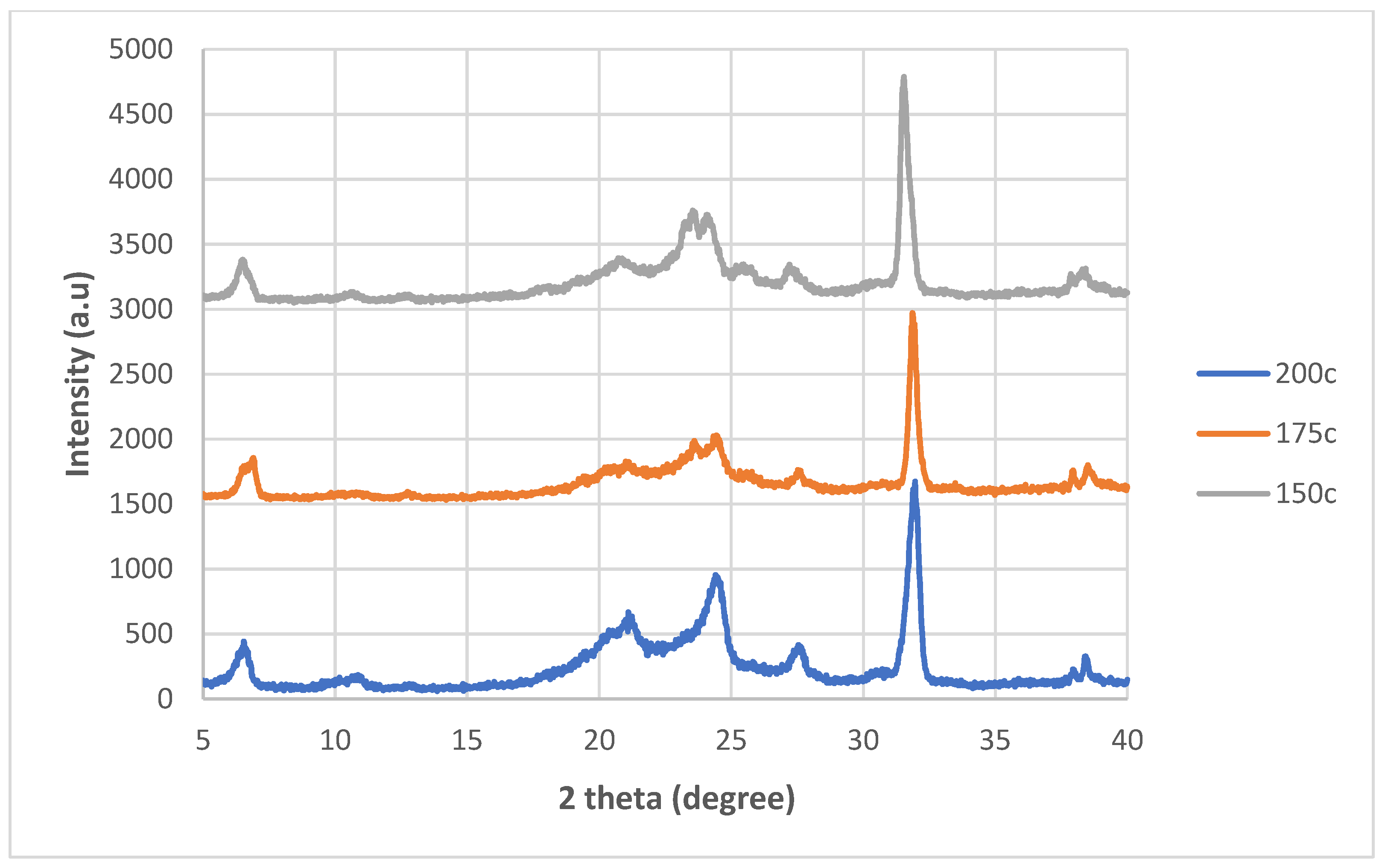
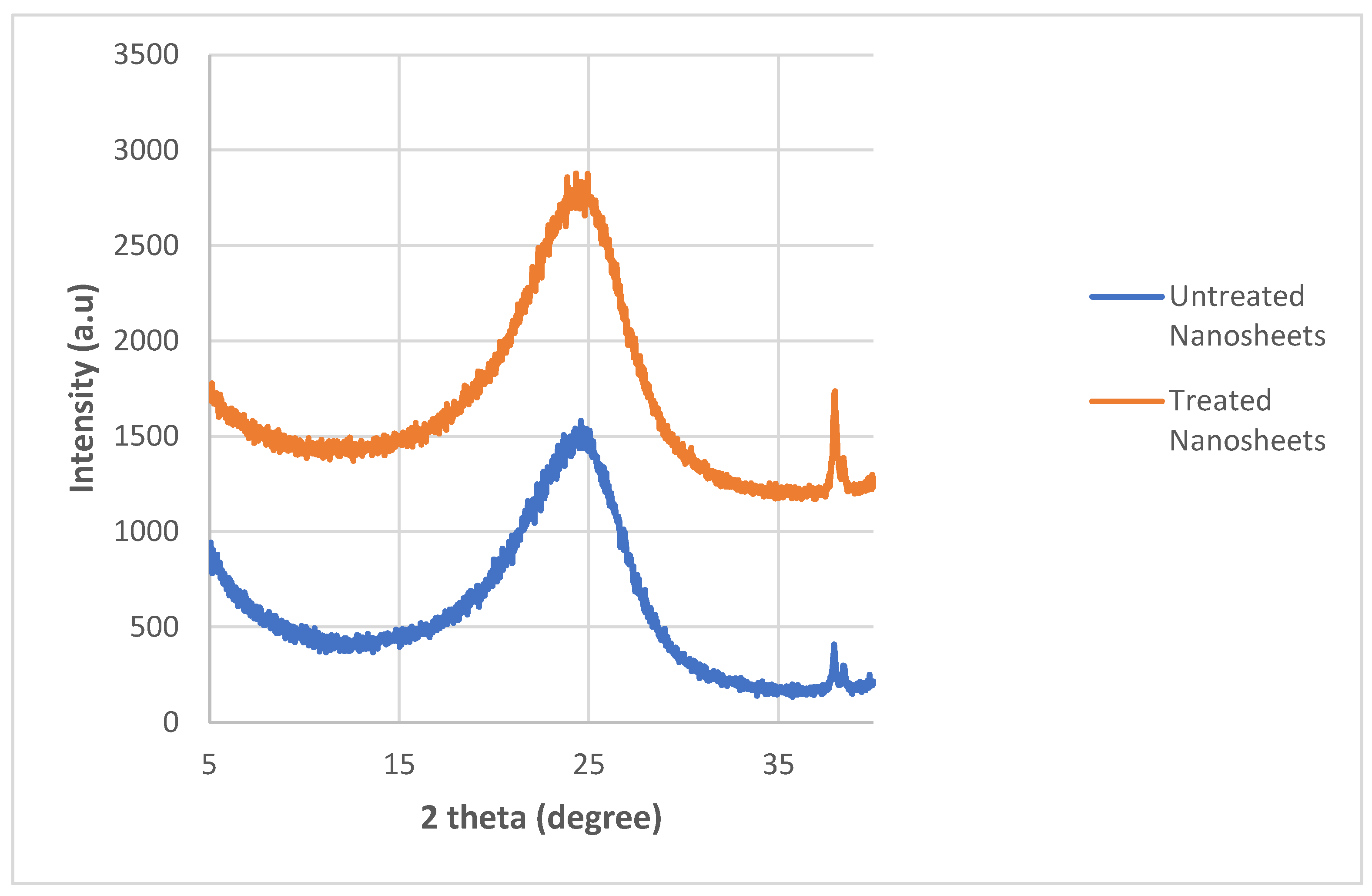
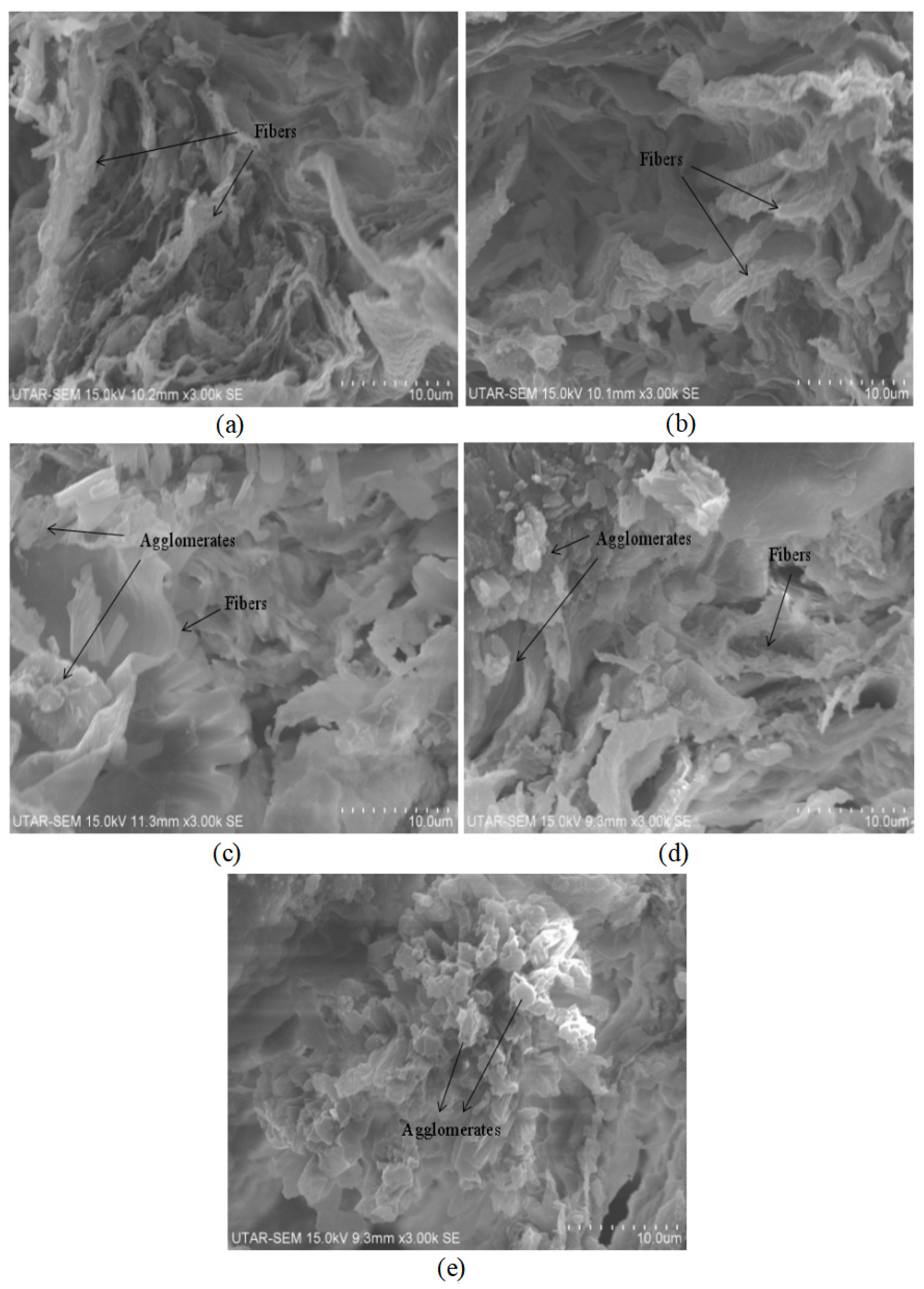
Appendix A
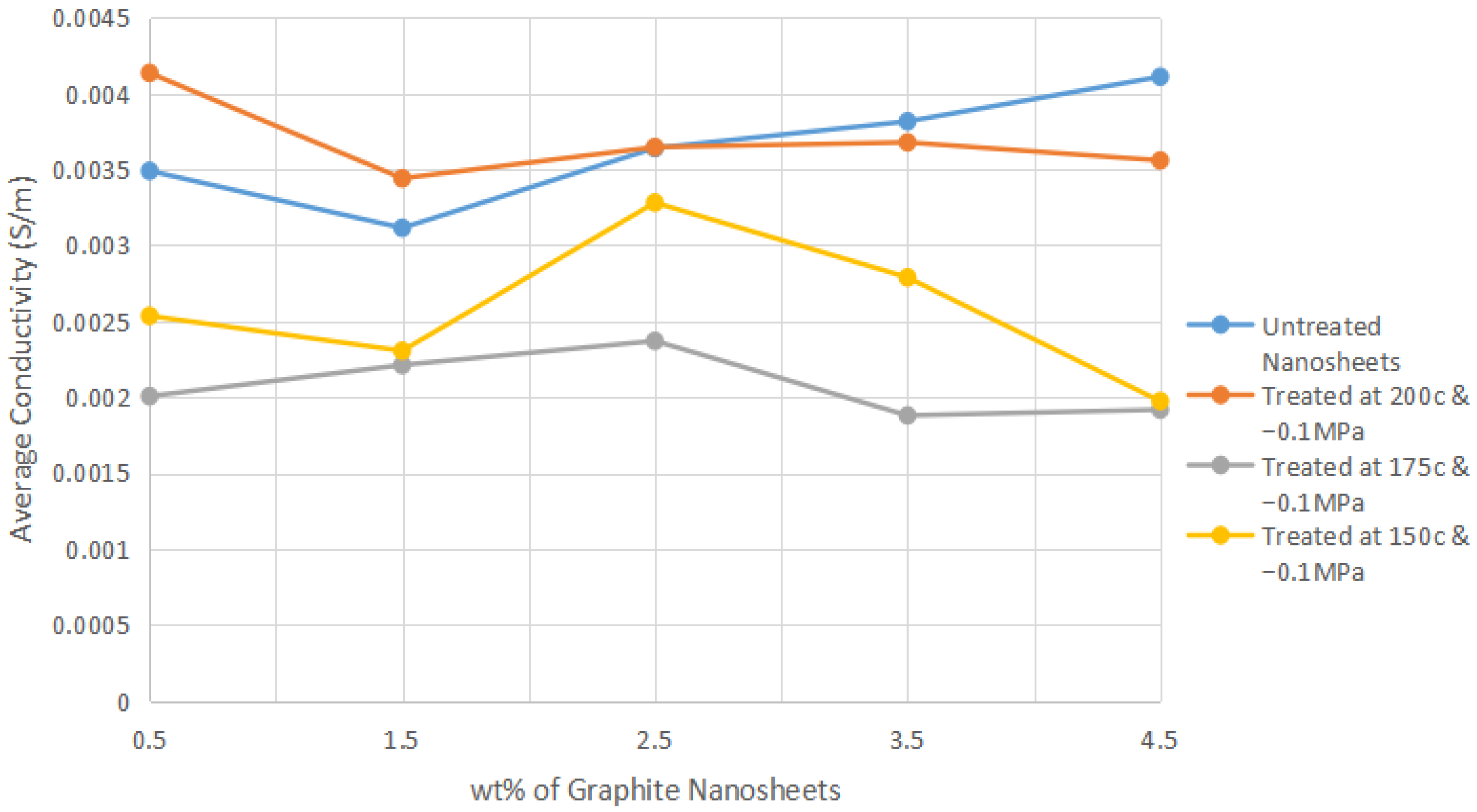
This appendix shows the bar chart of the conductivities for all 20 of the graphite nanosheets/nylon 610 nanocomposites. The purpose of this is to show the error bars for the conductivities measured. All of the results are the same as in
Figure 3.
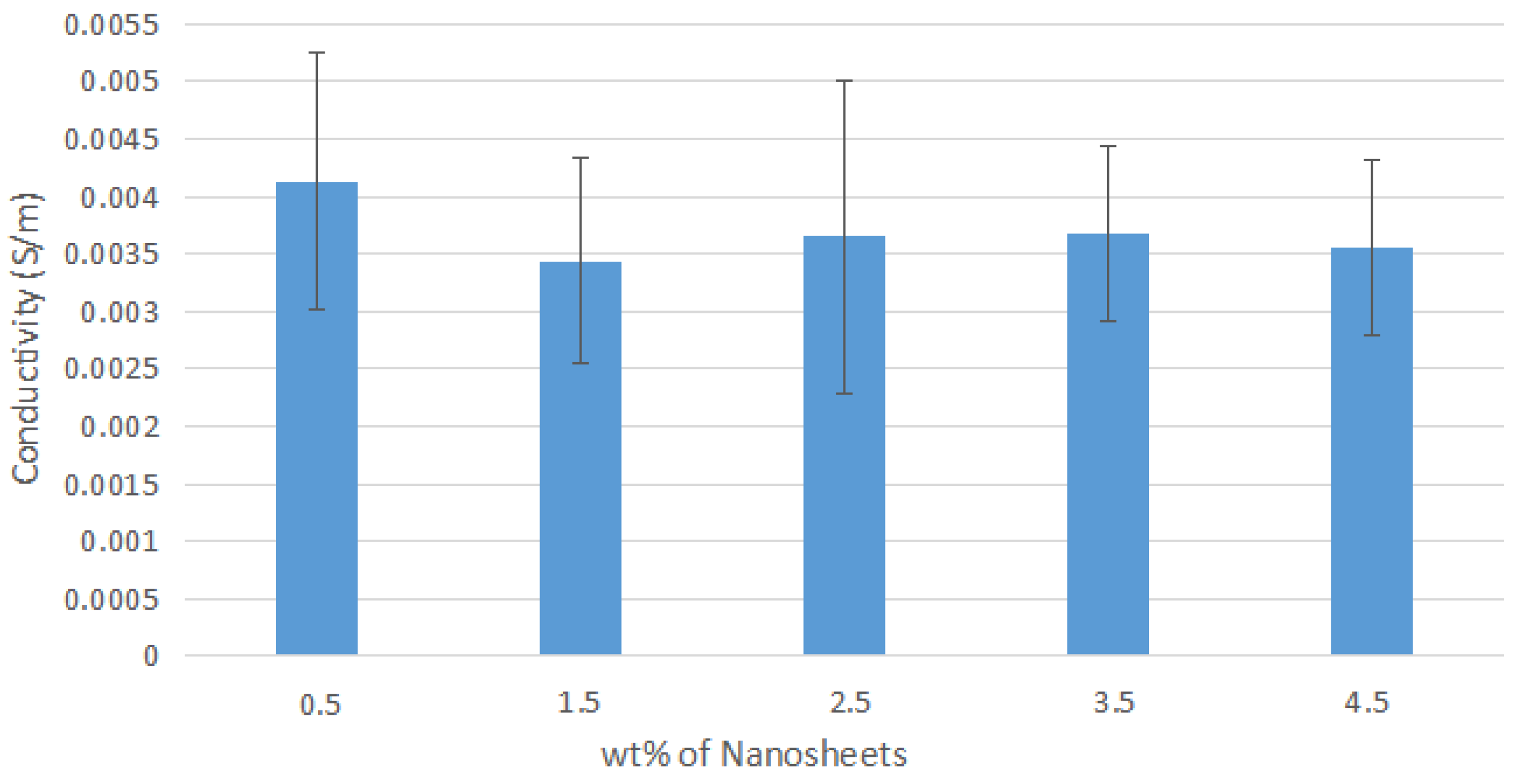
Figure A1.
Average Conductivity of Nylon 610 Nanocomposites with Nanosheets Treated at 200 °C and −0.1 MPa.
Figure A1.
Average Conductivity of Nylon 610 Nanocomposites with Nanosheets Treated at 200 °C and −0.1 MPa.
Figure A2.
Average Conductivity of Nylon 610 Nanocomposites with Nanosheets Treated at 175 °C and −0.1 MPa.
Figure A2.
Average Conductivity of Nylon 610 Nanocomposites with Nanosheets Treated at 175 °C and −0.1 MPa.
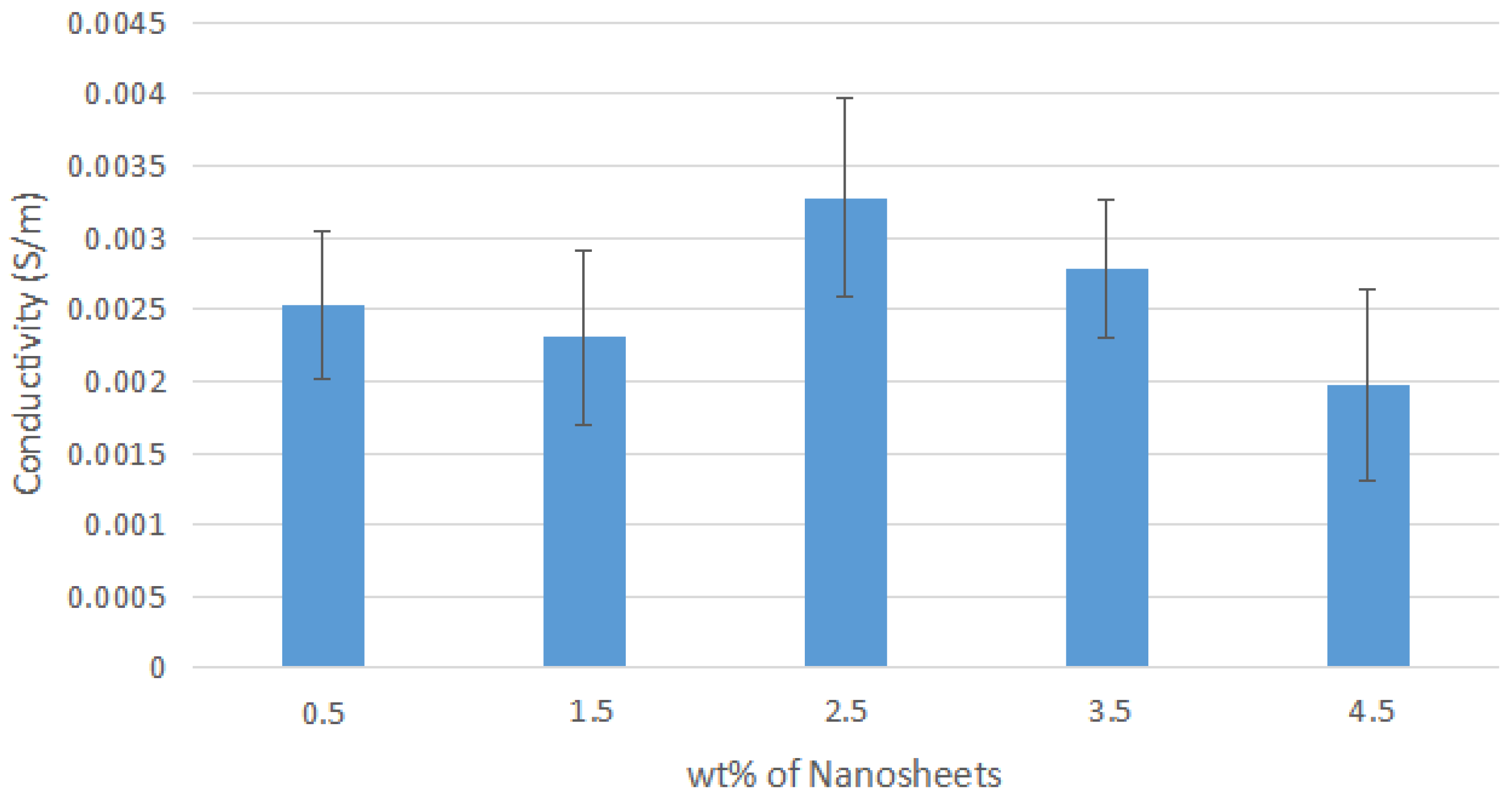
Figure A3.
Average Conductivity of Nylon 610 Nanocomposites with Nanosheets Treated at 150 °C and −0.1 MPa.
Figure A3.
Average Conductivity of Nylon 610 Nanocomposites with Nanosheets Treated at 150 °C and −0.1 MPa.
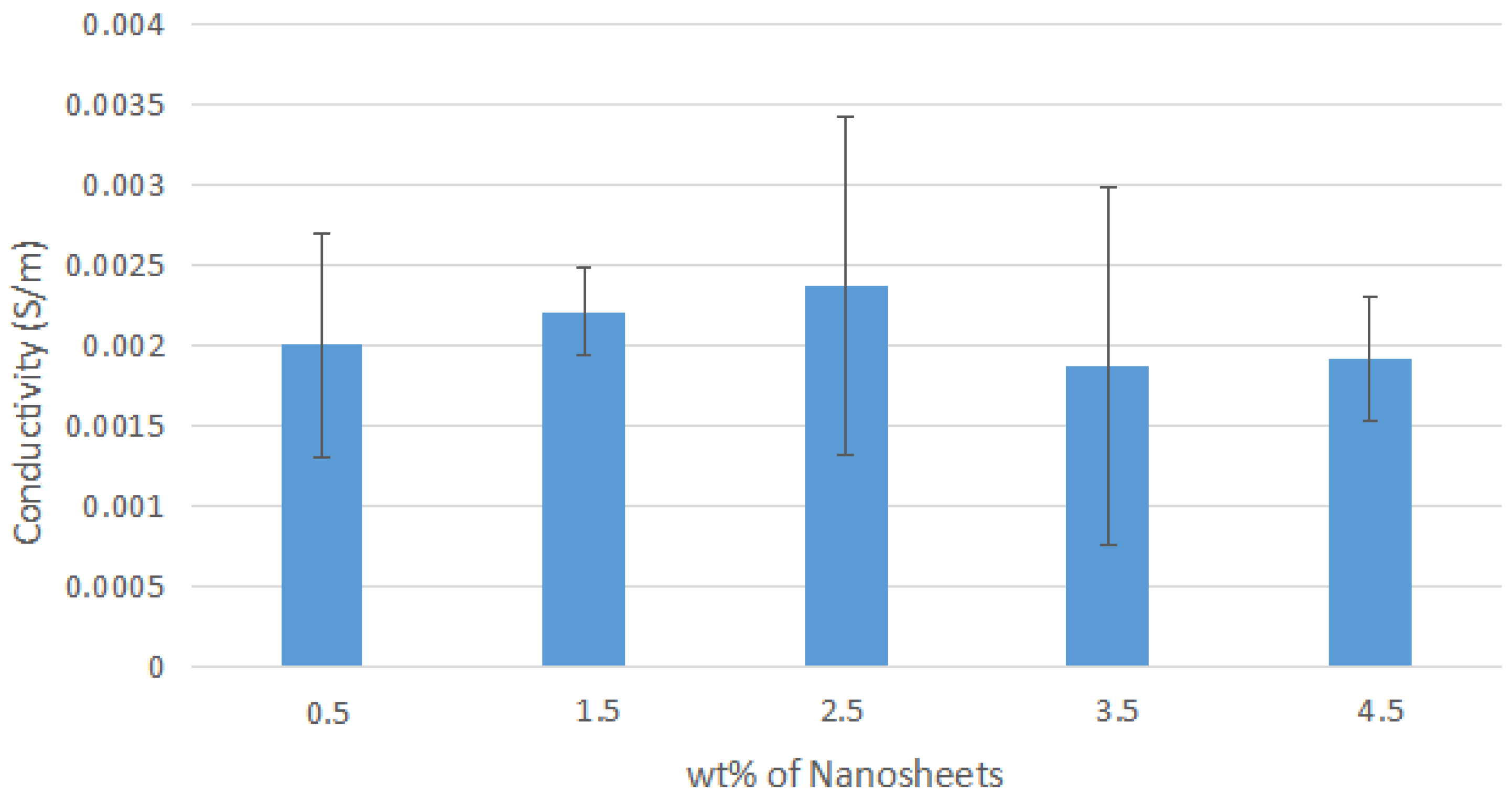
Figure A4.
Average Conductivity of Nylon 610 Nanocomposites with Untreated Nanosheets.
Figure A4.
Average Conductivity of Nylon 610 Nanocomposites with Untreated Nanosheets.
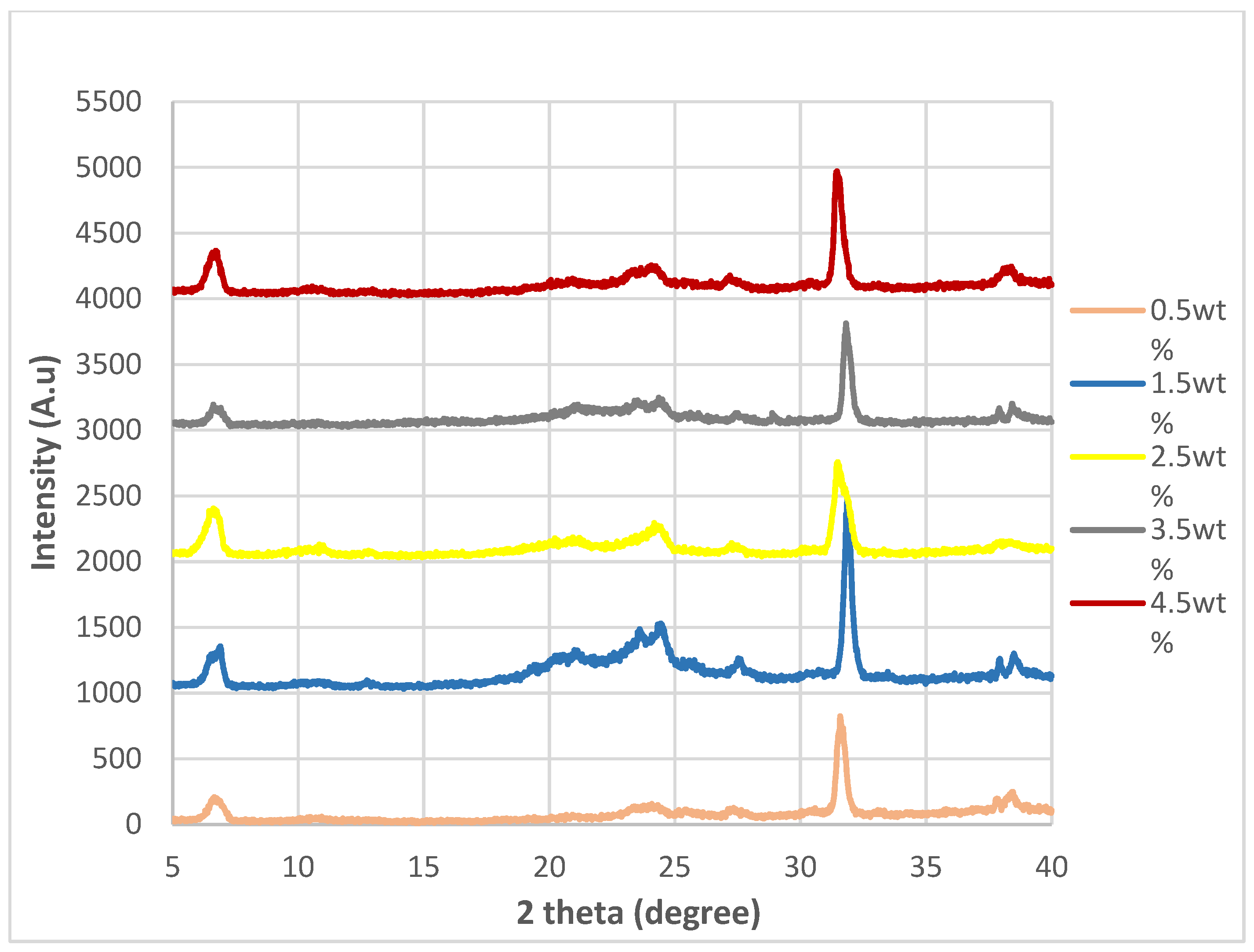
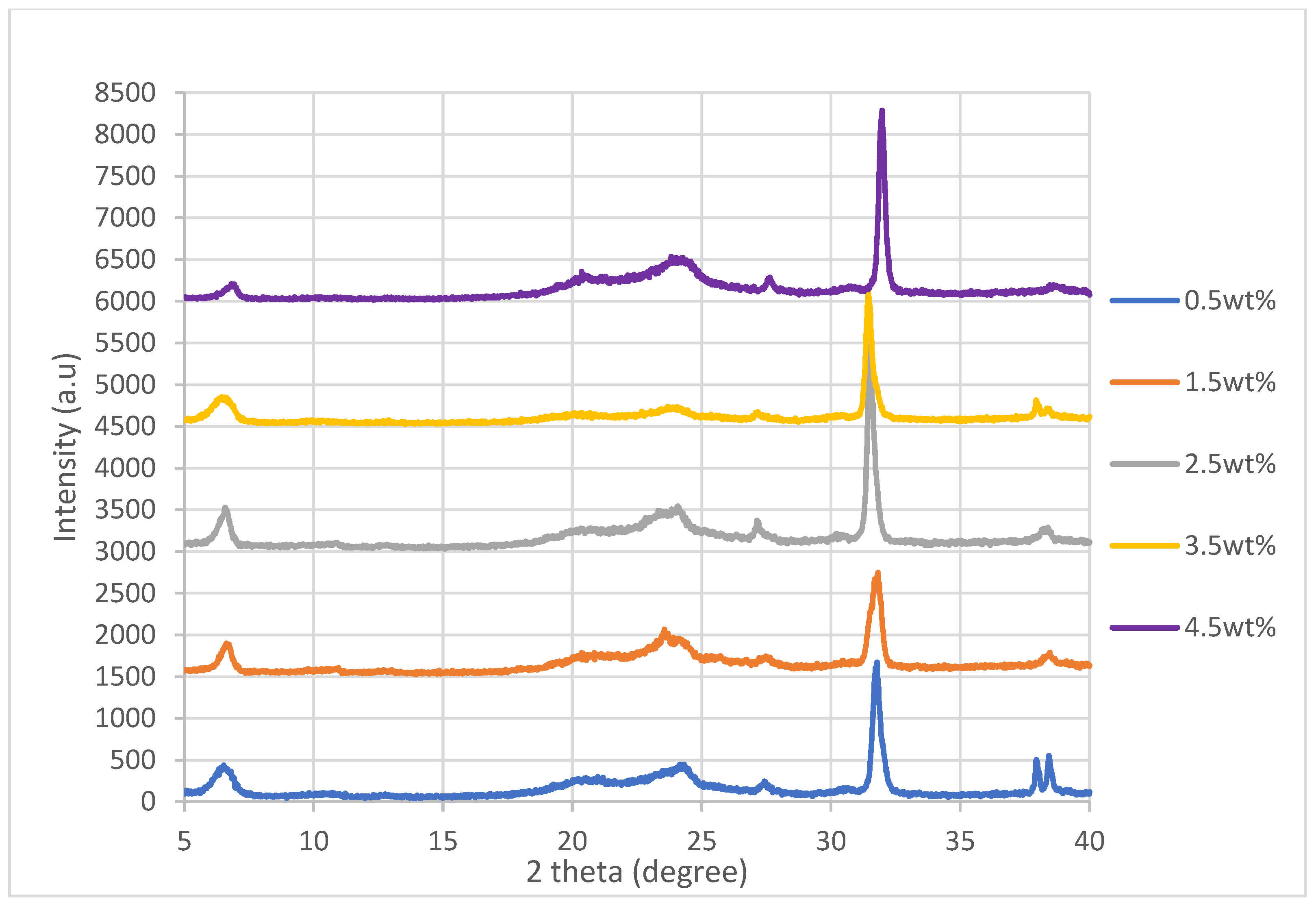
The tables below show the resistance and conductivity data for each of the five samples for all nanocomposites synthesized. The conductivity is then obtained by calculating the average from the conductivity data and rounding it up to 4 significant figures, which is found in
Table 1.
Table A1.
Conductivity Data for Nylon 610 Nanocomposites with 0.5 wt% Graphite Nanosheets Treated at 200 °C and −1 bar.
Table A1.
Conductivity Data for Nylon 610 Nanocomposites with 0.5 wt% Graphite Nanosheets Treated at 200 °C and −1 bar.
| Sample | 1 | 2 | 3 | 4 | 5 |
|---|
| Resistance (ohm) | 22,902.51471 | 22,902.51471 | 27,066.60829 | 29,773.26912 | 29,773.26912 |
| Conductivity (S/m) | 0.005352047 | 0.00507036 | 0.00407579 | 0.003528822 | 0.002646617 |
Table A2.
Conductivity Data for Nylon 610 Nanocomposites with 1.5 wt% Graphite Nanosheets Treated at 200 °C and −1 bar.
Table A2.
Conductivity Data for Nylon 610 Nanocomposites with 1.5 wt% Graphite Nanosheets Treated at 200 °C and −1 bar.
| Sample | 1 | 2 | 3 | 4 | 5 |
|---|
| Resistance (ohm) | 33,081.41014 | 33,081.41014 | 33,081.41014 | 29,773.26912 | 29,773.26912 |
| Conductivity (S/m) | 0.002778947 | 0.002381955 | 0.003510249 | 0.003900277 | 0.004631579 |
Table A3.
Conductivity Data for Nylon 610 Nanocomposites with 2.5 wt% Graphite Nanosheets Treated at 200 °C and −1 bar.
Table A3.
Conductivity Data for Nylon 610 Nanocomposites with 2.5 wt% Graphite Nanosheets Treated at 200 °C and −1 bar.
| Sample | 1 | 2 | 3 | 4 | 5 |
|---|
| Resistance (ohm) | 33,081.41014 | 33,081.41014 | 33,081.41014 | 33,081.41014 | 29,772.36911 |
| Conductivity (S/m) | 0.003334737 | 0.003510249 | 0.002899771 | 0.003705263 | 0.0049405 |
Table A4.
Conductivity Data for Nylon 610 Nanocomposites with 3.5 wt% Graphite Nanosheets Treated at 200 °C and −1 bar.
Table A4.
Conductivity Data for Nylon 610 Nanocomposites with 3.5 wt% Graphite Nanosheets Treated at 200 °C and −1 bar.
| Sample | 1 | 2 | 3 | 4 | 5 |
|---|
| Resistance (ohm) | 33,081.41014 | 29,773.26912 | 29,772.36911 | 33,081.41014 | 33,081.41014 |
| Conductivity (S/m) | 0.002778947 | 0.002744639 | 0.003900395 | 0.00392322 | 0.004446316 |
Table A5.
Conductivity Data for Nylon 610 Nanocomposites with 4.5 wt% Graphite Nanosheets Treated at 200 °C and −1 bar.
Table A5.
Conductivity Data for Nylon 610 Nanocomposites with 4.5 wt% Graphite Nanosheets Treated at 200 °C and −1 bar.
| Sample | 1 | 2 | 3 | 4 | 5 |
|---|
| Resistance (ohm) | 33,081.41014 | 33,081.41014 | 33,081.41014 | 33,081.41014 | 29,772.36911 |
| Conductivity (S/m) | 0.003334737 | 0.003510249 | 0.002899771 | 0.003705263 | 0.0049405 |
Table A6.
Conductivity Data for Nylon 610 Nanocomposites with 0.5 wt% Graphite Nanosheets Treated at 175 °C and −1 bar.
Table A6.
Conductivity Data for Nylon 610 Nanocomposites with 0.5 wt% Graphite Nanosheets Treated at 175 °C and −1 bar.
| Sample | 1 | 2 | 3 | 4 | 5 |
|---|
| Resistance (ohm) | 37,216.5864 | 42,533.2416 | 42,533.2416 | 42,533.2416 | 49,622.1152 |
| Conductivity (S/m) | 0.002694737 | 0.002255378 | 0.002074947 | 0.001621053 | 0.001389474 |
Table A7.
Conductivity Data for Nylon 610 Nanocomposites with 1.5 wt% Graphite Nanosheets Treated at 175 °C and −1 bar.
Table A7.
Conductivity Data for Nylon 610 Nanocomposites with 1.5 wt% Graphite Nanosheets Treated at 175 °C and −1 bar.
| Sample | 1 | 2 | 3 | 4 | 5 |
|---|
| Resistance (ohm) | 42,533.2416 | 49,622.1152 | 42,533.2416 | 49,622.1152 | 42,533.2416 |
| Conductivity (S/m) | 0.003242105 | 0.001933181 | 0.002074947 | 0.001646784 | 0.002161404 |
Table A8.
Conductivity Data for Nylon 610 Nanocomposites with 2.5 wt% Graphite Nanosheets Treated at 175 °C and −1 bar.
Table A8.
Conductivity Data for Nylon 610 Nanocomposites with 2.5 wt% Graphite Nanosheets Treated at 175 °C and −1 bar.
| Sample | 1 | 2 | 3 | 4 | 5 |
|---|
| Resistance (ohm) | 42,533.2416 | 49,622.1152 | 49,622.1152 | 42,533.2416 | 49,622.1152 |
| Conductivity (S/m) | 0.002255378 | 0.001482105 | 0.002223158 | 0.002470176 | 0.003420243 |
Table A9.
Conductivity Data for Nylon 610 Nanocomposites with 3.5 wt% Graphite Nanosheets Treated at 175 °C and −1 bar.
Table A9.
Conductivity Data for Nylon 610 Nanocomposites with 3.5 wt% Graphite Nanosheets Treated at 175 °C and −1 bar.
| Sample | 1 | 2 | 3 | 4 | 5 |
|---|
| Resistance (ohm) | 49,622.1152 | 49,622.1152 | 49,622.1152 | 49,622.1152 | 49,622.1152 |
| Conductivity (S/m) | 0.002470176 | 0.001482105 | 0.002021053 | 0.002117293 | 0.00130774 |
Table A10.
Conductivity Data for Nylon 610 Nanocomposites with 4.5 wt% Graphite Nanosheets Treated at 175 °C and −1 bar.
Table A10.
Conductivity Data for Nylon 610 Nanocomposites with 4.5 wt% Graphite Nanosheets Treated at 175 °C and −1 bar.
| Sample | 1 | 2 | 3 | 4 | 5 |
|---|
| Resistance (ohm) | 42,533.2416 | 49,622.1152 | 49,622.1152 | 42,533.2416 | 49,622.1152 |
| Conductivity (S/m) | 0.002881871 | 0.001111579 | 0.001778526 | 0.002255378 | 0.00158797 |
Table A11.
Conductivity Data for Nylon 610 Nanocomposites with 0.5 wt% Graphite Nanosheets Treated at 150 °C and −1 bar.
Table A11.
Conductivity Data for Nylon 610 Nanocomposites with 0.5 wt% Graphite Nanosheets Treated at 150 °C and −1 bar.
| Sample | 1 | 2 | 3 | 4 | 5 |
|---|
| Resistance (ohm) | 37,216.5864 | 37,216.5864 | 37,216.5864 | 33,081.41014 | 3,3081.41014 |
| Conductivity (S/m) | 0.00197614 | 0.002117293 | 0.002044283 | 0.003031579 | 0.003510249 |
Table A12.
Conductivity Data for Nylon 610 Nanocomposites with 1.5 wt% Graphite Nanosheets Treated at 150 °C and −1 bar.
Table A12.
Conductivity Data for Nylon 610 Nanocomposites with 1.5 wt% Graphite Nanosheets Treated at 150 °C and −1 bar.
| Sample | 1 | 2 | 3 | 4 | 5 |
|---|
| Resistance (ohm) | 29,773.26912 | 37,216.5864 | 33,081.41014 | 37,216.5864 | 33,081.41014 |
| Conductivity (S/m) | 0.002744639 | 0.002117293 | 0.002381955 | 0.002195712 | 0.002084211 |
Table A13.
Conductivity Data for Nylon 610 Nanocomposites with 2.5 wt% Graphite Nanosheets Treated at 150 °C and −1 bar.
Table A13.
Conductivity Data for Nylon 610 Nanocomposites with 2.5 wt% Graphite Nanosheets Treated at 150 °C and −1 bar.
| Sample | 1 | 2 | 3 | 4 | 5 |
|---|
| Resistance (ohm) | 17,513.68772 | 24,811.0576 | 29,773.26912 | 37,216.5864 | 37,216.5864 |
| Conductivity (S/m) | 0.004845344 | 0.003705263 | 0.002850203 | 0.002964211 | 0.002044283 |
Table A14.
Conductivity Data for Nylon 610 Nanocomposites with 3.5 wt% Graphite Nanosheets Treated at 150 °C and −1 bar.
Table A14.
Conductivity Data for Nylon 610 Nanocomposites with 3.5 wt% Graphite Nanosheets Treated at 150 °C and −1 bar.
| Sample | 1 | 2 | 3 | 4 | 5 |
|---|
| Resistance (ohm) | 42,533.2416 | 42,533.2416 | 49,622.1152 | 42,533.2416 | 42,533.2416 |
| Conductivity (S/m) | 0.001995142 | 0.002255378 | 0.001710122 | 0.003990284 | 0.003990284 |
Table A15.
Conductivity Data for Nylon 610 Nanocomposites with 4.5 wt% Graphite Nanosheets Treated at 150 °C and −1 bar.
Table A15.
Conductivity Data for Nylon 610 Nanocomposites with 4.5 wt% Graphite Nanosheets Treated at 150 °C and −1 bar.
| Sample | 1 | 2 | 3 | 4 | 5 |
|---|
| Resistance (ohm) | 42,533.2416 | 42,533.2416 | 42,533.2416 | 42,533.2416 | 42,533.2416 |
| Conductivity (S/m) | 0.002074947 | 0.001621053 | 0.001852632 | 0.002593684 | 0.001729123 |
Table A16.
Conductivity Data for Nylon 610 Nanocomposites with 0.5 wt% Untreated Graphite Nanosheets.
Table A16.
Conductivity Data for Nylon 610 Nanocomposites with 0.5 wt% Untreated Graphite Nanosheets.
| Sample | 1 | 2 | 3 | 4 | 5 |
|---|
| Resistance (ohm) | 27,066.60829 | 27,065.7901 | 29,773.26912 | 29,773.26912 | 29,773.26912 |
| Conductivity (S/m) | 0.003705263 | 0.003881822 | 0.003528822 | 0.003368421 | 0.002964211 |
Table A17.
Conductivity Data for Nylon 610 Nanocomposites with 1.5 wt% Untreated Graphite Nanosheets.
Table A17.
Conductivity Data for Nylon 610 Nanocomposites with 1.5 wt% Untreated Graphite Nanosheets.
| Sample | 1 | 2 | 3 | 4 | 5 |
|---|
| Resistance (ohm) | 29,773.26912 | 33,081.41014 | 29,773.26912 | 33,081.41014 | 33,081.41014 |
| Conductivity (S/m) | 0.003900277 | 0.002778947 | 0.002964211 | 0.003031579 | 0.002899771 |
Table A18.
Conductivity Data for Nylon 610 Nanocomposites with 2.5 wt% Untreated Graphite Nanosheets.
Table A18.
Conductivity Data for Nylon 610 Nanocomposites with 2.5 wt% Untreated Graphite Nanosheets.
| Sample | 1 | 2 | 3 | 4 | 5 |
|---|
| Resistance (ohm) | 33,081.41014 | 33,081.41014 | 33,081.41014 | 33,081.41014 | 33,081.41014 |
| Conductivity (S/m) | 0.004168421 | 0.002899771 | 0.003510249 | 0.00317594 | 0.004446316 |
Table A19.
Conductivity Data for Nylon 610 Nanocomposites with 3.5 wt% Untreated Graphite Nanosheets.
Table A19.
Conductivity Data for Nylon 610 Nanocomposites with 3.5 wt% Untreated Graphite Nanosheets.
| Sample | 1 | 2 | 3 | 4 | 5 |
|---|
| Resistance (ohm) | 33,081.41014 | 33,081.41014 | 29,773.26912 | 33,081.41014 | 29,773.26912 |
| Conductivity (S/m) | 0.004168421 | 0.002899771 | 0.003900277 | 0.00317594 | 0.004940351 |
Table A20.
Conductivity Data for Nylon 610 Nanocomposites with 4.5 wt% Untreated Graphite Nanosheets.
Table A20.
Conductivity Data for Nylon 610 Nanocomposites with 4.5 wt% Untreated Graphite Nanosheets.
| Sample | 1 | 2 | 3 | 4 | 5 |
|---|
| Resistance (ohm) | 29,773.26912 | 33,081.41014 | 29,773.26912 | 29,773.26912 | 29,773.26912 |
| Conductivity (S/m) | 0.004940351 | 0.004168421 | 0.002964211 | 0.004359133 | 0.004116959 |