Preparation of Citral Compound and Its Bamboo Antimildew Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Citral Compound
2.2.2. Fourier-Transform Infrared Spectroscopy Analysis of Citral Compounds
2.2.3. Bacteriostatic Properties of Citral Compounds against Bamboo Mold
2.2.4. Antimildew Treatment of Bamboo with Citral Compound
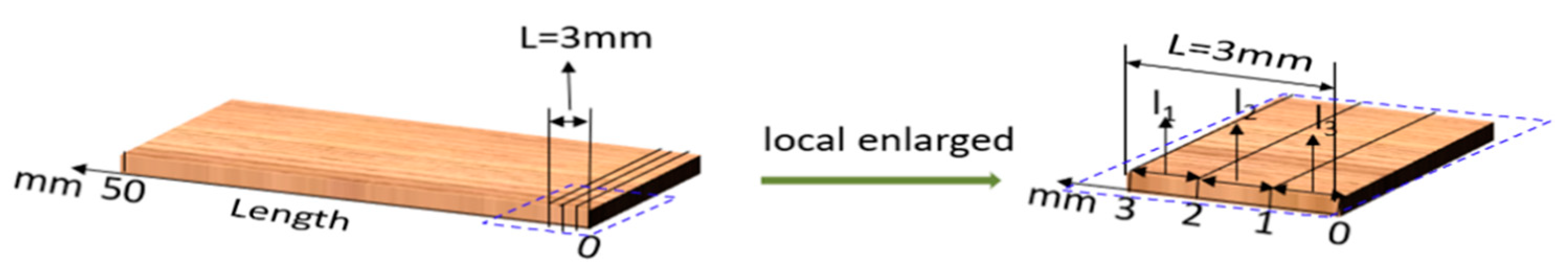
2.2.5. Distribution of Citral Compound in Antimildew-Treated Bamboo
2.2.6. Control Effect of the Citral Compound against Bamboo Mildew
3. Results and Discussion
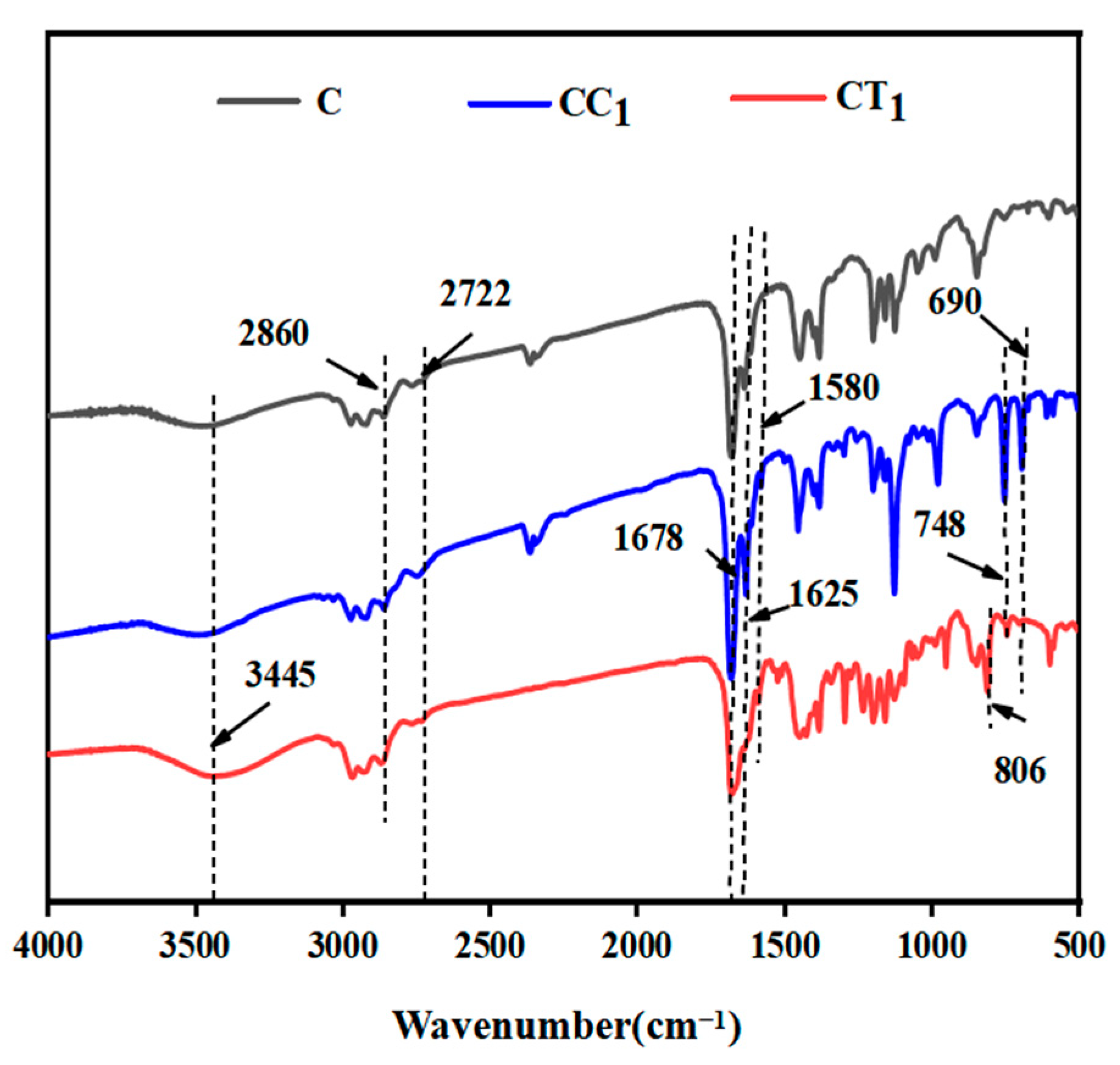
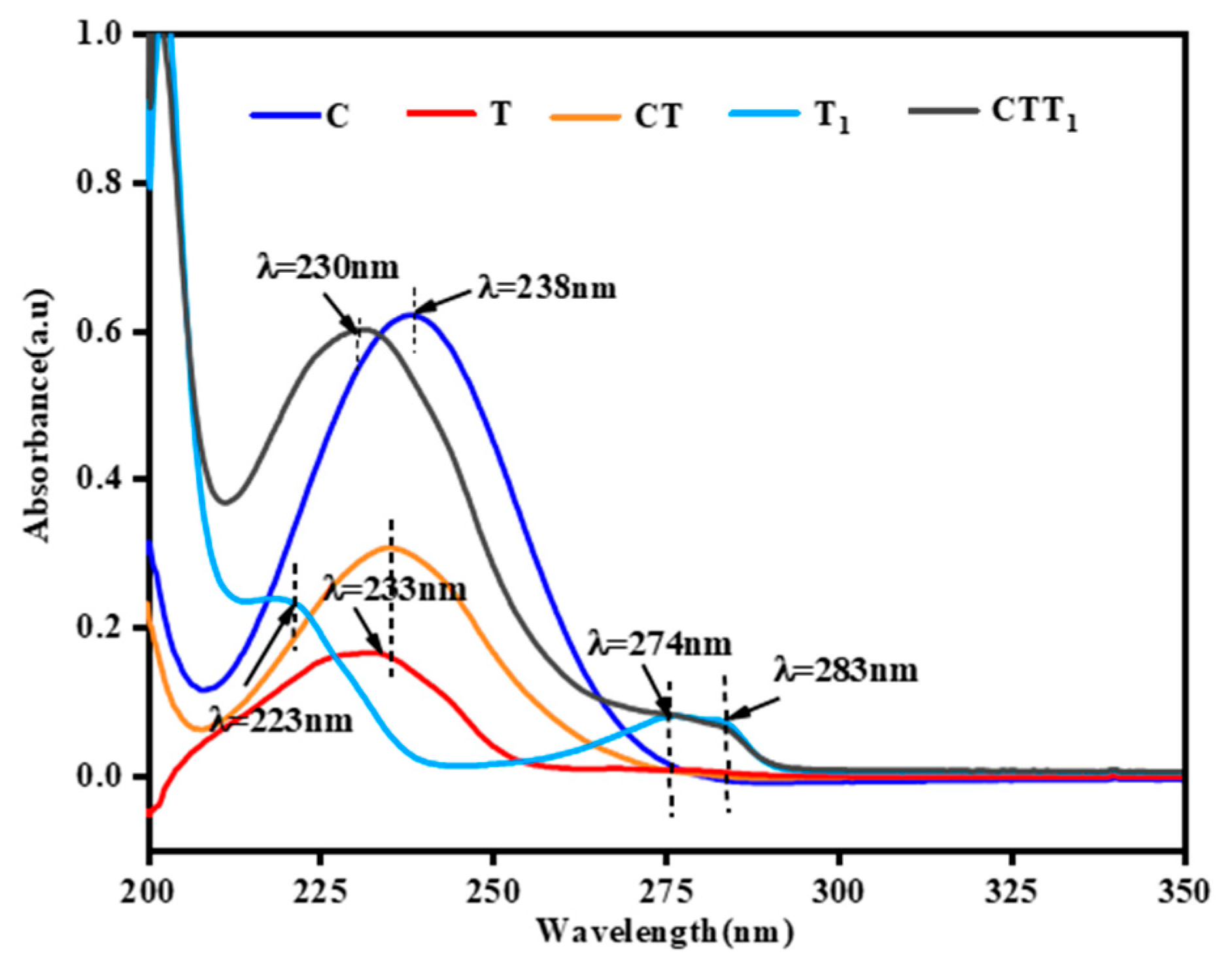
3.1. FT-IR Analysis of Citral and Its Compound Solutions
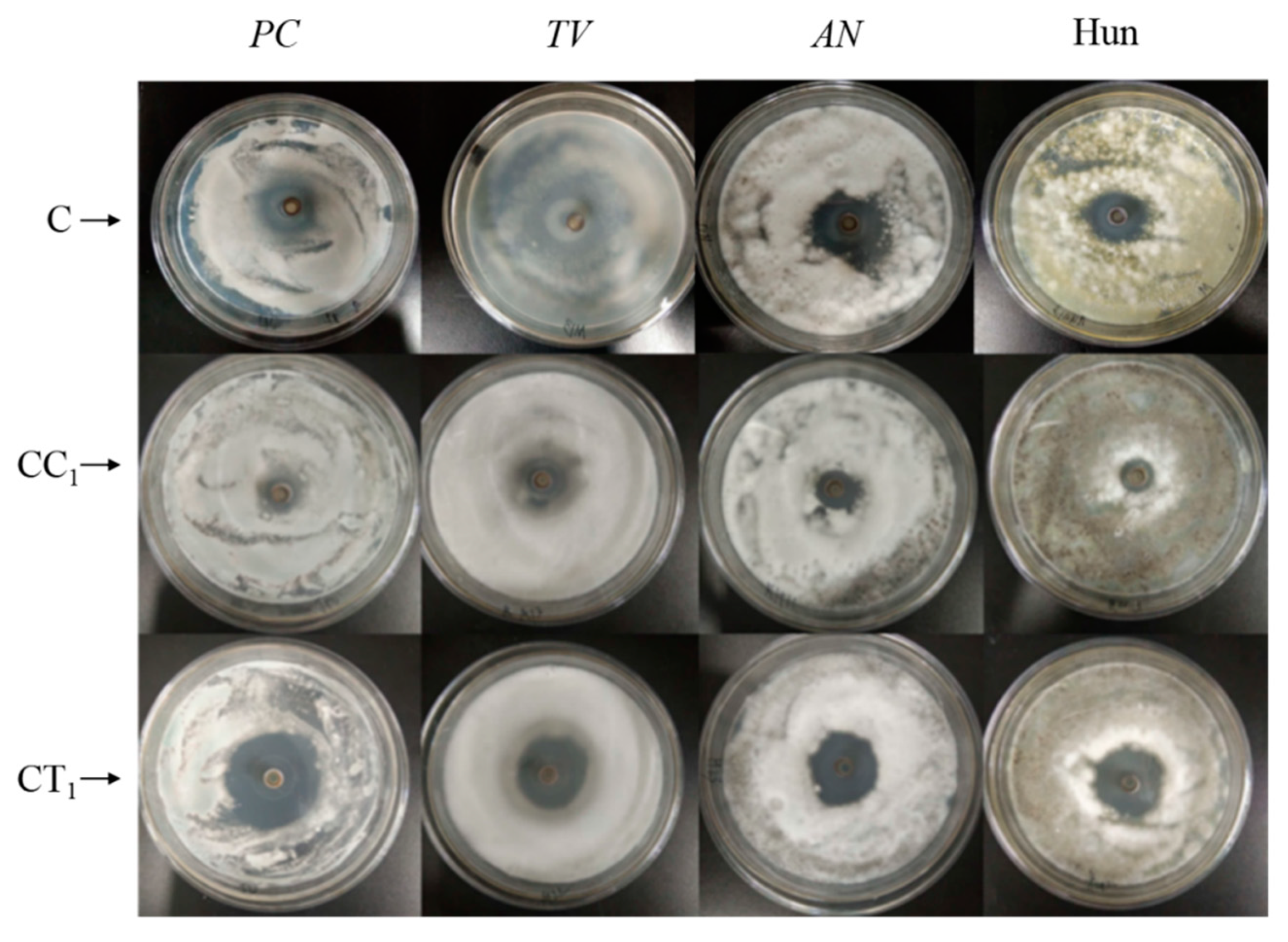
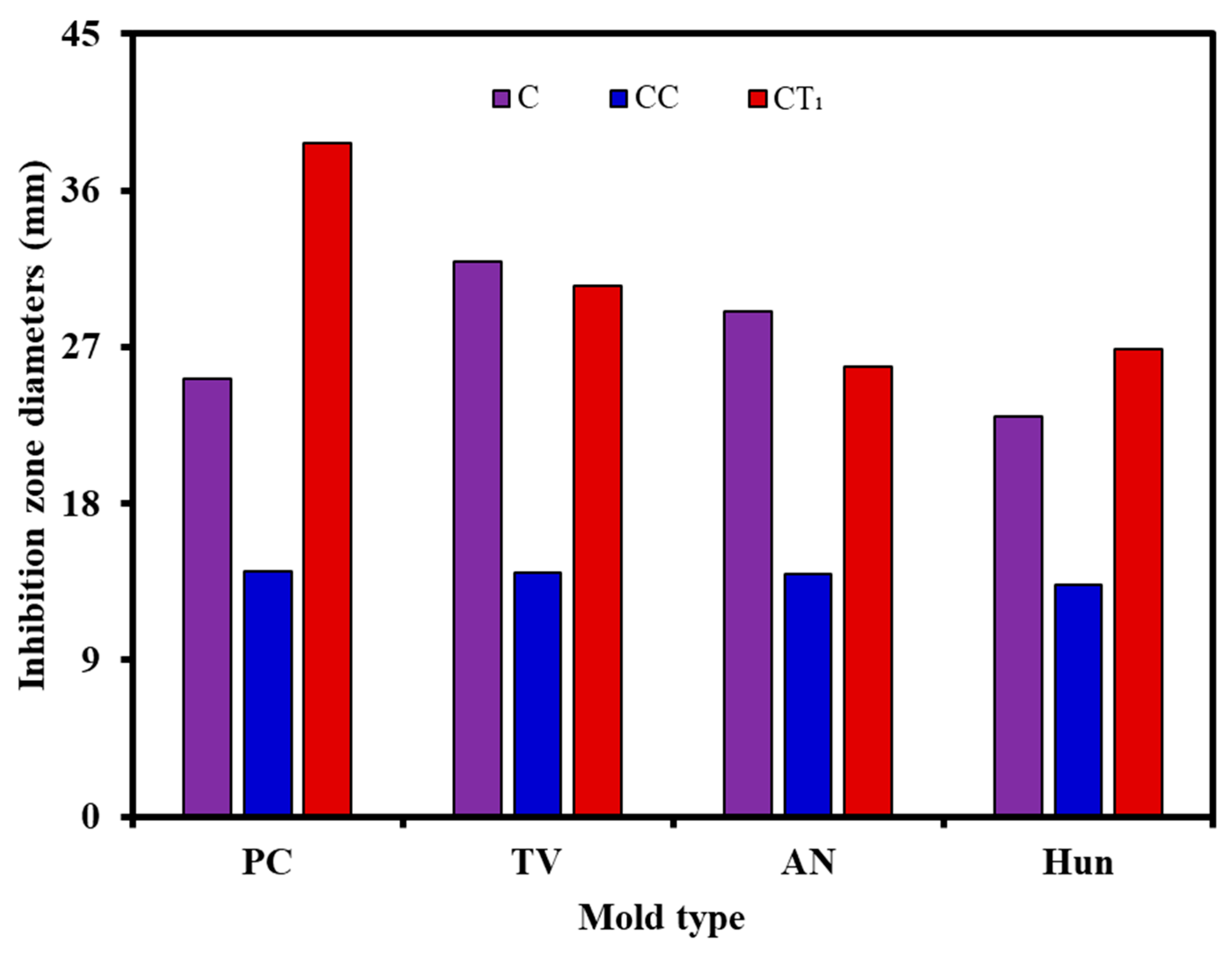
3.2. Bacteriostatic Properties of Citral Compounds
3.3. Distribution of Citral Compound in Antimildew-Agent-Treated Bamboo
3.4. Drug Loading of Citral Compound Agent in Antimildew-Treated Bamboo and Control Effect of Antimildew-Treated Bamboo
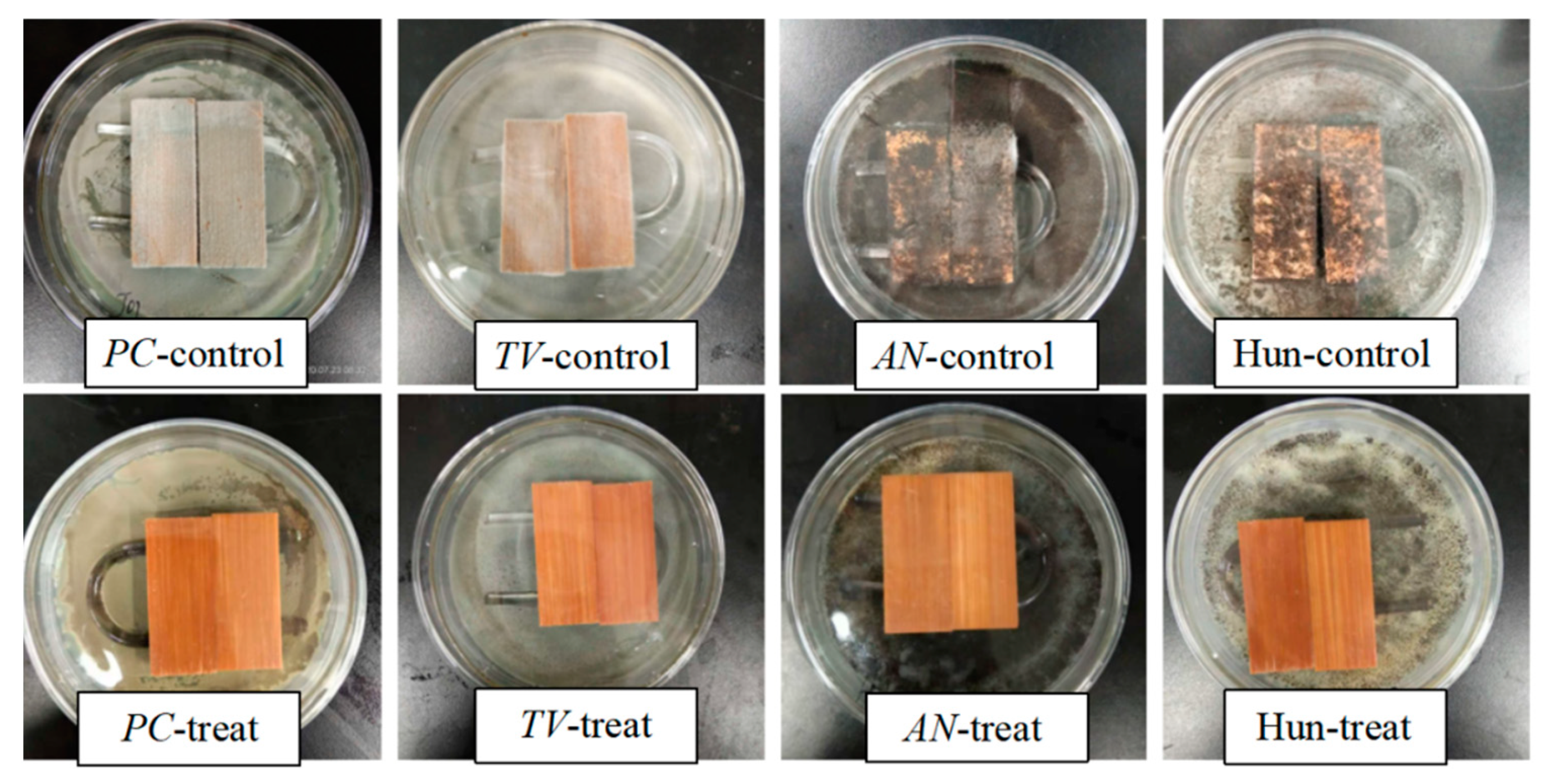
3.5. Antimildew Properties of Citral Compounds
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, Z.; Chen, C.; Mi, R.; Gan, W.; Dai, J.; Jiao, M.; Xie, H.; Yao, Y.; Xiao, S.; Hu, L. A strong, tough, and scalable structural material from fast-growing bamboo. Adv. Mater. 2020, 32, 1906308. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ma, Y.; Lin, H.; Zheng, Q.; Zhang, X.; Yang, W.; Li, R. Fabrication of hydrophobic ZnO/PMHS coatings on bamboo surfaces: The synergistic effect of ZnO and PMHS on anti-mildew properties. Coatings 2018, 9, 15. [Google Scholar] [CrossRef]
- Li, W.; Chen, L.; Li, Y.; Li, X. Bamboo modification with 1, 3-dimethylol-4, 5-dihydroxyethyleneurea (DMDHEU) catalyzed by maleic anhydride. J. Wood Chem. Technol. 2020, 40, 126–135. [Google Scholar] [CrossRef]
- Li, J.; Wu, Z.; Bao, Y.; Chen, Y.; Huang, C.; Li, N.; He, S.; Chen, Z. Wet chemical synthesis of ZnO nanocoating on the surface of bamboo timber with improved mould-resistance. J. Saudi Chem. Soc. 2017, 21, 920–928. [Google Scholar] [CrossRef]
- Ren, D.; Li, J.; Bao, Y.; Wu, Z.; He, S.; Wang, A.; Guo, F.; Chen, Y. Low-temperature synthesis of flower-like ZnO microstructures supported on TiO2 thin films as efficient antifungal coatings for bamboo protection under dark conditions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 381–388. [Google Scholar] [CrossRef]
- Yu, H.; Du, C.; Huang, Q.; Yao, X.; Hua, Y.; Zhang, W.; Zhou, Z.; Liu, H. Effects of extraction methods on anti-mould property of bamboo strips. BioResources 2018, 13, 2658–2669. [Google Scholar] [CrossRef]
- Yu, H.; Du, C.; Liu, H.; Wei, J.; Zhou, Z. Advance in Anti-mildew Research of Bamboo. J. Bamboo Res. 2016, 35, 46–51. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Thielmann, J.; Muranyi, P. Review on the chemical composition of Litsea cubeba essential oils and the bioactivity of its major constituents citral and limonene. J. Essent. Oil Res. 2019, 31, 361–378. [Google Scholar] [CrossRef]
- Qiuli, O.; Lei, J.; Nengguo, T. Citral Inhibits Mycelial Growth of Penicillium digitatum Involving Membrane Peroxidation. Food Sci. 2016, 37, 32–37. [Google Scholar]
- Xu, M. The Study on Composition Analysis and Antibacterial Activity from Cymbopogon Citratus (DC.) Stapf Oil; Central South University of Forestry & Technology: Changsha, China, 2015. [Google Scholar]
- Shi, C. The Antimicrobial Activity and Anti-Infection Effect against Cronobacter Sakazakii of Citral and Mechaniams of Action; Northwest A&F University: Yangling, China, 2017. [Google Scholar]
- Xia, S.; Jia, Q.; Wang, P.; Hu, X.; Zhu, L.; Zhu, P.; Wang, Z. Inhibition of Citral on Aspergillus flavus Isolated from Moldy Chinese Medicinal Materials. Biol. Disaster Sci. 2021, 44, 264–270. [Google Scholar]
- Tao, N.; Duan, X.; Fan, F.; Huang, S. Inhibitory effects of citral and octanal mixture on Penicillium digitatum. Mod. Food Sci. Technol 2015, 31, 73–76. [Google Scholar]
- Tao, N.; Jia, L.; Zhou, H. Anti-fungal activity of Citrus reticulata Blanco essential oil against Penicillium italicum and Penicillium digitatum. Food Chem. 2014, 153, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Du, C.; Li, Q.; Hu, A.; Peng, R.; Sun, F.; Zhang, W. Inhibition mechanism and antibacterial activity of natural antibacterial agent citral on bamboo mould and its anti-mildew effect on bamboo. R. Soc. Open Sci. 2021, 8, 202244. [Google Scholar] [CrossRef]
- Zhang, J.; Du, C.; Peng, R.; Hu, A.; Li, Q.; Liu, C.; Shan, Y.; Chen, S.; Yin, W. Antimildew Treatment and Control Effect of Citral on Bamboo. Int. J. Polym. Sci. 2021, 2021, 5949458. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wan, C.P.; Peng, X.; Chen, J.Y. Study on the Antifungal Mechanisms of the Main Active Ingredients of Ramulus cinnamomi against Penicillium italicum. Mod. Food Sci. Technol. 2016, 32, 45–51. [Google Scholar]
- Huang, M.; Zhao, W.; Bai, W.; Qian, M. Review on Antibacterial Effect of Cinnamaldehyde. China Condiment 2015, 40, 137–140. [Google Scholar]
- He, X.; Dai, Y.; Li, X.; He, J.; Li, C.; Chen, J.; Yi, L. Antibacterial Mechanism of Cinnamaldehyde on Salmonella typhimuriumin vitro. Acta Agric. Univ. Jiangxiensis 2020, 42, 150–156. [Google Scholar]
- Kim, J.H.; Faria, N.C.; Martins, M.D.L.; Chan, K.L.; Campbell, B.C. Enhancement of antimycotic activity of amphotericin B by targeting the oxidative stress response of Candida and Cryptococcus with natural dihydroxybenzaldehydes. Front. Microbiol. 2012, 3, 261. [Google Scholar] [CrossRef]
- Huang, F.; Kong, J.; Ju, J.; Zhang, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Xie, Y.; Yao, W. Membrane damage mechanism contributes to inhibition of trans-cinnamaldehyde on Penicillium italicum using Surface-Enhanced Raman Spectroscopy (SERS). Sci. Rep. 2019, 9, 490. [Google Scholar] [CrossRef]
- Sun, Q.; Shang, B.; Wang, L.; Lu, Z.; Liu, Y. Cinnamaldehyde inhibits fungal growth and aflatoxin B1 biosynthesis by modulating the oxidative stress response of Aspergillus flavus. Appl. Microbiol. 2016, 100, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Bensmira, M.; Jiang, B.; Nsabimana, C.; Jian, T. Effect of Lavender and Thyme incorporation in sunflower seed oil on its resistance to frying temperatures. Food Res. Int. 2007, 40, 341–346. [Google Scholar] [CrossRef]
- Solomakos, N.; Govaris, A.; Koidis, P.; Botsoglou, N. The antimicrobial effect of thyme essential oil, nisin, and their combination against Listeria monocytogenes in minced beef during refrigerated storage. Food Microbiol. 2008, 25, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Liang, Y.; Jiang, S.; Yang, L.; Shi, Y.; Guo, S.; Zhang, C. Physical, antioxidant and antimicrobial properties of modified peanut protein isolate based films incorporating thymol. RSC Adv. 2017, 7, 41610–41618. [Google Scholar] [CrossRef]
- Asprea, M.; Leto, I.; Bergonzi, M.C.; Bilia, A.R. Thyme essential oil loaded in nanocochleates: Encapsulation efficiency, invitro release study and antioxidant activity. LWT-Food Sci. Technol. 2017, 77, 497–502. [Google Scholar] [CrossRef]
- Bi, X.; Meng, R.; Guo, N.; Yu, L.; Ge, F.; Zeng, F.; Deng, X. Study on in Vitro Antifungal Activity of Thymol. Chin. Agric. Sci. Bull. 2009, 25, 21–24. [Google Scholar]
- Wang, D.; Xie, H.; Cao, K.; Ke, C.; Qian, S.; Shi, Y.; Ren, M. Study on antimicrobial activity of thymol and its influencing factors. Sci. Technol. Food Ind. 2012, 33, 96–99. [Google Scholar]
- Zhong, S.; Wu, K.; Chai, X.; Li, S. Study on bacteriostasis of seven isolate spices to food spoilage organisms. Sci. Technol. Food Ind. 2009, 30, 68–71. [Google Scholar]
- Walczak, M.; Michalska-Sionkowska, M.; Kaczmarek, B.; Sionkowska, A. Surface and antibacterial properties of thin films based on collagen and thymol. Mater. Today Commun. 2020, 22, 100949. [Google Scholar] [CrossRef]
- Wei, J.; Bi, Y.; Xue, H.; Wang, Y.; Zong, Y.; Prusky, D. Antifungal activity of cinnamaldehyde against Fusarium sambucinum involves inhibition of ergosterol biosynthesis. J. Appl. Microbiol. 2020, 129, 256–265. [Google Scholar] [CrossRef]
- Morsy, N.F. Production of thymol rich extracts from ajwain (Carum copticum L.) and thyme (Thymus vulgaris L.) using supercritical CO2. Ind. Crops Prod. 2020, 145, 112072. [Google Scholar] [CrossRef]
- Ruan, H. Application of Cinnamaldehydein Essence and Flavor Daily Chemical and Food Additive Industries. Fine Spec. Chem. 2005, Z1, 9–10. [Google Scholar]
- Singh, T.; Chittenden, C. Efficacy of essential oil extracts in inhibiting mould growth on panel products. Build. Environ. 2010, 45, 2336–2342. [Google Scholar] [CrossRef]
- Yang, D.; Wang, H.; Li, S.; Yuan, H. Decay and mould resistance of cinnamaldehyde and its derivatives. J. For. Eng. 2017, 2, 46–50. [Google Scholar]
- Tan, C.; Zhu, M.; Du, S.; Yao, Y. Study on the Inhibition Zone Method in Antimicrobial Test. Food Ind. 2016, 11, 122–125. [Google Scholar]
- GB/T 18261-2013; Test Method for Anti-Mildew Agents in Controlling Wood Mould and Stain Fungi. National Standardization Technical Committee Wood: Beijing China, 2013.
- Tian, H.X.; Lu, Z.Y.; Li, D.F.; Hu, J. Preparation and characterization of citral-loaded solid lipid nanoparticles. Food Chem. 2018, 248, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.A.; Lima, B.S.; Trindade, G.G.; Souza, E.P.; Mota, D.S.; Heimfarth, L.; Quintans, J.S.; Quintans-Júnior, L.J.; Sussuchi, E.M.; Sarmento, V.H. Anti-hyperalgesic and anti-inflammatory effects of citral with β-cyclodextrin and hydroxypropyl-β-cyclodextrin inclusion complexes in animal models. Life Sci. 2019, 229, 139–148. [Google Scholar] [CrossRef]
- Higueras, L.; Lopez-Carballo, G.; Gavara, R.; Hernandez-Munoz, P. Reversible Covalent Immobilization of Cinnamaldehyde on Chitosan Films via Schiff Base Formation and Their Application in Active Food Packaging. Food Bioprocess Technol. 2015, 8, 526–538. [Google Scholar] [CrossRef]
- Bian, Y. Synthesis Research of Thymol and Its Derivatives; Nanjing Forestry University: Nanjing, China, 2015. [Google Scholar]
- Qu, C.; Pan, L.; Tu, K.; Yang, L. Research of the inhibition activities effect of complex essential oil on Aspergillus flavus. Sci. Technol. Food Ind. 2012, 33, 128–130. [Google Scholar]
- Lv, H.; Zhao, L.; Huo, S.; Wang, R. Cinnamon-mountain fang complex plant essential oil against moldy peanuts Antibacterial effect of aspergillus niger BQM. J. Chin. Inst. Food Sci. Technol. 2021, 21, 222–229. [Google Scholar]
- Wu, K.; Luo, M.; Wei, H. Evaluation of the In Vitro Antimicrobial Effects of Eight Plant Essential Oils against Common Intestinal Microorganisms. Mod. Food Sci. Technol. 2017, 33, 133–141. [Google Scholar]
- Cheng, S.-S.; Liu, J.-Y.; Chang, E.-H.; Chang, S. Antifungal activity of cinnamaldehyde and eugenol congeners against wood-rot fungi. Bioresour. Technol. 2008, 99, 5145–5149. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Alfonso, C.; Martínez-Romero, D.; Zapata, P.; Serrano, M.; Valero, D.; Castillo, S. The effects of essential oils carvacrol and thymol on growth of Penicillium digitatum and P. italicum involved in lemon decay. Int. J. Food Microbiol. 2012, 158, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Ju, J. Study on the Synergistic Inhibitory Mechanism of Eugenol and Citral against Penicillium Roqueforti and Aspergillus Niger; Jiangnan University: Wuxi, China, 2021. [Google Scholar]

| Name of the Citral Compound | Quality of Citral/mg | Quality of Cinnamaldehyde/mg | Quality of Thymol/mg | Tween 80 Volume/mL |
|---|---|---|---|---|
| CC1 (citral + cinnamaldehyde) | 5000 | 2500 | – | 2 |
| CT1 (citral + thymol) | 5000 | – | 2500 | 2 |
| Infection Value | Specimen Infection Area |
|---|---|
| 0 | No hyphae or mildew on the sample surface |
| 1 | Infected area of sample < 1/4 |
| 2 | Infected area of sample 1/4–1/2 |
| 3 | Infected area of sample 1/2–3/4 |
| 4 | Infected area of sample > 3/4 |
| Name | The Bacteriostatic Rate of Bamboo Mold (%) | |||
|---|---|---|---|---|
| PC | TV | AN | Hun | |
| C | 140.06 | 356.28 | 290.12 | 231.12 |
| CC1 | 33.39 | 100.57 | 86.96 | 91.50 |
| CT1 | 269.30 | 335.86 | 247.31 | 287.18 |
| Name | Drug Loading (g/m2) | Antimildew Efficiency (%) | |||
|---|---|---|---|---|---|
| PC | TV | AN | Hun | ||
| C | 119.250 | 100 | 100 | 100 | 100 |
| CC1 | 108.219 | 100 | 100 | 99.65 | 100 |
| CT1 | 116.270 | 100 | 100 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, Y.; Chen, S.; Zhang, J.; Du, C.; Liu, C.; Yang, F.; Yin, W.; Shao, Y.; Wang, Y. Preparation of Citral Compound and Its Bamboo Antimildew Properties. Polymers 2022, 14, 4691. https://doi.org/10.3390/polym14214691
Shan Y, Chen S, Zhang J, Du C, Liu C, Yang F, Yin W, Shao Y, Wang Y. Preparation of Citral Compound and Its Bamboo Antimildew Properties. Polymers. 2022; 14(21):4691. https://doi.org/10.3390/polym14214691
Chicago/Turabian StyleShan, Yingying, Shiqin Chen, Jingjing Zhang, Chungui Du, Chunlin Liu, Fei Yang, Wenxiu Yin, Yuran Shao, and Yuting Wang. 2022. "Preparation of Citral Compound and Its Bamboo Antimildew Properties" Polymers 14, no. 21: 4691. https://doi.org/10.3390/polym14214691
APA StyleShan, Y., Chen, S., Zhang, J., Du, C., Liu, C., Yang, F., Yin, W., Shao, Y., & Wang, Y. (2022). Preparation of Citral Compound and Its Bamboo Antimildew Properties. Polymers, 14(21), 4691. https://doi.org/10.3390/polym14214691









