S.O.; Jones, O.A.H.; Smooker, P.M.; Brkljača, R.; Sherkat, F.; Blanch, E.W.; Gupta, A.; Shanks, R.A. Extraction of keratin from waste chicken feathers using sodium sulfide and L-cysteine. Process Biochem. 2019, 82, 205–214. [Google Scholar] [CrossRef]
- Ma, B.; Qiao, X.; Hou, X.; Yang, Y. Pure keratin membrane and fibers from chicken feather. Int. J. Biol. Macromol. 2016, 89, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Dai, X.; Chen, K.; Li, R.; Xing, Y. Fabrication of regenerated wool keratin/polycaprolactone nanofiber membranes for cell culture. Biomacromol. Mass Spectrom. 2018, 114, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhu, S.; Yang, H.; Liao, Z.; Gong, Z.; Zhao, W.; Li, Y.; Gu, J.; Wei, Z.; Yang, J. Keratin-based composite bioactive films and their preservative effects on cherry tomato. Molecules 2022, 27, 6331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wu, Z.; Jiang, Z.; Zhou, M.; Yu, Y.; Wang, P.; Wang, Q. pH mediated L-cysteine aqueous solution for wool reduction and urea-free keratin extraction. J. Polym. Environ. 2022, 30, 2714–2726. [Google Scholar] [CrossRef]
- Chen, M.; Ren, X.; Dong, L.; Li, X.; Cheng, H. Preparation of dynamic covalently crosslinking keratin hydrogels based on thiol/disulfide bonds exchange strategy. Int. J. Biol. Macromol. 2021, 182, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Fagbemi, O.D.; Sithole, B.; Tesfaye, T. Optimization of keratin protein extraction from waste chicken feathers using hybrid pre-treatment techniques. Sustainable Chem. Pharm. 2020, 17, 100267–100278. [Google Scholar] [CrossRef]
- Pace, L.A.; Plate, J.F.; Smith, T.L.; Dyke, M.E.V. The effect of human hair keratin hydrogel on early cellular response to sciatic nerve injury in a rat model. Biomaterials 2013, 34, 5907–5914. [Google Scholar] [CrossRef]
- Agarwal, V.; Panicker, A.G.; Indrakumar, S.; Chatterjee, K. Comparative study of keratin extraction from human hair. Int. J. Biol. Macromol. 2019, 133, 382–390. [Google Scholar] [CrossRef]
- Shavandi, A.; Carne, A.; Bekhit, A.A.; Bekhit, A.E.D.A. An improved method for solubilisation of wool keratin using peracetic acid. J. Environ. Chem. Eng. 2017, 5, 1977–1984. [Google Scholar] [CrossRef]
- Fontoura, R.; Daroit, D.J.; Corrêa, A.P.F.; Moresco, K.S.; Santi, L.; Beys-da-Silva, W.O.; Yates, J.R.; Moreira, J.C.F.; Brandelli, A. Characterization of a novel antioxidant peptide from feather keratin hydrolysates. New Biotechnol. 2019, 49, 71–76. [Google Scholar] [CrossRef]
- Kshetri, P.; Singh, P.L.; Chanu, S.B.; Singh, T.S.; Rajiv, C.; Tamreihao, K.; Singh, H.N.; Chongtham, T.; Devi, A.K.; Sharma, S.K.; et al. Biological activity of peptides isolated from feather keratin waste through microbial and enzymatic hydrolysis. Electron. J. Biotechnol. 2022, 60, 11–18. [Google Scholar] [CrossRef]
- Yan, R.R.; Xue, D.; Su, C.; Xu, Y.; Gong, J.S.; Liu, Y.L.; Jiang, M.; Geng, Y.; Lv, G.Z.; Zheng, H.X.; et al. A keratin/chitosan sponge with excellent hemostatic performance for uncontrolled bleeding. Colloids Surf. B 2022, 218, 112770–112784. [Google Scholar]
- Azmi, N.A.; Idris, A.; Yusof, N.S.M. Ultrasonic technology for value added products from feather keratin. Ultrason. Sonochem. 2018, 47, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Cassoni, A.C.; Freixo, R.; Pintado, A.; Amorim, M.; Pereira, C.D.; Madureira, A.; Pintado, M. A novel eco-friendly method to extract keratin from hair. ACS Sustain. Chem. Eng. 2018, 6, 12268–12274. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, T. Revisiting greenness of ionic liquids and deep eutectic solvents. Green Chem. Eng. 2021, 2, 174–186. [Google Scholar] [CrossRef]
- Zhong, X.; Li, R.; Wang, Z. Eco-fabrication of antibacterial nanofibrous membrane with high moisture permeability from wasted wool fabrics. Waste Manag. 2020, 102, 404–411. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, X. Extracting keratin from chicken feathers by using a hydrophobic ionic liquid. Process Biochem. 2012, 47, 896–899. [Google Scholar] [CrossRef]
- Ghosh, A.; Clerens, S.; Deb-Choudhury, S.; Dyer, J. Thermal effects of ionic liquid dissolution on the structures and properties of regenerated wool keratin. Polym. Degrad. Stab. 2014, 108, 108–115. [Google Scholar] [CrossRef]
- Ji, Y.; Chen, J.; Lv, J.; Li, Z.; Xing, L.; Ding, S. Extraction of keratin with ionic liquids from poultry feather. Sep. Purif. Technol. 2014, 132, 577–583. [Google Scholar] [CrossRef]
- Liu, X.; Nie, Y.; Meng, X.; Zhang, Z.; Zhang, X.; Zhang, S. DBN-based ionic liquids with high capability for the dissolution of wool keratin. RSC Adv. 2017, 7, 1981–1988. [Google Scholar] [CrossRef]
- Zhang, Z.; Nie, Y.; Zhang, Q.; Liu, X.; Tu, W.; Zhang, X.; Zhang, S. Quantitative change in disulfide bonds and microstructure variation of regenerated wool keratin from various ionic liquids. ACS Sustain. Chem. Eng. 2017, 5, 2614–2622. [Google Scholar] [CrossRef]
- Li, X.; Guo, Z.; Li, J.; Yang, M.; Yao, S. Swelling and microwave-assisted hydrolysis of animal keratin in ionic liquids. J. Mol. Liq. 2021, 341, 117306–117320. [Google Scholar] [CrossRef]
- Feroz, S.; Muhammad, N.; Dias, G.; Alsaiari, M.A. Extraction of keratin from sheep wool fibres using aqueous ionic liquids assisted probe sonication technology. J. Mol. Liq. 2022, 350, 118595–118604. [Google Scholar] [CrossRef]
- Ding, S.; Sun, Y.; Chen, H.; Xu, C.; Hu, Y. An ultrasonic-ionic liquid process for the efficient acid catalyzed hydrolysis of feather keratin. Chin. J. Chem. Eng. 2019, 27, 660–667. [Google Scholar] [CrossRef]
- Salama, A.; Guarino, V. Ionic liquids to process silk fibroin and wool keratin for bio-sustainable and biomedical applications. J. Polym. Environ. 2022, 30, 1–17. [Google Scholar] [CrossRef]
- Wils, L.; Hilali, S.; Boudesocque-Delaye, L. Biomass valorization using natural deep eutectic solvents: What’s new in France? Molecules 2021, 26, 6556. [Google Scholar] [CrossRef]
- Nuutinen, E.M.; Willberg-Keyriläinen, P.; Virtanen, T.; Mija, A.; Kuutti, L.; Lantto, R.; Jääskeläinen, A.S. Green process to regenerate keratin from feathers with an aqueous deep eutectic solvent. RSC Adv. 2019, 9, 19720–19728. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, Y.; Yang, X. Extraction of keratin from poultry feathers with choline chloride-oxalic acid deep eutectic solvent. Fibers Polym. 2021, 22, 3326–3335. [Google Scholar] [CrossRef]
- Chao, S.J.; Chung, K.H.; Lai, Y.F.; Lai, Y.K.; Chang, S.H. Keratin particles generated from rapid hydrolysis of waste feathers with green DES/KOH: Efficient adsorption of fluoroquinolone antibiotic and its reuse. Int. J. Biol. Macromol. 2021, 173, 211–218. [Google Scholar] [CrossRef]
- Okoro, O.V.; Jafari, H.; Hobbi, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Enhanced keratin extraction from wool waste using a deep eutectic solvent. Chem. Pap. 2022, 76, 2637–2648. [Google Scholar] [CrossRef]
- Wang, D.; Yang, X.; Tang, R.; Yao, F. Extraction of keratin from rabbit hair by a deep eutectic solvent and its characterization. Polymers 2018, 10, 993. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Yuan, J.; Wang, P.; Fan, X.; Xu, J.; Wang, Q.; Zhang, L. Dissolution and regeneration of wool keratin in the deep eutectic solvent of choline chloride-urea. Int. J. Biol. Macromol. 2018, 119, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, W.; Zhang, N.; Soladoye, O.P.; Zhang, Y.; Fu, Y. Deep eutectic solvents as new media for green extraction of food proteins: Opportunity and challenges. Food Res. Int. 2022, 161, 111842–111856. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.; Lee, Y.J.; Song, K.; Jin, H.; Lee, J.; Kim, D.; Lee, D.; Kang, N. Low-molecular weight keratins with anti-skin aging activity produced by anaerobic digestion of poultry feathers with Fervidobacterium islandicum AW-1. J. Biotechnol. 2018, 271, 17–25. [Google Scholar] [CrossRef]
- Sharma, S.; Rostamabadi, H.; Gupta, S.; Nadda, A.K.; Kharazmi, M.S.; Jafari, S.M. Nano/micro-formulations of keratin in biocomposites, wound healing and drug delivery systems; recent advances in biomedical applications. Eur. Polym. J. 2022, 180, 111614–111635. [Google Scholar] [CrossRef]
- Pourjavaheri, F.; Jones, O.A.; Czajka, M.; Martinez-Pardo, I.; Blanch, E.W.; Shanks, R.A. Design and characterization of sustainable bio-composites from waste chicken feather keratin and thermoplastic polyurethane. Polym. Compos. 2018, 39, 620–632. [Google Scholar] [CrossRef]
- Wu, S.; Chen, X.; Li, T.; Cui, Y.; Yi, M.; Ge, J.; Yin, G.; Li, X.; He, M. Improving the performance of feather keratin/polyvinyl alcohol/tris (hydroxymethyl) aminomethane nanocomposite films by incorporating graphene oxide or graphene. Nanomaterials 2020, 10, 327. [Google Scholar] [CrossRef]
- Wang, R. Performance and Structure Evaluation of Gln-Lys Isopeptide Bond Crosslinked USYK-SPI Bioplastic Film Derived from Discarded Yak Hair. Polymers 2022, 14, 2471. [Google Scholar] [CrossRef]
- Jus, S.; Stachel, I.; Schloegl, W.; Pretzler, M.; Friess, W.; Meyer, M.; Birner-Grünberger, R.; Gübitz, G. Cross-linking of collagen with laccases and tyrosinases. Mat. Sci. Eng. C 2011, 31, 1068–1077. [Google Scholar] [CrossRef]
- Fan, L.; Wu, H.; Cao, M.; Zhou, X.; Peng, M.; Xie, W.; Liu, S. Enzymatic synthesis of collagen peptide-carboxymethylated chitosan copolymer and its characterization. React. Funct. Polym. 2014, 76, 26–31. [Google Scholar] [CrossRef]
- Wu, S.; Chen, X.; Yi, M.; Ge, J.; Yin, G.; Li, X. Improving the water resistance and mechanical properties of feather keratin/polyvinyl alcohol/tris (hydroxymethyl) aminomethane blend films by cross-linking with transglutaminase, CaCl2, and genipin. Materials 2018, 11, 2203. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.L.; Anandhavelu, S.; Swathy, M. Preparation and characterization of goat hoof keratin/gelatin/sodium alginate base biofilm for tissue engineering application. Integr. Ferroelectr. 2019, 202, 1–12. [Google Scholar] [CrossRef]
- Caringella, R.; Bhavsar, P.; Dalla Fontana, G.; Patrucco, A.; Tonin, C.; Pozzo, P.D.; Zoccola, M. Fabrication and properties of keratoses/sericin blend films. Polym. Bull. 2022, 79, 2189–2204. [Google Scholar] [CrossRef]
- Das, A.; Das, A.; Basu, A.; Datta, P.; Gupta, M.; Mukherjee, A. Newer guar gum ester/chicken feather keratin interact films for tissue engineering. Int. J. Biol. Macromol. 2021, 180, 339–354. [Google Scholar] [CrossRef]
- Ocak, B. Collagen hydrolysate/chitosan/keratin-based ternary films from bio-wastes of the leather and poultry industries against plastic waste generation. Biomass Conv. Bioref. 2022, 12, 1–14. [Google Scholar] [CrossRef]
- Deng, L.; Yue, W.; Zhang, L.; Guo, Y.; Xie, H.; Zheng, Q.; Zhou, G.; Chen, P. Biobased protic ionic liquids as sustainable solvents for wool keratin/cellulose simultaneous dissolution: Solution properties and composited membrane preparation. ACS Sustain. Chem. Eng. 2022, 10, 2158–2168. [Google Scholar] [CrossRef]
- Khaliq, T.; Sohail, M.; Shah, S.A.; Mahmood, A.; Kousar, M.; Jabeen, N. Bioactive and multifunctional keratin-pullulan based hydrogel membranes facilitate re-epithelization in diabetic model. Int. J. Biol. Macromol. 2022, 209, 1826–1836. [Google Scholar] [CrossRef]
- Goyal, S.; Dotter, M.; Diestelhorst, E.; Storck, J.L.; Ehrmann, A.; Mahltig, B. Extraction of keratin from wool and its use as biopolymer in film formation and in electrospinning for composite material processing. J. Eng. Fibers Fabr. 2022, 17, 1–11. [Google Scholar] [CrossRef]
- Shen, B.; Zhang, D.; Wei, Y.; Zhao, Z.; Ma, X.; Zhao, X.; Wang, S.; Yang, W. Preparation of Ag doped keratin/PA6 nanofiber membrane with enhanced air filtration and antimicrobial properties. Polymers 2019, 11, 1511. [Google Scholar] [CrossRef]
- Schifino, G.; Gasparini, C.; Drudi, S.; Giannelli, M.; Sotgiu, G.; Posati, T.; Zamboni, R.; Treossi, E.; Maccaferri, E.; Giorgini, L.; et al. Keratin/polylactic acid/graphene oxide composite nanofibers for drug delivery. Int. J. Pharm. 2022, 623, 121888. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Bondi, E.; Posati, T.; Sotgiu, G.; Zamboni, R.; Torreggiani, A.; Corticelli, F.; Lotti, N.; Aluigi, A. Effects of the blending ratio on the design of keratin/poly (butylene succinate) nanofibers for drug delivery applications. Biomolecules 2021, 11, 1194. [Google Scholar] [CrossRef] [PubMed]
- Aluigi, A.; Varesano, A.; Vineis, C.; Del Rio, A. Electrospinning of immiscible systems: The wool keratin/polyamide-6 case study. Mater. Des. 2017, 127, 144–153. [Google Scholar] [CrossRef]
- Ma, H.; Shen, J.; Cao, J.; Wang, D.; Yue, B.; Mao, Z.; Wu, W.; Zhang, H. Fabrication of wool keratin/polyethylene oxide nano-membrane from wool fabric waste. J. Cleaner Prod. 2017, 161, 357–361. [Google Scholar] [CrossRef]
- Monavari, M.; Zohoori, S.; Davodiroknabadi, A. Anti-inflammatory and bactericidal effect of keratin/harmaline/ginkgo biloba electrospun nano fibres as band aid. Micro Nano Lett. 2022, 17, 210–215. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, H.; Yuan, X.; Cui, S. Wool keratin: A novel building block for layer-by-layer self-assembly. J. Colloid Interface Sci. 2009, 336, 756–760. [Google Scholar] [CrossRef]
- Ferraris, S.; Prato, M.; Vineis, C.; Varesano, A.; Confiengo, G.; Spriano, S. Coupling of keratin with titanium: A physico-chemical characterization of functionalized or coated surfaces. Surf. Coat. Technol. 2020, 397, 126057–126067. [Google Scholar] [CrossRef]
- Posati, T.; Sotgiu, G.; Varchi, G.; Ferroni, C.; Zamboni, R.; Corticelli, F.; Puglia, D.; Torre, L.; Terenzi, A.; Aluigi, A. Developing keratin sponges with tunable morphologies and controlled antioxidant properties induced by doping with polydopamine (PDA) nanoparticles. Mater. Des. 2016, 110, 475–484. [Google Scholar] [CrossRef]
- Posati, T.; Listwan, A.; Sotgiu, G.; Torreggiani, A.; Zamboni, R.; Aluigi, A. Keratin/Hydrotalcites hybrid sponges as promising adsorbents for cationic and anionic dyes. Front. Bioeng. Biotechnol. 2020, 8, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Wu, X.; Cao, Z.; Zhao, X.; Zhou, M.; Gao, P. Preparation and characterization of sponge film made from feathers. Mater. Sci. Eng. C 2013, 33, 4732–4738. [Google Scholar] [CrossRef]
- Kakkar, P.; Verma, S.; Manjubala, I.; Madhan, B. Development of keratin-chitosan-gelatin composite scaffold for soft tissue engineering. Mater. Sci. Eng. C 2014, 45, 343–347. [Google Scholar] [CrossRef]
- Ramadoss, P.; Arul, K.T.; Ramya, J.R.; Begam, M.R.; Chandra, V.S.; Manikandan, E. Enhanced mechanical strength and sustained drug release of gelatin/keratin scaffolds. Mater. Lett. 2017, 186, 109–121. [Google Scholar] [CrossRef]
- Dai, F.; Li, T.; Zhang, Y.; He, Y.; Song, P.; Wang, R. A flexible and moisturizing multilayer microporous sponge copolymerized with keratin and alginate. ChemistrySelect 2021, 6, 10848–10853. [Google Scholar] [CrossRef]
- Guiza, K.; Ben Arfi, R.; Mougin, K.; Vaulot, C.; Michelin, L.; Josien, L.; Schrodj, G.; Ghorbal, A. Development of novel and ecological keratin/cellulose-based composites for absorption of oils and organic solvents. Environ. Sci. Pollut. Res. 2021, 28, 46655–46668. [Google Scholar] [CrossRef]
- Feroz, S.; Dias, G. Hydroxypropylmethyl cellulose (HPMC) crosslinked keratin/hydroxyapatite (HA) scaffold fabrication, characterization and in vitro biocompatibility assessment as a bone graft for alveolar bone regeneration. Heliyon 2021, 7, 8294–8303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dai, C.; Yuan, J.; Wang, P. A bacteriostatic and hemostatic medical dressing based on PEG modified keratin/carboxymethyl chitosan. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 1–9. [Google Scholar] [CrossRef]
- Mohamed, J.M.M.; Alqahtani, A.; Al Fatease, A.; Alqahtani, T.; Khan, B.A.; Ashmitha, B.; Vijaya, R. Human hair keratin composite scaffold: Characterisation and biocompatibility study on NIH3T3 fibroblast cells. Pharmaceuticals 2021, 14, 781. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Luo, W.; Zeng, W. Preparation of free-standing micropatterned keratin films by soft lithography. Acta Chim. Sin. 2019, 77, 533–538. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Z.; Shang, L.; Zhao, Y. Structural color materials from natural polymers. Adv. Mater. Technol. 2021, 6, 2100296–2100314. [Google Scholar] [CrossRef]
- Jia, M.; Kim, J.; Nguyen, T.; Duong, T.; Rolandi, M. Natural biopolymers as proton conductors in bioelectronics. Biopolymers 2021, 112, 23433–23447. [Google Scholar] [CrossRef]
- Tan, H.B.; Wang, F.Y.; Ding, W.; Zhang, Y.; Ding, J.; Cai, D.X.; Yu, K.F.; Yang, J.; Yang, L.; Xu, Y.Q. Fabrication and evaluation of porous keratin/chitosan (KCS) scaffolds for effectively accelerating wound healing. Biomed. Environ. Sci. 2015, 28, 178–189. [Google Scholar]
- Mi, X.; Chang, Y.; Xu, H.; Yang, Y. Valorization of keratin from food wastes via crosslinking using non-toxic oligosaccharide derivatives. Food Chem. 2019, 300, 125181–125188. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Pant, B.; Kim, B.; Jang, R.; Park, S.; Park, M.; Park, S.; Kim, H. Carbon quantum dots incorporated keratin/polyvinyl alcohol hydrogels: Preparation and photoluminescent assessment. Mater. Lett. 2017, 207, 57–61. [Google Scholar] [CrossRef]
- Navarro, J.; Swayambunathan, J.; Lerman, M.; Santoro, M.; Fisher, J.P. Development of keratin-based membranes for potential use in skin repair. Acta Biomater. 2019, 83, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Vakilian, S.; Jamshidi-adegani, F.; Al-Shidhani, S.; Anwar, M.U.; Al-Harrasi, R.; Al-Wahaibi, N.; Qureshi, A.; Alyaqoobi, S.; Al-Amri, I.; Al-Harrasi, A.; et al. A keratin-based biomaterial as a promising dresser for skin wound healing. Wound Med. 2019, 25, 100155–100163. [Google Scholar] [CrossRef]
- Shanmugasundaram, O.L.; Syed, Z.A.K.; Sujatha, K.; Ponnmurugan, P.; Srivastava, A.; Ramesh, R.; Sukumar, R.; Elanithi, K. Fabrication and characterization of chicken feather keratin/polysaccharides blended polymer coated nonwoven dressing materials for wound healing applications. Mater. Sci. Eng. C 2018, 92, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Nayak, K.K.; Gupta, P. Study of the keratin-based therapeutic dermal patches for the delivery of bioactive molecules for wound treatment. Mater. Sci. Eng. C 2017, 77, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Reichl, S. Films based on human hair keratin as substrates for cell culture and tissue engineering. Biomaterials 2009, 30, 6854–6866. [Google Scholar] [CrossRef]
- Reichl, S.; Borrelli, M.; Geerling, G. Keratin films for ocular surface reconstruction. Biomaterials 2011, 32, 3375–3386. [Google Scholar] [CrossRef]
- Teresa, K.; Justyna, B. Biodegradation of keratin waste: Theory and practical aspects. Waste Manag. 2011, 31, 1689–1701. [Google Scholar]
- Tamreihao, K.; Mukherjee, S.; Khunjamayum, R.; Devi, L.J.; Asem, R.S.; Ningthoujam, D.S. Feather degradation by keratinolytic bacteria and biofertilizing potential for sustainable agricultural production. J. Basic Microbiol. 2019, 59, 4–13. [Google Scholar] [CrossRef]
- Kaur, M.; Bhari, R.; Singh, R.S. Chicken feather waste-derived protein hydrolysate as a potential biostimulant for cultivation of mung beans. Biologia 2021, 76, 1807–1815. [Google Scholar] [CrossRef]
- Timorshina, S.; Popova, E.; Osmolovskiy, A. Sustainable applications of animal waste proteins. Polymers 2022, 14, 1601. [Google Scholar] [CrossRef] [PubMed]
- Li, Q. Perspectives on converting keratin-containing wastes into biofertilizers for sustainable agriculture. Front. Microbiol. 2022, 13, 918262–918274. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, Y.; Zhang, S. Hydrolysis of abandoned bovine hair by pulping spent liquor and preparation of degradable keratin-based sprayable mulch film. BioResources 2020, 15, 5058–5071. [Google Scholar] [CrossRef]
- Natali, M.; Campana, A.; Posati, T.; Benvenuti, E.; Prescimone, F.; Ramirez, D.O.; Varesano, A.; Vineis, C.; Zamboni, R.; Muccini, M.; et al. Engineering of keratin functionality for the realization of bendable all biopolymeric micro-electrode array as humidity sensor. Biosens. Bioelectron. 2019, 141, 111480–111488. [Google Scholar] [CrossRef]
- Hamouche, H.; Makhlouf, S.; Chaouchi, A.; Laghrouche, M. Humidity sensor based on keratin biopolymer film. Sens. Actuators A 2018, 282, 132–141. [Google Scholar] [CrossRef]
- Wang, J.; Wang, N.; Xu, D.; Tang, L.; Sheng, B. Flexible humidity sensors composed with electrodes of laser induced graphene and sputtered sensitive films derived from poly (ether-ether-ketone). Sens. Actuators B 2022, 375, 132846–132870. [Google Scholar] [CrossRef]
- de Aguiar, M.F.; Leal, A.N.; de Melo, C.P.; Alves, K.G. Polypyrrole-coated electrospun polystyrene films as humidity sensors. Talanta 2021, 234, 122636–122643. [Google Scholar] [CrossRef]
- Wang, N.; Tong, J.; Wang, J.; Wang, Q.; Chen, S.; Sheng, B. Polyimide-sputtered and polymerized films with ultrahigh moisture sensitivity for respiratory monitoring and contactless sensing. ACS Appl. Mater. Interfaces 2022, 14, 11842–11853. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Z.; Li, Y.; Yang, M. Single-sided and integrated polyaniline/poly (vinylidene fluoride) flexible membrane with micro/nanostructures as breathable, nontoxic and fast response wearable humidity sensor. J. Colloid Interface Sci. 2022, 607, 367–377. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, Y.; Lei, L.; Hu, S. Moist-electric generators based on electrospun cellulose acetate nanofiber membranes with tree-like structure. J. Membr. Sci. 2022, 662, 120962–120976. [Google Scholar] [CrossRef]
- Song, K.; Qian, X.; Zhu, X.; Li, X.; Hong, X. Fabrication of mechanical robust keratin film by mesoscopic molecular network reconstruction and its performance for dye removal. J. Colloid Interface Sci. 2020, 579, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Aluigi, A.; Rombaldoni, F.; Tonetti, C.; Jannoke, L. Study of methylene blue adsorption on keratin nanofibrous membranes. J. Hazard. Mater. 2014, 268, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, H.; Jin, X.; Wang, H.; Chen, L.; Wang, W.; Lin, T.; Zhu, Z. Preparation of keratin/PET nanofiber membrane and its high adsorption performance of Cr(VI). Sci. Total Environ. 2019, 710, 135546–135578. [Google Scholar] [CrossRef] [PubMed]
- Aluigi, A.; Zoccola, M.; Vineis, C.; Tonin, C.; Ferrero, F.; Canetti, M. Study on the structure and properties of wool keratin regenerated from formic acid. Int. J. Biol. Macromol. 2007, 41, 266–273. [Google Scholar] [CrossRef]
- Zahara, I.; Arshad, M.; Naeth, M.A.; Naeth, A.; Siddique, T.; Ullah, A. Feather keratin derived sorbents for the treatment of wastewater produced during energy generation processes. ACS Med. Chem. Lett. 2021, 273, 128545–128582. [Google Scholar] [CrossRef]
- Zubair, M.; Roopesh, M.S.; Ullah, A. Nano-modified feather keratin derived green and sustainable biosorbents for the remediation of heavy metals from synthetic wastewater. Chemosphere 2022, 308, 136339. [Google Scholar] [CrossRef]
- Olawale, S.A.; Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I.; Okafor, C.C.; Sellaoui, L.; Badawi, M. Thermodynamics and mechanism of the adsorption of heavy metal ions on keratin biomasses for wastewater detoxification. Adsorpt. Sci. Technol. 2022, 2022, 7384924. [Google Scholar] [CrossRef]
- David, P.S.; Karunanithi, A.; Fathima, N.N. Improved filtration for dye removal using keratin–polyamide blend nanofibrous membranes. Environ. Sci. Pollut. Res. 2020, 27, 45629–45638. [Google Scholar] [CrossRef]
- Qian, X.; Zhu, X.; Zhu, W.; Yu, Z.; Song, K. Fabrication of cellulose nanocrystals/keratin composite film and its dye adsorption performance. J. Funct. Polym. 2020, 33, 200–206. [Google Scholar]
- Aluigi, A.; Tonetti, C.; Vineis, C.; Tonin, G.; Mazzuchetti, G. Adsorption of copper(II) ions by keratin/PA6 blend nanofibres. Eur. Polym. J. 2011, 47, 1756–1764. [Google Scholar] [CrossRef]

| Extract Methods | Solvent(s) | Process Conditions | Extraction Theory | Merit | Defect | Ref. |
|---|---|---|---|---|---|---|
| Acidic hydrolysis | HCl solution | Wool was placed in 4 M HCl solution and incubated at 95 °C. | RSSR’ + OH−↔RSH + R’SH | High efficiency, Low molecular weight, Simple and practicable, Low cost. | Significant acid pollution Strong acids destroy the keratin structure and some important amino acids, such as tryptophan, asparagine, arginine, serine, and glutamine. Other amino acids are racemised. | [30,31] |
| Alkaline hydrolysis | NaOH solution | Duck feathers were immersed in 2% (wt) NaOH solution at 60–70 °C for 2 h. | RSSR’ + OH−↔RSSO− + R’H | Keratin amino acid damage during alkaline hydrolysis is substantially lower, as compared to acidic hydrolysis. | Cannot be recycled, Difficult to handle, Time-consuming, Low yield. | [32,33,34,35,36,37] |
| Reduction | 2-mercaptoethanol | Human hair was immersed in the Shindai solution of 25 mM Tris-HCl, 2.6 M thiourea, 5 M urea, 5% (v/v) 2-mercaptoethanol, and then incubated at 50 °C for 72 h. | RSSR’ + 2HOCH2CH2SH↔RSH + R’SH + HOCH2CH2S-SCH2CH2OH | High yield, High efficiency, High molecular weight keratin, The keratin structure is undamaged. | Toxic, Harmful, Expensive, multi-steps, Cannot be recycled, Not industrially viable. | [38,39,40,41,42,43,44,45,46,47,48] |
| Sulphitolysis | Metabisulphite | Wool was placed in a solution of urea (8 M) and metabisulphite (0.5 M), adjusted to pH 6.5, and agitated at 65 °C for 2 h. | RSSR’ + SO32−↔RS− + R’S-SO3− | Highly efficient, Low molecular weight keratin, Short extraction time. | Low solubility, High extract temperature. | [22,49,50,51,52,53] |
| Sodium sulphide | Human hair was immersed in 0.125 M sodium sulphide solution and incubated at 40 °C for 4 h. | Na2S + H2O↔2Na+ + HS− + OH− RSSR’ + HS−↔RSH + R’S-S− | Efficient, Economical, High yield, Low extract temperature, The antioxidant potency and the secondary structure of keratin are saved. | Time-consuming, Low solubility. | [54,55,56,57,58,59] | |
| L-cysteine | Chicken feathers were placed in the solutions of 8 M urea and 0.165 M of L-cysteine, adjusted to pH 10.5 using NaOH (2 M). | RSSR’ + 2−SCH2CH(NH2)COO−↔RS− + R’S− + −OOC(NH2)CHCH2S-SCH2CH(NH2)COO− | Eco-friendly, More crystalline structure, The structure of keratin is better saved. Low-cost, Mild treatment conditions, L-cysteine is non-toxic. | Low yield. | [60,61,62,63,64,65] | |
| Sodium bisulphite | Chicken feathers were added to a solution of 1.78% NaOH and 0.5% NaHSO3, and incubated at 87 °C for 111 min. | RSSR’ + HSO3−↔RSH + R’S- SO3− | Highly effective, Low-cost, Moderate molecular weight keratin, Moderate solubility, Non-toxic. | High extract temperature. | [9,66] | |
| oxidation | Peracetic acid | Human hair was placed in 2.5% (w/v) peracetic acid solution overnight at room temperature. | RSSR’ + 3O2−↔RSO3− + R’SO3− | Low molecular weight keratin, Mild extraction conditions, Good solubility. | Low yield. | [67,68,69] |
| Enzymatic hydrolysis | Keratinases | Wool was immersed in 100 mL of a water solution containing 100 kU of keratinase and vigorously stirred for 24 h at 50 °C. | The peptide bonds on the keratin backbone are interrupted using an enzymatic lytic response. | Bioactive keratins, Eco-friendly, Few species alterations, Safe, Mild treatment conditions, No contaminants. | Expensive, Low efficiency, Time-consuming. | [23,70,71,72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Tong, H. Preparation Methods and Functional Characteristics of Regenerated Keratin-Based Biofilms. Polymers 2022, 14, 4723. https://doi.org/10.3390/polym14214723
Wang R, Tong H. Preparation Methods and Functional Characteristics of Regenerated Keratin-Based Biofilms. Polymers. 2022; 14(21):4723. https://doi.org/10.3390/polym14214723
Chicago/Turabian StyleWang, Ruirui, and Hui Tong. 2022. "Preparation Methods and Functional Characteristics of Regenerated Keratin-Based Biofilms" Polymers 14, no. 21: 4723. https://doi.org/10.3390/polym14214723
APA StyleWang, R., & Tong, H. (2022). Preparation Methods and Functional Characteristics of Regenerated Keratin-Based Biofilms. Polymers, 14(21), 4723. https://doi.org/10.3390/polym14214723








