Solid-State NMR Spectroscopy: Towards Structural Insights into Starch-Based Materials in the Food Industry
Abstract
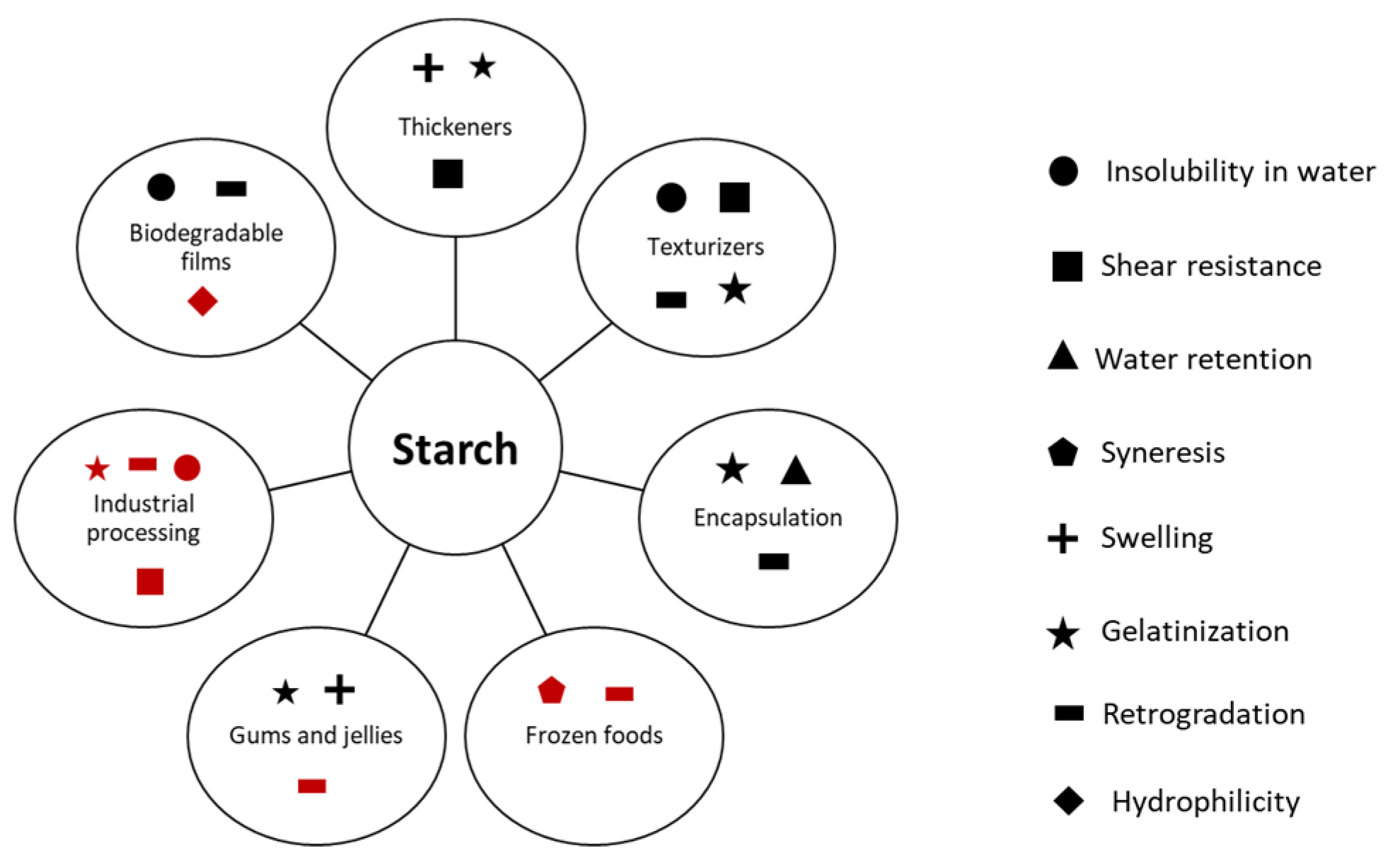
1. Introduction
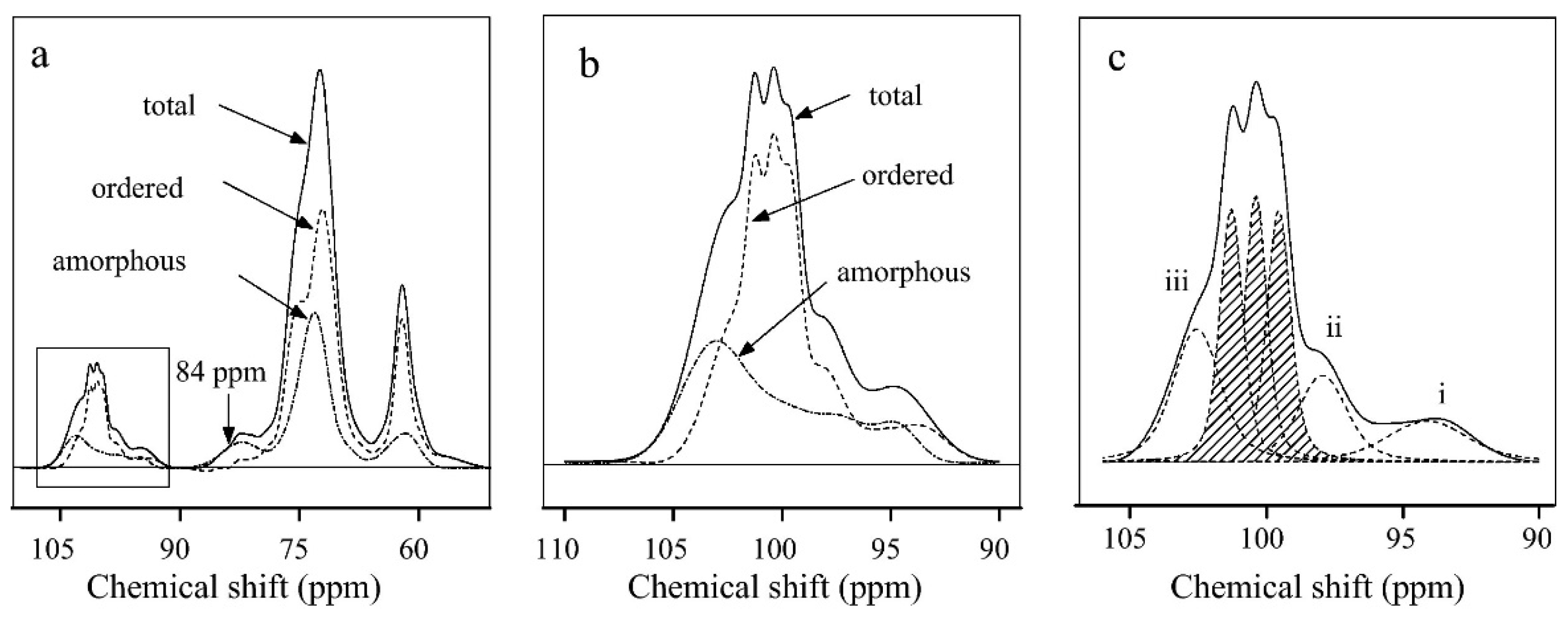
2. Study of Starch Polymorphism
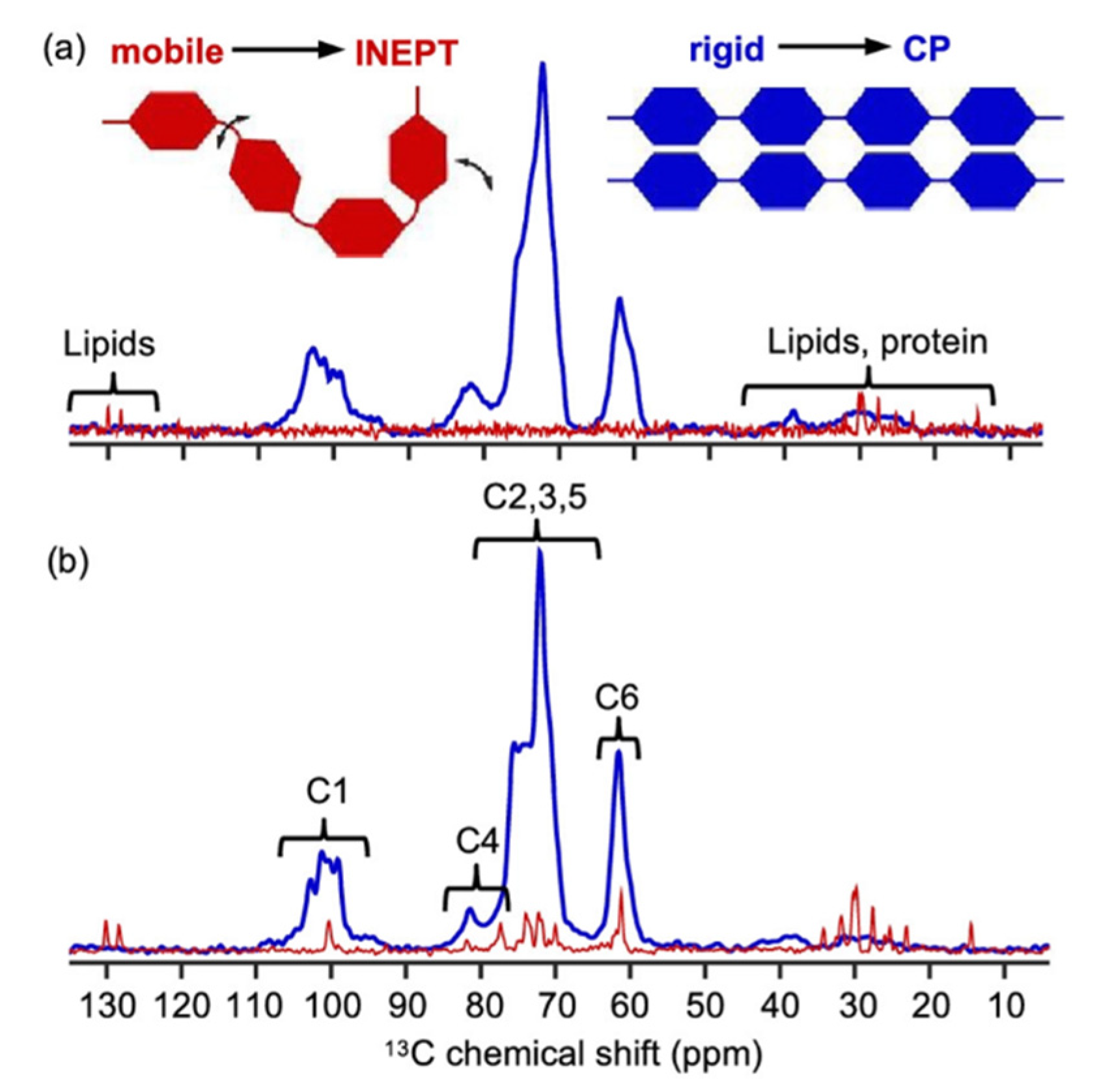
3. Study of Structural and Dynamic Heterogeneity in Starch
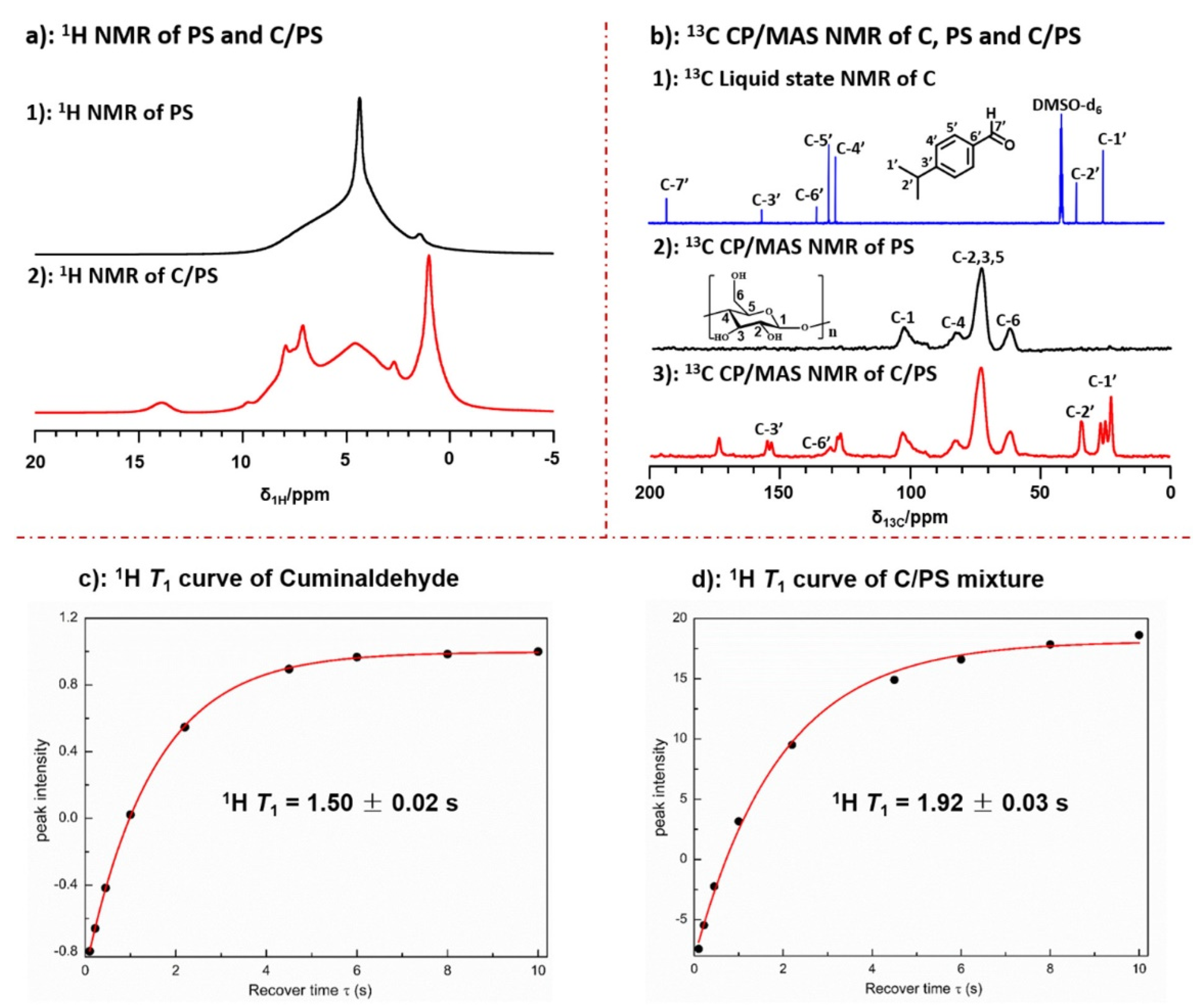
4. Study of Dynamics in Starch in the Presence of Plasticizers and Structural Modifications
5. Future Perspectives and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- García-Oliveira, P.; Fraga-Corral, M.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Solutions for the Sustainability of the Food Production and Consumption System. Crit. Rev. Food Sci. Nutr. 2022, 62, 1765–1781. [Google Scholar] [CrossRef] [PubMed]
- Blazek, J.; Gilbert, E.P. Application of Small-Angle X-ray and Neutron Scattering Techniques to the Characterisation of Starch Structure: A Review. Carbohydr. Polym. 2011, 85, 281–293. [Google Scholar] [CrossRef]
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and Functionality of Starch. Food Hydrocoll. 2009, 23, 1527–1534. [Google Scholar] [CrossRef]
- Bangar, S.P.; Ashogbon, A.O.; Singh, A.; Chaudhary, V.; Whiteside, W.S. Enzymatic Modification of Starch: A Green Approach for Starch Applications. Carbohydr. Polym. 2022, 287, 119265. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, I.; Pooja, N.; Mal, S.S.; Paul, U.C.; Rahman, M.H.; Mazumder, N. An Insight into the Gelatinization Properties Influencing the Modified Starches Used in Food Industry: A Review. Food Bioprocess Technol. 2022, 15, 1195–1223. [Google Scholar] [CrossRef]
- Cui, C.; Jia, Y.; Sun, Q.; Yu, M.; Ji, N.; Dai, L.; Wang, Y.; Qin, Y.; Xiong, L.; Sun, Q. Recent Advances in the Preparation, Characterization, and Food Application of Starch-Based Hydrogels. Carbohydr. Polym. 2022, 291, 119624. [Google Scholar] [CrossRef] [PubMed]
- Obadi, M.; Xu, B. Review on the Physicochemical Properties, Modifications, and Applications of Starches and Its Common Modified Forms Used in Noodle Products. Food Hydrocoll. 2021, 112, 106286. [Google Scholar] [CrossRef]
- Fan, Y.; Picchioni, F. Modification of Starch: A Review on the Application of “Green” Solvents and Controlled Functionalization. Carbohydr. Polym. 2020, 241, 116350. [Google Scholar] [CrossRef]
- Boutboul, A.; Giampaoli, P.; Feigenbaum, A.; Ducruet, V. Influence of the Nature and Treatment of Starch on Aroma Retention. Carbohydr. Polym. 2002, 47, 73–82. [Google Scholar] [CrossRef]
- Baysal, G.; Doğan, F. Investigation and Preparation of Biodegradable Starch-Based Nanofilms for Potential Use of Curcumin and Garlic in Food Packaging Applications. J. Biomater. Sci. 2020, 31, 1127–1143. [Google Scholar] [CrossRef]
- Baysal, G.; Çelik, B.Y. Synthesis and Characterization of Antibacterial Bio-Nano Films for Food Packaging. J. Environ. Sci. Health Part B 2019, 54, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Qiao, D.; Zhao, S.; Zhang, B.; Xie, F. Starch-Based Materials Encapsulating Food Ingredients: Recent Advances in Fabrication Methods and Applications. Carbohydr. Polym. 2021, 270, 118358. [Google Scholar] [CrossRef] [PubMed]
- Calvert, P. The Structure of Starch. Nature 1997, 389, 338–339. [Google Scholar] [CrossRef]
- Karim, A.A.; Norziah, M.H.; Seow, C.C. Methods for the Study of Starch Retrogradation. Food Chem. 2000, 71, 9–36. [Google Scholar] [CrossRef]
- Liu, P.; Wang, R.; Kang, X.; Cui, B.; Yu, B. Effects of Ultrasonic Treatment on Amylose-Lipid Complex Formation and Properties of Sweet Potato Starch-Based Films. Ultrason. Sonochem. 2018, 44, 215–222. [Google Scholar] [CrossRef]
- Pokhrel, S. A Review on Introduction and Applications of Starch and Its Biodegradable Polymers. Int. J. Environ. 2015, 4, 114–125. [Google Scholar] [CrossRef]
- Wu, A.C.; Witt, T.; Gilbert, R.G. Characterization Methods for Starch-Based Materials: State of the Art and Perspectives. Aust. J. Chem. 2013, 66, 1550–1563. [Google Scholar] [CrossRef]
- Zhu, F. NMR Spectroscopy of Starch Systems. Food Hydrocoll. 2017, 63, 611–624. [Google Scholar] [CrossRef]
- Santana, Á.L.; Angela, A.; Meireles, M. New Starches Are the Trend for Industry Applications: A Review. Food Public Health 2014, 4, 229–241. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, L.; McClements, D.J.; Yang, T.; Zhang, Z.; Ren, F.; Miao, M.; Tian, Y.; Jin, Z. Starch-Based Biodegradable Packaging Materials: A Review of Their Preparation, Characterization and Diverse Applications in the Food Industry. Trends Food Sci. Technol. 2021, 114, 70–82. [Google Scholar] [CrossRef]
- Ashogbon, A.O.; Akintayo, E.T. Recent Trend in the Physical and Chemical Modification of Starches from Different Botanical Sources: A Review. Starch 2014, 66, 41–57. [Google Scholar] [CrossRef]
- Himashree, P.; Sengar, A.S.; Sunil, C.K. Food Thickening Agents: Sources, Chemistry, Properties and Applications—A Review. Int. J. Gastron. Food Sci. 2022, 27, 100468. [Google Scholar] [CrossRef]
- Ding, Y.; Lin, Q.; Kan, J. Development and Characteristics Nanoscale Retrograded Starch as an Encapsulating Agent for Colon-Specific Drug Delivery. Colloids Surf. B Biointerfaces 2018, 171, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Benavent-Gil, Y.; Rosell, C.M. Comparison of Porous Starches Obtained from Different Enzyme Types and Levels. Carbohydr. Polym. 2017, 157, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Blennow, A. Starch Bioengineering. Starch 2018, 70, 1870006. [Google Scholar] [CrossRef]
- Luallen, T. 13—Utilizing Starches in Product Development. In Starch in Food; Eliasson, A.-C., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2004; pp. 393–424. ISBN 978-1-85573-731-0. [Google Scholar]
- Dong, H.; Zhang, Q.; Gao, J.; Chen, L.; Vasanthan, T. Comparison of Morphology and Rheology of Starch Nanoparticles Prepared from Pulse and Cereal Starches by Rapid Antisolvent Nanoprecipitation. Food Hydrocoll. 2021, 119, 106828. [Google Scholar] [CrossRef]
- Blazek, J.; Gilbert, E.P.; Copeland, L. Effects of Monoglycerides on Pasting Properties of Wheat Starch after Repeated Heating and Cooling. J. Cereal Sci. 2011, 54, 151–159. [Google Scholar] [CrossRef]
- Lian, X.; Cheng, K.; Wang, D.; Zhu, W.; Wang, X. Analysis of Crystals of Retrograded Starch with Sharp X-Ray Diffraction Peaks Made by Recrystallization of Amylose and Amylopectin. Int. J. Food Prop. 2017, 20, S3224–S3236. [Google Scholar] [CrossRef]
- Liu, X.; Luan, H.; Jinglin, Y.; Wang, S.; Wang, S.; Copeland, L. A Method for Characterizing Short-Range Molecular Order in Amorphous Starch. Carbohydr. Polym. 2020, 242, 116405. [Google Scholar] [CrossRef]
- Oleyaei, S.A.; Zahedi, Y.; Ghanbarzadeh, B.; Moayedi, A.A. Modification of Physicochemical and Thermal Properties of Starch Films by Incorporation of TiO2 Nanoparticles. Int. J. Biol. Macromol. 2016, 89, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Domene-López, D.; García-Quesada, J.C.; Martin-Gullon, I.; Montalbán, M.G. Influence of Starch Composition and Molecular Weight on Physicochemical Properties of Biodegradable Films. Polymers 2019, 11, 1084. [Google Scholar] [CrossRef] [PubMed]
- Korkut, A.; Kahraman, K. Production of Cross-Linked Resistant Starch from Tapioca Starch and Effect of Reaction Conditions on the Functional Properties, Morphology, X ray Pattern, FT-IR Spectra and Digestibility. J. Food Meas. Charact. 2021, 15, 1693–1702. [Google Scholar] [CrossRef]
- Sujka, M.; Jamroz, J. Ultrasound-Treated Starch: SEM and TEM Imaging, and Functional Behaviour. Food Hydrocoll. 2013, 31, 413–419. [Google Scholar] [CrossRef]
- Genkina, N.K.; Kurkovskaya, L.N. A Novel Method for the Determination of Phospholipids in Starch Matrixes. J. Anal. Chem. 2013, 68, 170–172. [Google Scholar] [CrossRef]
- Lim, Y.-M.; Hoobin, P.; Ying, D.; Burgar, I.; Gooley, P.R.; Augustin, M.A. Physical Characterisation of High Amylose Maize Starch and Acylated High Amylose Maize Starches. Carbohydr. Polym. 2015, 117, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, P.; Nitschke, F.; Steup, M.; Mallow, K.; Specker, E. Determination of Glucan Phosphorylation Using Heteronuclear 1H, 13C Double and 1H, 13C, 31P Triple-Resonance NMR Spectra: Glucan Phosphorylation. Magn. Reson. Chem. 2013, 51, 655–661. [Google Scholar] [CrossRef]
- Tizzotti, M.J.; Sweedman, M.C.; Tang, D.; Schaefer, C.; Gilbert, R.G. New 1H NMR Procedure for the Characterization of Native and Modified Food-Grade Starches. J. Agric. Food Chem. 2011, 59, 6913–6919. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, Q.; Chen, Z.; Xiao, H. The Interaction between Tea Polyphenols and Rice Starch during Gelatinization. Food Sci. Technol. Int. 2011, 17, 569–577. [Google Scholar] [CrossRef]
- Lian, X.; Zhang, K.; Luo, Q.; Wang, C.; Liu, X. A Possible Structure of Retrograded Maize Starch Speculated by UV and IR Spectra of It and Its Components. Int. J. Biol. Macromol. 2012, 50, 119–124. [Google Scholar] [CrossRef]
- Almeida, M.R.; Alves, R.S.; Nascimbem, L.B.L.R.; Stephani, R.; Poppi, R.J.; de Oliveira, L.F.C. Determination of Amylose Content in Starch Using Raman Spectroscopy and Multivariate Calibration Analysis. Anal. Bioanal. Chem. 2010, 397, 2693–2701. [Google Scholar] [CrossRef] [PubMed]
- Mutungi, C.; Passauer, L.; Onyango, C.; Jaros, D.; Rohm, H. Debranched Cassava Starch Crystallinity Determination by Raman Spectroscopy: Correlation of Features in Raman Spectra with X-Ray Diffraction and 13C CP/MAS NMR Spectroscopy. Carbohydr. Polym. 2012, 87, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Lin, L.; Wang, J.; Liu, Q.; Wei, C. Long Branch-Chains of Amylopectin with B-Type Crystallinity in Rice Seed with Inhibition of Starch Branching Enzyme I and IIb Resist in Situ Degradation and Inhibit Plant Growth during Seedling Development. BMC Plant Biol. 2018, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, Y.; Xu, X.; Jin, Z. Starch Retrogradation Studied by Thermogravimetric Analysis (TGA). Carbohydr. Polym. 2011, 84, 1165–1168. [Google Scholar] [CrossRef]
- Curvelo, A.A.S.; de Carvalho, A.J.F.; Agnelli, J.A.M. Thermoplastic Starch–Cellulosic Fibers Composites: Preliminary Results. Carbohydr. Polym. 2001, 45, 183–188. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Rayas-Duarte, P. The Effect of Mixing and Wheat Protein/Gluten on the Gelatinization of Wheat Starch. Food Chem. 2003, 81, 533–545. [Google Scholar] [CrossRef]
- Ghassemi, N.; Poulhazan, A.; Deligey, F.; Mentink-Vigier, F.; Marcotte, I.; Wang, T. Solid-State NMR Investigations of Extracellular Matrixes and Cell Walls of Algae, Bacteria, Fungi, and Plants. Chem. Rev. 2022, 122, 10036–10086. [Google Scholar] [CrossRef]
- El Hariri El Nokab, M.; van der Wel, P.C.A. Use of Solid-State NMR Spectroscopy for Investigating Polysaccharide-Based Hydrogels: A Review. Carbohydr. Polym. 2020, 240, 116276. [Google Scholar] [CrossRef]
- Teng, C.; Chen, D.; Wu, G.; Campanella, O.H. Non-Invasive Techniques to Study Starch Structure and Starchy Products Properties. Curr. Opin. Food Sci. 2021, 38, 196–202. [Google Scholar] [CrossRef]
- El Hariri El Nokab, M.; Habib, M.H.; Alassmy, Y.A.; Abduljawad, M.M.; Alshamrani, K.M.; Sebakhy, K.O. Solid State NMR a Powerful Technique for Investigating Sustainable/Renewable Cellulose-Based Materials. Polymers 2022, 14, 1049. [Google Scholar] [CrossRef]
- Gidley, M.J. High-Resolution Solid-State NMR of Food Materials. Trends Food Sci. Technol. 1992, 3, 231–236. [Google Scholar] [CrossRef]
- Marcone, M.F.; Wang, S.; Albabish, W.; Nie, S.; Somnarain, D.; Hill, A. Diverse Food-Based Applications of Nuclear Magnetic Resonance (NMR) Technology. Food Res. Int. 2013, 51, 729–747. [Google Scholar] [CrossRef]
- Courtier-Murias, D.; Farooq, H.; Longstaffe, J.G.; Kelleher, B.P.; Hart, K.M.; Simpson, M.J.; Simpson, A.J. Cross Polarization-Single Pulse/Magic Angle Spinning (CPSP/MAS): A Robust Technique for Routine Soil Analysis by Solid-State NMR. Geoderma 2014, 226–227, 405–414. [Google Scholar] [CrossRef]
- Zhang, R.; Mroue, K.H.; Ramamoorthy, A. Hybridizing Cross-Polarization with NOE or Refocused-INEPT Enhances the Sensitivity of MAS NMR Spectroscopy. J. Magn. Reson. 2016, 266, 59–66. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, Z.; Kümmerle, R.; Stevens, J.C.; Redwine, D.; He, Y.; Qiu, X.; Cong, R.; Klosin, J.; Montañez, N.; Roof, G. 13C NMR of Polyolefins with a New High Temperature 10mm Cryoprobe. J. Magn. Reson. 2009, 200, 328–333. [Google Scholar] [CrossRef]
- Zhao, W.; Kirui, A.; Deligey, F.; Mentink-Vigier, F.; Zhou, Y.; Zhang, B.; Wang, T. Solid-State NMR of Unlabeled Plant Cell Walls: High-Resolution Structural Analysis without Isotopic Enrichment. Biotechnol. Biofuels 2021, 14, 14. [Google Scholar] [CrossRef]
- El Hariri El Nokab, M.; Lasorsa, A.; Sebakhy, K.O.; Picchioni, F.; van der Wel, P.C.A. Solid-State NMR Spectroscopy Insights for Resolving Different Water Pools in Alginate Hydrogels. Food Hydrocoll. 2022, 127, 107500. [Google Scholar] [CrossRef]
- Reif, B.; Ashbrook, S.E.; Emsley, L.; Hong, M. Solid-State NMR Spectroscopy. Nat. Rev. Methods Prim. 2021, 1, 23. [Google Scholar] [CrossRef]
- Weingarth, M.; Baldus, M. Solid-State NMR-Based Approaches for Supramolecular Structure Elucidation. Acc. Chem. Res. 2013, 46, 2037–2046. [Google Scholar] [CrossRef]
- Zhao, W.; Fernando, L.D.; Kirui, A.; Deligey, F.; Wang, T. Solid-State NMR of Plant and Fungal Cell Walls: A Critical Review. Solid State Nucl. Magn. Reson. 2020, 107, 101660. [Google Scholar] [CrossRef]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—Composition, Fine Structure and Architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Imberty, A.; Buléon, A.; Tran, V.; Péerez, S. Recent Advances in Knowledge of Starch Structure. Starch 1991, 43, 375–384. [Google Scholar] [CrossRef]
- Buléon, A.; Véronèse, G.; Putaux, J.-L. Self-Association and Crystallization of Amylose. Aust. J. Chem. 2007, 60, 706–718. [Google Scholar] [CrossRef]
- Gidley, M.J.; Bociek, S.M. Molecular Organization in Starches: A Carbon 13CP/MAS NMR Study. J. Am. Chem. Soc. 1985, 107, 7040–7044. [Google Scholar] [CrossRef]
- Flanagan, B.M.; Gidley, M.J.; Warren, F.J. Rapid Quantification of Starch Molecular Order through Multivariate Modelling of 13C CP/MAS NMR Spectra. Chem. Commun. 2015, 51, 14856–14858. [Google Scholar] [CrossRef]
- Katoh, E.; Murata, K.; Fujita, N. 13C CP/MAS NMR Can Discriminate Genetic Backgrounds of Rice Starch. ACS Omega 2020, 5, 24592–24600. [Google Scholar] [CrossRef]
- Veregin, R.P.; Fyfe, C.A.; Marchessault, R.H.; Taylor, M.G. Characterization of the Crystalline A and B Starch Polymorphs and Investigation of Starch Crystallization by High-Resolution Carbon-13CP/MAS NMR. Macromolecules 1986, 19, 1030–1034. [Google Scholar] [CrossRef]
- Gidley, M.J.; Bociek, S.M. Carbon-13CP/MAS NMR Studies of Amylose Inclusion Complexes, Cyclodextrins, and the Amorphous Phase of Starch Granules: Relationships between Glycosidic Linkage Conformation and Solid-State Carbon-13 Chemical Shifts. J. Am. Chem. Soc. 1988, 110, 3820–3829. [Google Scholar] [CrossRef]
- Paris, M.; Bizot, H.; Emery, J.; Buzaré, J.Y.; Buléon, A. NMR Local Range Investigations in Amorphous Starchy Substrates I. Structural Heterogeneity Probed by 13C CP–MAS NMR. Int. J. Biol. Macromol. 2001, 29, 127–136. [Google Scholar] [CrossRef]
- Cheetham, N.W.H.; Tao, L. Solid State NMR Studies on the Structural and Conformational Properties of Natural Maize Starches. Carbohydr. Polym. 1998, 36, 285–292. [Google Scholar] [CrossRef]
- Kulik, A.S.; Haverkamp, J. Molecular Mobility of Polysaccharide Chains in Starch Investigated by Two-Dimensional Solid-State NMR Spectroscopy. Carbohydr. Polym. 1997, 34, 49–54. [Google Scholar] [CrossRef]
- Tang, H.; Hills, B.P. Use of 13C MAS NMR to Study Domain Structure and Dynamics of Polysaccharides in the Native Starch Granules. Biomacromolecules 2003, 4, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Paris, M.; Bizot, H.; Emery, J.; Buzaré, J.Y.; Buléon, A. NMR Local Range Investigations in Amorphous Starchy Substrates: II-Dynamical Heterogeneity Probed by 1H/13C Magnetization Transfer and 2D WISE Solid State NMR. Int. J. Biol. Macromol. 2001, 29, 137–143. [Google Scholar] [CrossRef]
- Tan, I.; Flanagan, B.M.; Halley, P.J.; Whittaker, A.K.; Gidley, M.J. A Method for Estimating the Nature and Relative Proportions of Amorphous, Single, and Double-Helical Components in Starch Granules by 13C CP/MAS NMR. Biomacromolecules 2007, 8, 885–891. [Google Scholar] [CrossRef]
- Poulhazan, A.; Arnold, A.; Warschawski, D.; Marcotte, I. Unambiguous Ex Situ and in Cell 2D 13C Solid-State NMR Characterization of Starch and Its Constituents. Int. J. Mol. Sci. 2018, 19, 3817. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.H.; Kasprzak, M.M.; Lærke, H.N.; Knudsen, K.E.B.; Pedersen, S.; Jørgensen, A.S.; Blennow, A. Hydration Properties and Phosphorous Speciation in Native, Gelatinized and Enzymatically Modified Potato Starch Analyzed by Solid-State MAS NMR. Carbohydr. Polym. 2013, 97, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dickinson, L.C.; Chinachoti, P. Mobility of “Unfreezable” and “Freezable” Water in Waxy Corn Starch by 2H and 1H NMR. J. Agric. Food Chem. 1998, 46, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.J.; Baianu, I.C.; Steinberg, M.P. Mobility of Water in Corn Starch Suspensions Determined by Nuclear Magnetic Resonance. Starch 1987, 39, 79–83. [Google Scholar] [CrossRef]
- Ritota, M.; Gianferri, R.; Bucci, R.; Brosio, E. Proton NMR Relaxation Study of Swelling and Gelatinisation Process in Rice Starch–Water Samples. Food Chem. 2008, 110, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Atichokudomchai, N.; Varavinit, S.; Chinachoti, P. A Study of Ordered Structure in Acid-Modified Tapioca Starch by 13C CP/MAS Solid-State NMR. Carbohydr. Polym. 2004, 58, 383–389. [Google Scholar] [CrossRef]
- Cai, J.; Yang, Y.; Man, J.; Huang, J.; Wang, Z.; Zhang, C.; Gu, M.; Liu, Q.; Wei, C. Structural and Functional Properties of Alkali-Treated High-Amylose Rice Starch. Food Chem. 2014, 145, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Cai, C.; Man, J.; Yang, Y.; Zhang, F.; Wei, C. Crystalline and Structural Properties of Acid-Modified Lotus Rhizome C-Type Starch. Carbohydr. Polym. 2014, 102, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wei, B.; Zhang, B.; Li, H.; Xu, X.; Jin, Z.; Tian, Y. Interaction between Amylose and 1-Butanol during 1-Butanol-Hydrochloric Acid Hydrolysis of Normal Rice Starch. Int. J. Biol. Macromol. 2013, 61, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Baik, M.-Y.; Dickinson, L.C.; Chinachoti, P. Solid-State 13C CP/MAS NMR Studies on Aging of Starch in White Bread. J. Agric. Food Chem. 2003, 51, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Bathista, A.L.B.S.; da Silva, E.O.; Tavares, M.I.B.; Prado, R.J. Solid-State NMR to Evaluate the Molecular Changes in the Mango Starch after 8 Years of Storage. J. Appl. Polym. Sci. 2012, 126, E123–E126. [Google Scholar] [CrossRef]
- Flores-Morales, A.; Jiménez-Estrada, M.; Mora-Escobedo, R. Determination of the Structural Changes by FT-IR, Raman, and CP/MAS 13C NMR Spectroscopy on Retrograded Starch of Maize Tortillas. Carbohydr. Polym. 2012, 87, 61–68. [Google Scholar] [CrossRef]
- Smits, A.L.M.; Ruhnau, F.C.; Vliegenthart, J.F.G.; van Soest, J.J.G. Ageing of Starch Based Systems as Observed with FT-IR and Solid State NMR Spectroscopy. Starch 1998, 50, 478–483. [Google Scholar] [CrossRef]
- Deng, Y.; Jin, Y.; Luo, Y.; Zhong, Y.; Yue, J.; Song, X.; Zhao, Y. Impact of Continuous or Cycle High Hydrostatic Pressure on the Ultrastructure and Digestibility of Rice Starch Granules. J. Cereal Sci. 2014, 60, 302–310. [Google Scholar] [CrossRef]
- Guo, Z.; Zeng, S.; Zhang, Y.; Lu, X.; Tian, Y.; Zheng, B. The Effects of Ultra-High Pressure on the Structural, Rheological and Retrogradation Properties of Lotus Seed Starch. Food Hydrocoll. 2015, 44, 285–291. [Google Scholar] [CrossRef]
- Le Bail, P.; Chauvet, B.; Simonin, H.; Rondeau-Mouro, C.; Pontoire, B.; de Carvalho, M.; Le-Bail, A. Formation and Stability of Amylose Ligand Complexes Formed by High Pressure Treatment. Innov. Food Sci. Emerg. Technol. 2013, 18, 1–6. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, Y.; Zhang, F.; Zhao, Q.; Xue, Y.; Hu, J.; Shen, Q. Comparison of the Molecular Structure of Heat and Pressure-Treated Corn Starch Based on Experimental Data and Molecular Dynamics Simulation. Food Hydrocoll. 2022, 125, 107371. [Google Scholar] [CrossRef]
- Htoon, A.; Shrestha, A.K.; Flanagan, B.M.; Lopez-Rubio, A.; Bird, A.R.; Gilbert, E.P.; Gidley, M.J. Effects of Processing High Amylose Maize Starches under Controlled Conditions on Structural Organisation and Amylase Digestibility. Carbohydr. Polym. 2009, 75, 236–245. [Google Scholar] [CrossRef]
- Khatoon, S.; Sreerama, Y.N.; Raghavendra, D.; Bhattacharya, S.; Bhat, K.K. Properties of Enzyme Modified Corn, Rice and Tapioca Starches. Food Res. Int. 2009, 42, 1426–1433. [Google Scholar] [CrossRef]
- Man, J.; Yang, Y.; Huang, J.; Zhang, C.; Chen, Y.; Wang, Y.; Gu, M.; Liu, Q.; Wei, C. Effect of Simultaneous Inhibition of Starch Branching Enzymes I and IIb on the Crystalline Structure of Rice Starches with Different Amylose Contents. J. Agric. Food Chem. 2013, 61, 9930–9937. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.K.; Blazek, J.; Flanagan, B.M.; Dhital, S.; Larroque, O.; Morell, M.K.; Gilbert, E.P.; Gidley, M.J. Molecular, Mesoscopic and Microscopic Structure Evolution during Amylase Digestion of Maize Starch Granules. Carbohydr. Polym. 2012, 90, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Nowacka-Perrin, A.; Steglich, T.; Topgaard, D.; Bernin, D. In Situ 13C Solid-State Polarization Transfer NMR to Follow Starch Transformations in Food. Magn. Reson. Chem. 2022, 60, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, S.; Alves, L.; Lindman, B.; Topgaard, D. Polarization Transfer Solid-State NMR: A New Method for Studying Cellulose Dissolution. RSC Adv. 2014, 4, 31836–31839. [Google Scholar] [CrossRef]
- Koev, T.T.; Muñoz-García, J.C.; Iuga, D.; Khimyak, Y.Z.; Warren, F.J. Structural Heterogeneities in Starch Hydrogels. Carbohydr. Polym. 2020, 249, 116834. [Google Scholar] [CrossRef]
- Nessi, V.; Rolland-Sabaté, A.; Lourdin, D.; Jamme, F.; Chevigny, C.; Kansou, K. Multi-Scale Characterization of Thermoplastic Starch Structure Using Second Harmonic Generation Imaging and NMR. Carbohydr. Polym. 2018, 194, 80–88. [Google Scholar] [CrossRef]
- Pushpadass, H.A.; Kumar, A.; Jackson, D.S.; Wehling, R.L.; Dumais, J.J.; Hanna, M.A. Macromolecular Changes in Extruded Starch-Films Plasticized with Glycerol, Water and Stearic Acid. Starch 2009, 61, 256–266. [Google Scholar] [CrossRef]
- Smits, A.L.M.; Kruiskamp, P.H.; van Soest, J.J.G.; Vliegenthart, J.F.G. Interaction between Dry Starch and Plasticisers Glycerol or Ethylene Glycol, Measured by Differential Scanning Calorimetry and Solid State NMR Spectroscopy. Carbohydr. Polym. 2003, 53, 409–416. [Google Scholar] [CrossRef]
- Šoltýs, A.; Hronský, V.; Šmídová, N.; Olčák, D.; Ivanič, F.; Chodák, I. Solid-State 1H and 13C NMR of Corn Starch Plasticized with Glycerol and Urea. Eur. Polym. J. 2019, 117, 19–27. [Google Scholar] [CrossRef]
- Vrábel, P.; Baran, A.; Kovaľaková, M.; Fričová, O.; Hutníková, M.; Olčák, D. Characterization of Native and Plasticized Starch Using Solid State NMR. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2018; Volume 1996, p. 20049. [Google Scholar]
- van Duynhoven, J.; Voda, A.; Witek, M.; van As, H. Chapter 3—Time-Domain NMR Applied to Food Products. In Annual Reports on NMR Spectroscopy; Academic Press: Cambridge, MA, USA, 2010; Volume 69, pp. 145–197. ISBN 0066-4103. [Google Scholar]
- Vallurupalli, P.; Hansen, D.F.; Lundström, P.; Kay, L.E. CPMG Relaxation Dispersion NMR Experiments Measuring Glycine 1Hα and 13Cα Chemical Shifts in the ‘Invisible’ Excited States of Proteins. J. Biomol. NMR 2009, 45, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Cioica, N.; Fechete, R.; Cota, C.; Nagy, E.M.; David, L.; Cozar, O. NMR Relaxation Investigation of the Native Corn Starch Structure with Plasticizers. J. Mol. Struct. 2013, 1044, 128–133. [Google Scholar] [CrossRef]
- Brito, L.M.; Sebastião, P.J.O.; Bruno Tavares, M.I. NMR Relaxometry Evaluation of Nanostructured Starch-PLA Blends. Polym. Test. 2015, 45, 161–167. [Google Scholar] [CrossRef]
- Cheng, W.; Luo, Z.; Li, L.; Fu, X. Preparation and Characterization of Debranched-Starch/Phosphatidylcholine Inclusion Complexes. J. Agric. Food Chem. 2015, 63, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Fričová, O.; Hutníková, M.; Kovaľaková, M.; Baran, A. Influence of Aging on Molecular Motion in PBAT-Thermoplastic Starch Blends Studied Using Solid-State NMR. Int. J. Polym. Anal. Charact. 2020, 25, 275–282. [Google Scholar] [CrossRef]
- Martini, F.; Hughes, D.J.; Badolato Bönisch, G.; Zwick, T.; Schäfer, C.; Geppi, M.; Alam, M.A.; Ubbink, J. Antiplasticization and Phase Behavior in Phase-Separated Modified Starch-Sucrose Blends: A Positron Lifetime and Solid-State NMR Study. Carbohydr. Polym. 2020, 250, 116931. [Google Scholar] [CrossRef]
- Spěváček, J.; Brus, J.; Divers, T.; Grohens, Y. Solid-State NMR Study of Biodegradable Starch/Polycaprolactone Blends. Eur. Polym. J. 2007, 43, 1866–1875. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, K.; Wang, P. Study on Structure and Molecular Dynamics of Starch/Poly(Sodium Acrylate)-Grafted Superabsorbent by 13C Solid State NMR. Fibers Polym. 2008, 9, 271–275. [Google Scholar] [CrossRef]
- Zhang, X.; Dean, K.; Burgar, I.M. A High-Resolution Solid-State NMR Study on Starch–Clay Nanocomposites and the Effect of Aging on Clay Dispersion. Polym. J. 2010, 42, 689–695. [Google Scholar] [CrossRef][Green Version]
- Hughes, D.; Tedeschi, C.; Leuenberger, B.; Roussenova, M.; Coveney, A.; Richardson, R.; Bönisch, G.B.; Alam, M.A.; Ubbink, J. Amorphous-Amorphous Phase Separation in Hydrophobically-Modified Starch–Sucrose Blends II. Crystallinity and Local Free Volume Investigation Using Wide-Angle X-Ray Scattering and Positron Annihilation Lifetime Spectroscopy. Food Hydrocoll. 2016, 58, 316–323. [Google Scholar] [CrossRef]
- Hughes, D.J.; Bönisch, G.B.; Zwick, T.; Schäfer, C.; Tedeschi, C.; Leuenberger, B.; Martini, F.; Mencarini, G.; Geppi, M.; Alam, M.A.; et al. Phase Separation in Amorphous Hydrophobically Modified Starch–Sucrose Blends: Glass Transition, Matrix Dynamics and Phase Behavior. Carbohydr. Polym. 2018, 199, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, Z.; Wang, Y.; Zhang, S. Molecular Insight into the Interactions between Starch and Cuminaldehyde Using Relaxation and 2D Solid-State NMR Spectroscopy. Carbohydr. Polym. 2022, 278, 118932. [Google Scholar] [CrossRef]
- Kumar, A.; Durand, H.; Zeno, E.; Balsollier, C.; Watbled, B.; Sillard, C.; Fort, S.; Baussanne, I.; Belgacem, N.; Lee, D.; et al. The Surface Chemistry of a Nanocellulose Drug Carrier Unravelled by MAS-DNP. Chem. Sci. 2020, 11, 3868–3877. [Google Scholar] [CrossRef] [PubMed]
- Kirui, A.; Ling, Z.; Kang, X.; Widanage, M.C.D.; Mentink-Vigier, F.; French, A.D.; Wang, T. Atomic Resolution of Cotton Cellulose Structure Enabled by Dynamic Nuclear Polarization Solid-State NMR. Cellulose 2019, 26, 329–339. [Google Scholar] [CrossRef]
- El Hariri El Nokab, M.; Sebakhy, K.O. Solid State NMR Spectroscopy a Valuable Technique for Structural Insights of Advanced Thin Film Materials: A Review. Nanomaterials 2021, 11, 1494. [Google Scholar] [CrossRef]



| Broad Technique | Analytical Method | Property Analyzed | Description | Reference |
|---|---|---|---|---|
| Rheology | Rheometer | Viscosity | Continuous shear tests performed on starch nanoparticles to measure apparent viscosity | [28] |
| Microscopy | Scanning electron microscope(SEM) | Granule morphology | SEM morphology comparison between potato, corn, wheat, and rice as well as enzymatically modified starches | [25,33] |
| Transmission electron microscopy (TEM) | Granule shape and surface features | Ultrasonically treated (modified) starch analyzed in thin cross-sections of granules obtained by ultramicrotome | [35] | |
| Atomic force microscopy (AFM) | Morphology of films | Starch-based biodegradable film surfaces analyzed by AFM in tapping model | [33] | |
| X-ray technique | Small angle neutron scattering (SANS) | Lamellar structure | Lamellar architecture and crystalline structures of starch during hydrolysis | [2] |
| Small angle X-ray scattering (SANS) | Nanostructure | Nanostructure of the freeze-dried wheat starch pastes after repeated heating and cooling | [29] | |
| X-ray diffraction | Crystallite morphology | X-ray diffraction patterns of sweet potato amylose before and after retrogradation using copper, nickel foil-filtered and Ka radiation | [30] | |
| Thermal analysis | Differential scanning calorimetry (DSC) | Glass transition temperature and melting point | Starch-TiO2 nanocomposite films glass transition temperature and melting point analysis by DSC | [32] |
| Spectroscopic | Nuclear magnetic resonance (NMR) | Structural features | Characterization of native and modified starch and starch gelatinization procedure | [39,40] |
| Infra-red (IR) spectroscopy | Structural features | Analysis of the structure of retrograded maize starch | [41] | |
| Raman spectroscopy | Amylose content | Determination of amylose content in starch FT-Raman spectroscopy with germanium detector | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Nokab, M.E.H.; Alassmy, Y.A.; Abduljawad, M.M.; Al-shamrani, K.M.; Alnafisah, M.S.; Asgar Pour, Z.; Tucker, C.L.; Sebakhy, K.O. Solid-State NMR Spectroscopy: Towards Structural Insights into Starch-Based Materials in the Food Industry. Polymers 2022, 14, 4686. https://doi.org/10.3390/polym14214686
El Nokab MEH, Alassmy YA, Abduljawad MM, Al-shamrani KM, Alnafisah MS, Asgar Pour Z, Tucker CL, Sebakhy KO. Solid-State NMR Spectroscopy: Towards Structural Insights into Starch-Based Materials in the Food Industry. Polymers. 2022; 14(21):4686. https://doi.org/10.3390/polym14214686
Chicago/Turabian StyleEl Nokab, Mustapha El Hariri, Yasser A. Alassmy, Marwan M. Abduljawad, Khalid M. Al-shamrani, Mohammed S. Alnafisah, Zahra Asgar Pour, Chelsea L. Tucker, and Khaled O. Sebakhy. 2022. "Solid-State NMR Spectroscopy: Towards Structural Insights into Starch-Based Materials in the Food Industry" Polymers 14, no. 21: 4686. https://doi.org/10.3390/polym14214686
APA StyleEl Nokab, M. E. H., Alassmy, Y. A., Abduljawad, M. M., Al-shamrani, K. M., Alnafisah, M. S., Asgar Pour, Z., Tucker, C. L., & Sebakhy, K. O. (2022). Solid-State NMR Spectroscopy: Towards Structural Insights into Starch-Based Materials in the Food Industry. Polymers, 14(21), 4686. https://doi.org/10.3390/polym14214686








