Preparation of Temperature-Responsive Antibody–Nanoparticles by RAFT-Mediated Grafting from Polymerization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
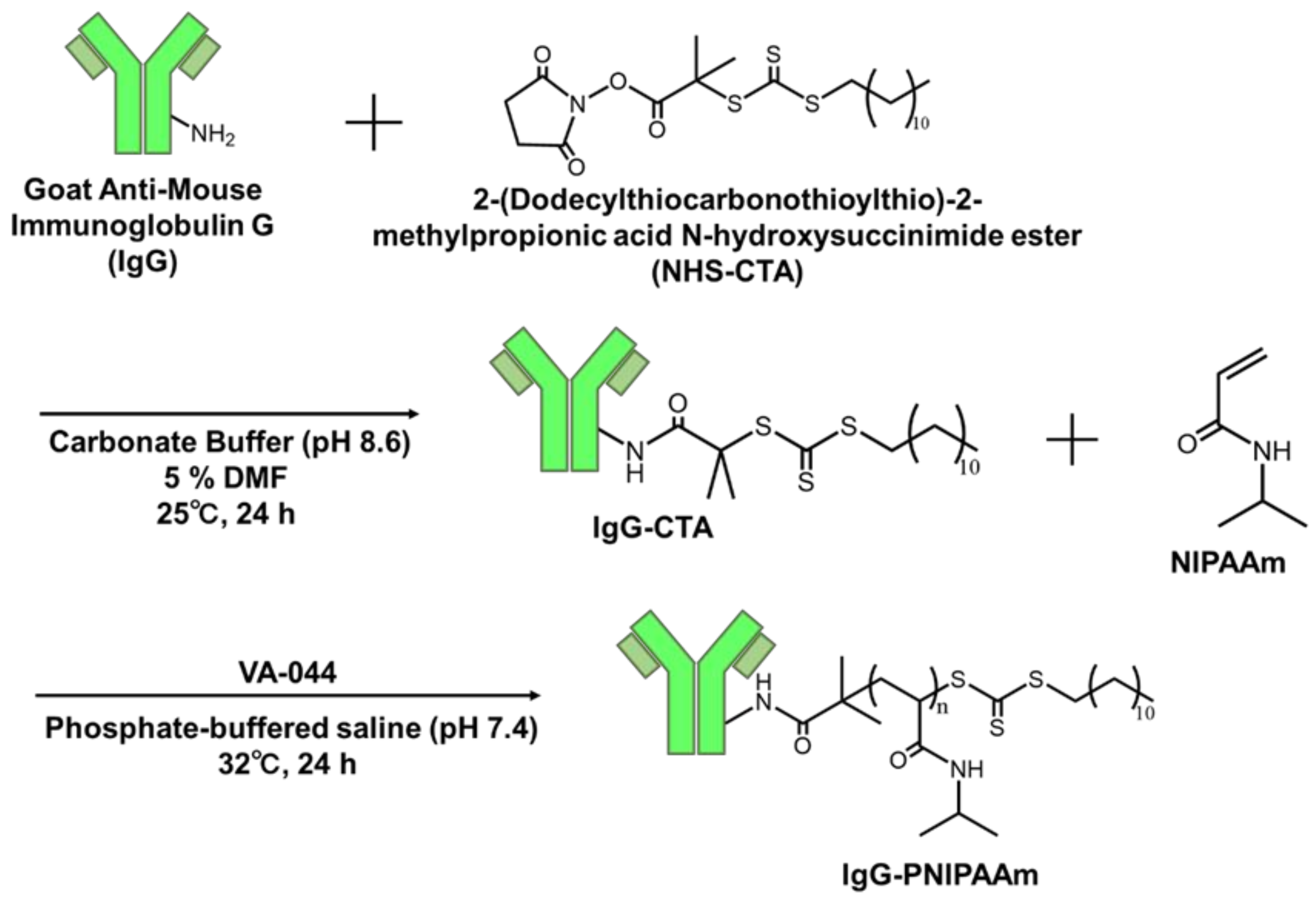
2.2.1. Synthesis of IgG-CTA
2.2.2. Investigation of the Adjustment Conditions of the IgG–PNIPAAm Conjugates
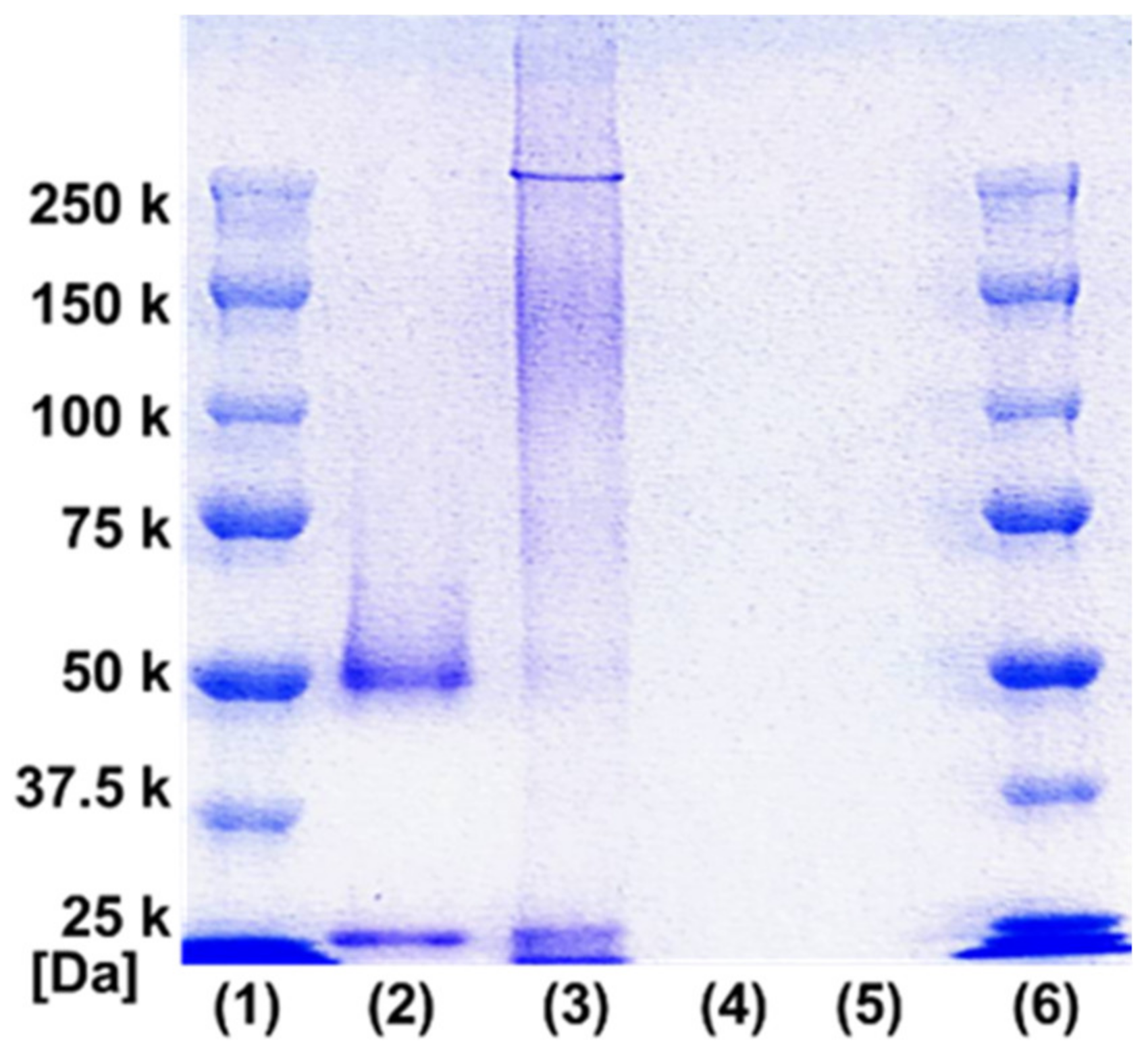
2.2.3. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.2.4. Field Flow Fractionation (FFF) Measurement
2.2.5. GPC Measurements
2.2.6. DLS Measurement
2.2.7. Lower Critical Solution Temperature Measurement
2.2.8. IgG Recovery Ratio Evaluation
2.2.9. BCA Method
2.2.10. Enzymatic Degradation Test of IgG–PNIPAAm Using Trypsin
2.2.11. Evaluation of the Binding Constant of Antibody–Temperature Responsive Polymer Conjugate to Antigen
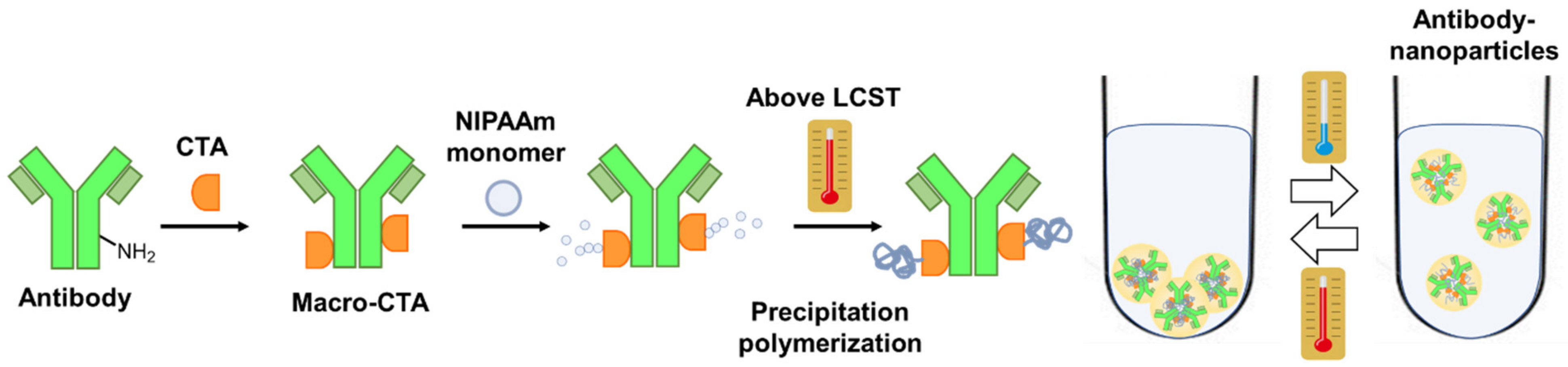
3. Results and Discussion
3.1. Synthesis of IgG-CTA
3.2. Synthesis of IgG–PNIPAAm Conjugates
3.3. Characterization of IgG–PNIPAAm Using FFF
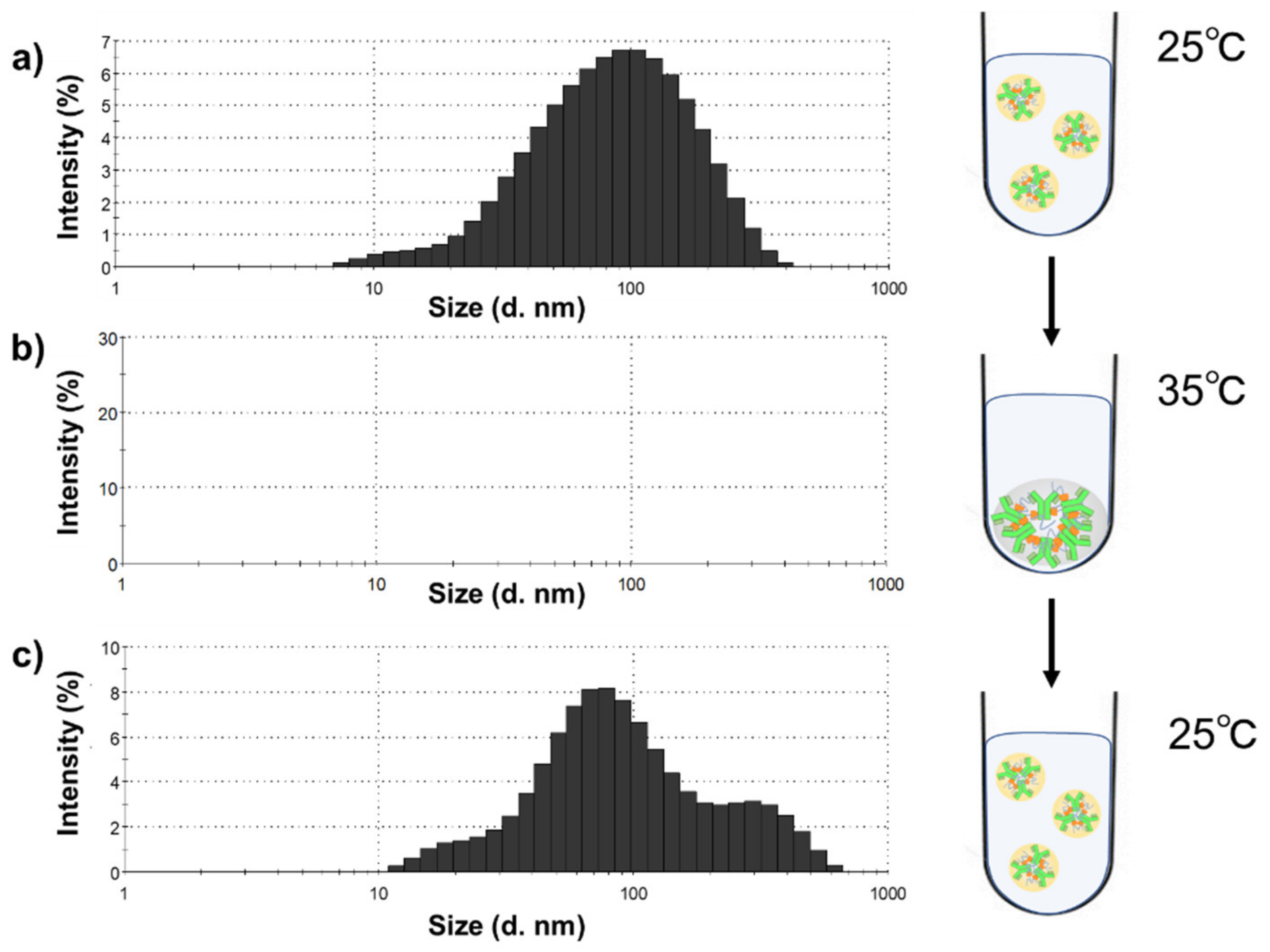
3.4. Evaluation of Antibody—Polymer Conjugates as a Particle Form
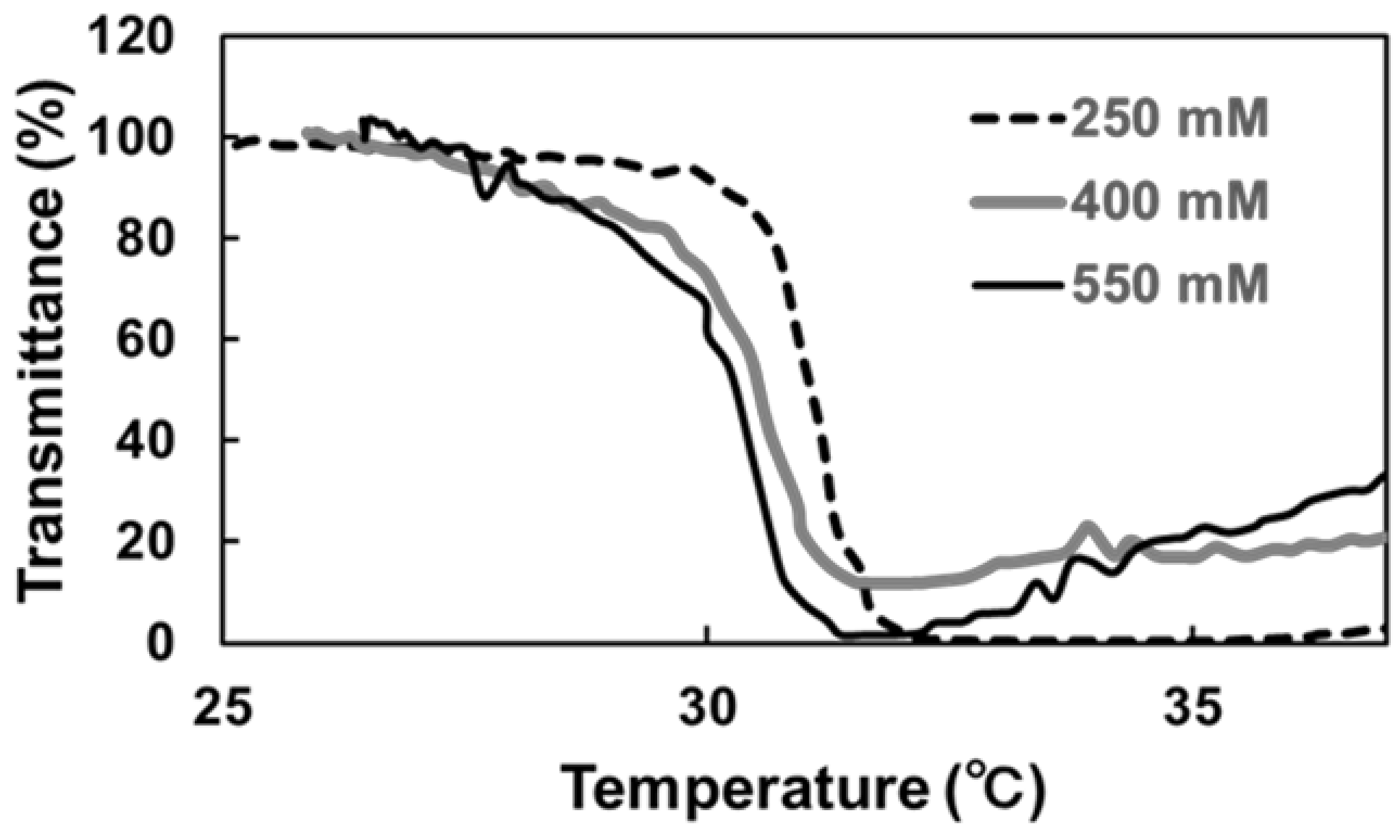
3.5. Temperature-Responsive Phase Transition Behavior
3.6. Response Evaluation of IgG–PNIPAAm Nanoparticles
3.7. Thermal Precipitation and Recovery of IgG–PNIPAAm
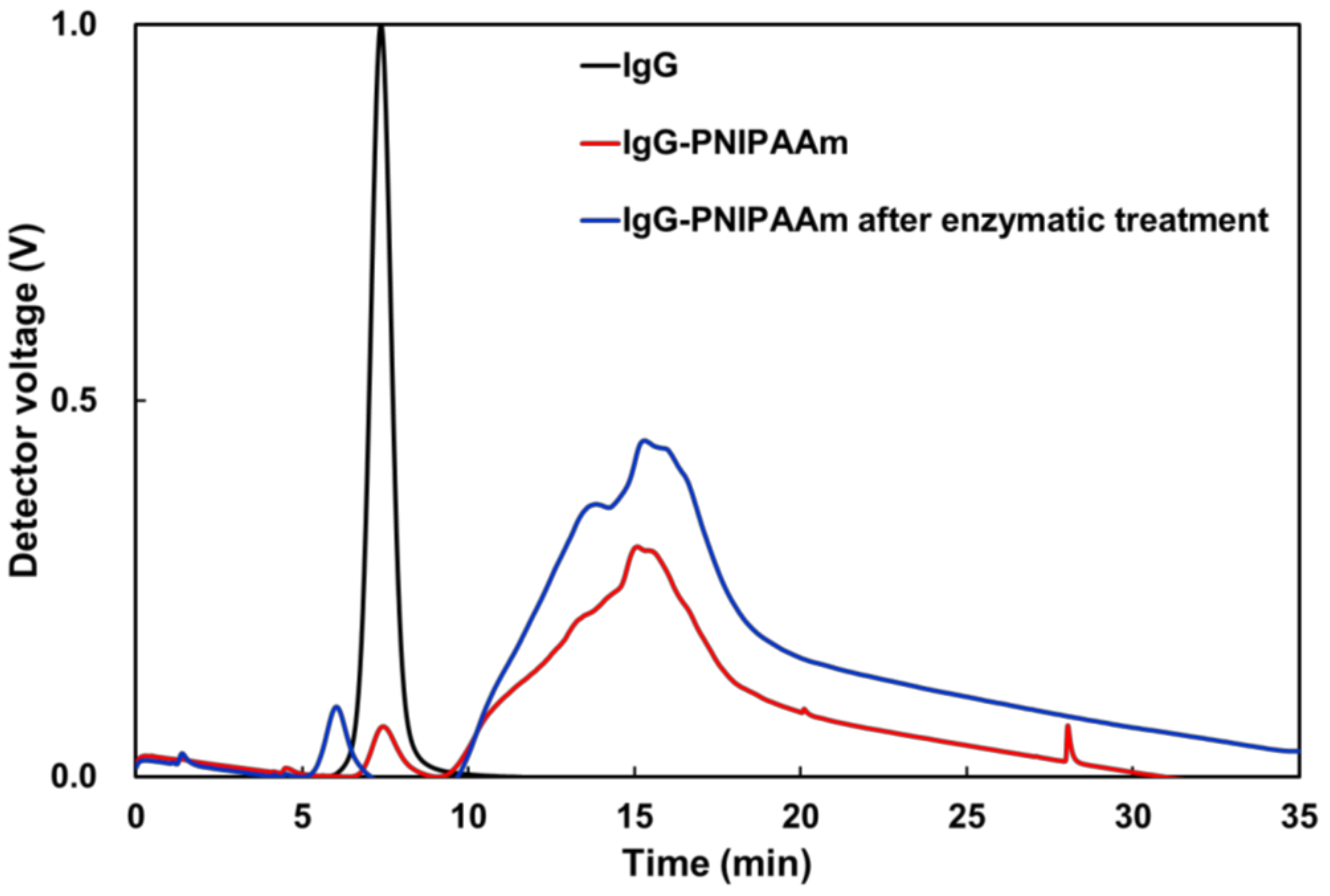
3.8. Evaluation of the Stability of the Nanoparticles against Enzymes
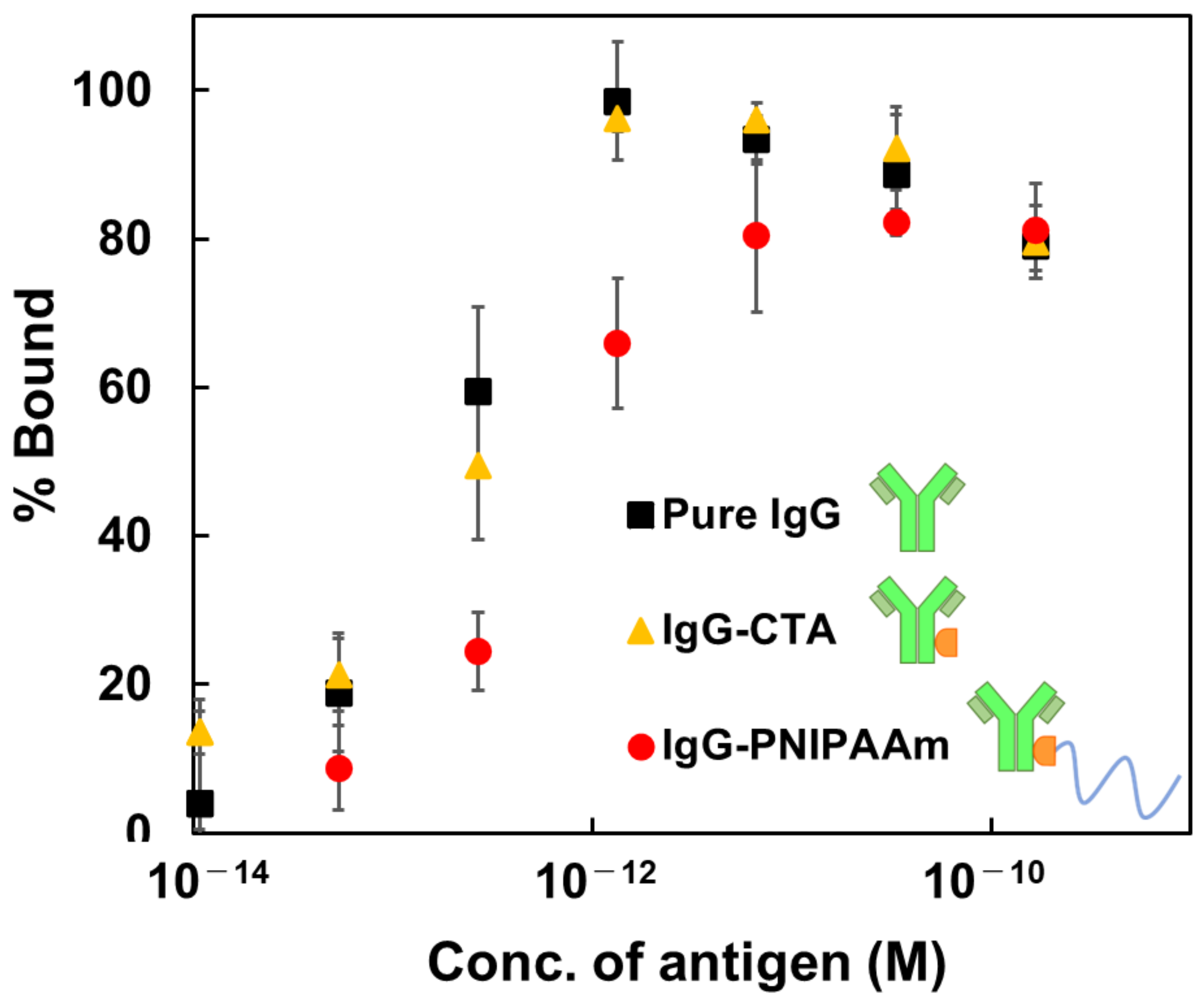
3.9. Evaluation of the Binding Constant of the Antibody-Temperature Responsive Polymer Conjugate to Antigen
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wright, T.A.; Page, R.C.; Konkolewicz, D. Polymer conjugation of proteins as a synthetic post-translational modification to impact their stability and activity. Polym. Chem. 2019, 10, 434–454. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Lu, H. Protein PEPylation: A new paradigm of protein-polymer conjugation. Bioconjug. Chem. 2019, 30, 1604–1616. [Google Scholar] [CrossRef] [PubMed]
- Lyon, R.P.; Bovee, T.D.; Doronina, S.O.; Burke, P.J.; Hunter, J.H.; Neff-Laford, H.D.; Jonas, M.; Anderson, M.E.; Setter, J.R.; Senter, P.D. Reducing hydrophobicity of homogeneous antibody-drug conjugates improves pharmacokinetics and therapeutic index. Nat. Biotechnol. 2015, 33, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Vicent, M.J. Do HPMA copolymer conjugates have a future as clinically useful nanomedicines? A critical overview of current status and future opportunities. Adv. Drug Deliv. Rev. 2010, 62, 272–282. [Google Scholar]
- Smorodinsky, N.; Von Specht, B.-U.; Cesla, R.; Shaltiel, S. A Conjugate between a Purified Timothy Allergen and Poly(N-Vinylpyrrolidone) Suppresses the Specific IgE Response in Mice. Immunol. Lett. 1981, 2, 305–309. [Google Scholar] [CrossRef]
- Sedlacek, O.; de la Rosa, V.R.; Hoogenboom, R. Poly(2-Oxazoline)-protein conjugates. Eur. Polym. J. 2019, 120, 109246. [Google Scholar]
- Stayton, P.S.; Shimoboji, T.; Long, C.; Chilkoti, A.; Ghen, G.; Harris, J.M.; Hoffman, A.S. Control of protein–ligand recognition using a stimuli-responsive polymer. Nature 1995, 378, 472–474. [Google Scholar]
- Shimoboji, T.; Ding, Z.L.; Stayton, P.S.; Hoffman, A.S. Photoswitching of ligand association with a photoresponsive polymer-protein conjugate. Bioconjug. Chem. 2002, 13, 915–919. [Google Scholar] [CrossRef]
- Chiu, M.L.; Goulet, D.R.; Teplyakov, A.; Gilliland, G.L. Antibody structure and function: The basis for engineering therapeutics. Antibodies 2019, 8, 55. [Google Scholar]
- Goel, N.; Stephens, S. Certolizumab Pegol. MAbs 2010, 2, 137–147. [Google Scholar]
- Lu, H.; Wang, D.; Kazane, S.; Javahishvili, T.; Tian, F.; Song, F.; Sellers, A.; Barnett, B.; Schultz, P.G. Site-Specific Antibody–polymer conjugates for SiRNA delivery. J. Am. Chem. Soc. 2013, 135, 13885–13891. [Google Scholar] [PubMed]
- Yurkovetskiy, A.V.; Yin, M.; Bodyak, N.; Stevenson, C.A.; Thomas, J.D.; Hammond, C.E.; Qin, L.L.; Zhu, B.; Gumerov, D.R.; Ter-Ovanesyan, E.; et al. A polymer-based antibody-vinca drug conjugate platform: Characterization and preclinical efficacy. Cancer Res. 2015, 75, 3365–3372. [Google Scholar] [CrossRef] [PubMed]
- Satchi-Fainaro, R.; Wrasidlo, W.; Lode, H.N.; Shabat, D. Synthesis and characterization of a catalytic antibody-HPMA copolymer-conjugate as a tool for tumor selective prodrug activation. Bioorganic Med. Chem. 2002, 10, 3023–3029. [Google Scholar]
- Aaron, A.J.; Bumb, A.; Brechbiel, M.W. Macromolecules, dendrimers, and nanomaterials in magnetic resonance imaging: The interplay between size, function, and pharmacokinetics. Chem. Rev. 2010, 110, 2921–2959. [Google Scholar]
- Zhang, L.; Zhao, W.; Liu, X.; Wang, G.; Wang, Y.; Li, D.; Xie, L.; Gao, Y.; Deng, H.; Gao, W. Site-selective in situ growth of fluorescent polymer-antibody conjugates with enhanced antigen detection by signal amplification. Biomaterials 2015, 64, 2–9. [Google Scholar] [PubMed]
- Nastyshyn, S.; Stetsyshyn, Y.; Raczkowska, J.; Nastishin, Y.; Melnyk, Y.; Panchenko, Y.; Budkowski, A. Temperature-Responsive Polymer Brush Coatings for Advanced Biomedical Applications. Polymers 2022, 14, 4245. [Google Scholar]
- Gajos, K.; Petrou, P.; Budkowski, A. Comparison of Physical Adsorption and Covalent Coupling Methods for Surface Density-Dependent Orientation of Antibody on Silicon. Molecules 2022, 27, 3672. [Google Scholar] [CrossRef]
- Awsiuk, K.; Stetsyshyn, Y.; Raczkowska, J.; Lishchynskyi, O.; Dabczyński, P.; Kostruba, A.; Ohar, H.; Shymborska, Y.; Nastyshyn, S.; Budkowski, A. Temperature-Controlled Orientation of Proteins on Temperature-Responsive Grafted Polymer Brushes: Poly(Butyl Methacrylate) vs Poly(Butyl Acrylate): Morphology, Wetting, and Protein Adsorption. Biomacromolecules 2019, 20, 2185–2197. [Google Scholar] [CrossRef]
- Gao, S.; Guisán, J.M.; Rocha-Martin, J. Oriented Immobilization of Antibodies onto Sensing Platforms-A Critical Review. Anal. Chim. Acta 2022, 1189, 338907. [Google Scholar] [CrossRef]
- Richards, D.A.; Maruani, A.; Chudasama, V. Antibody Fragments as Nanoparticle Targeting Ligands: A Step in the Right Direction. Chem. Sci. 2016, 8, 63–77. [Google Scholar]
- Johnston, M.C.; Scott, C.J. Antibody Conjugated Nanoparticles as a Novel Form of Antibody Drug Conjugate Chemotherapy. Drug Discov. Today Technol. 2018, 30, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Cobo, I.; Li, M.; Sumerlin, B.S.; Perrier, S. Smart hybrid materials by conjugation of responsive polymers to biomacromolecules. Nat. Mater. 2015, 14, 143–159. [Google Scholar] [PubMed]
- Ding, Z.; Chen, G.; Hoffman, A.S. Synthesis and purification of thermally sensitive oligomer−enzyme conjugates of poly(N-isopropylacrylamide)-trypsin. Bioconjug. Chem. 1996, 7, 121–125. [Google Scholar] [CrossRef]
- Takei, Y.G.; Matsukata, M.; Aoki, T.; Sanui, K.; Ogata, N.; Kikuchi, A.; Sakurai, Y.; Okano, T. Temperature-responsive bioconjugates. 3. Antibody—Poly (N-isopropylacrylamide) conjugates for temperature-modulated precipitations and affinity bioseparation. Bioconjug. Chem. 1994, 5, 577–582. [Google Scholar]
- Chilkoti, A.; Tan, P.H.; Stayton, P.S. Site-directed mutagenesis studies of the high-affinity streptavidin-biotin complex: Contributions of tryptophan residues 79, 108, and 120. Proc. Natl. Acad. Sci. USA 1995, 92, 1754–1758. [Google Scholar] [CrossRef] [PubMed]
- Fong, R.B.; Ding, Z.; Long, C.J.; Hoffman, A.S.; Stayton, P.S. Thermoprecipitation of streptavidin via oligonucleotide-mediated self-assembly with poly(N-isopropylacrylamide). Bioconjug. Chem. 1999, 10, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Phan, J.C.; Nehilla, B.J.; Srinivasan, S.; Coombs, R.W.; Woodrow, K.A.; Lai, J.J. Human immunodeficiency virus (HIV) separation and enrichment via the combination of antiviral lectin recognition and a thermoresponsive reagent system. Pharm. Res. 2016, 33, 2411–2420. [Google Scholar]
- Pan, P.; Fujita, M.; Ooi, W.Y.; Sudesh, K.; Takarada, T.; Goto, A. DNA-functionalized thermoresponsive bioconjugates synthesized via ATRP and click chemistry. Polymer 2011, 52, 895–900. [Google Scholar]
- Hironaka, K.; Yoshihara, E.; Nabil, A.; Lai, J.J.; Kikuchi, A.; Ebara, M. Conjugation of antibody with temperature-responsive polymer via in situ click reaction to enable biomarker enrichment for increased diagnostic sensitivity. Biomater. Sci. 2021, 9, 4870–4879. [Google Scholar] [CrossRef]
- Nabil, A.; Yoshihara, E.; Hironaka, K.; Hassan, A.A.; Shiha, G.; Ebara, M. Temperature responsive smart polymer for enabling affinity enrichment of current coronavirus (SARS-CoV-2) to improve its diagnostic sensitivity. Comput. Struct. Biotechnol. J. 2021, 19, 3609–3617. [Google Scholar]
- De, P.; Li, M.; Gondi, S.R.; Sumerlin, B.S. Temperature-regulated activity of responsive polymer-protein conjugates prepared by grafting-from via RAFT polymerization. J. Am. Chem. Soc. 2008, 130, 11288–11289. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Hou, W.; Liu, Y.; Liu, L.; Zhao, H. Biosurfaces fabricated by polymerization-induced surface self-assembly. Langmuir 2020, 36, 12649–12657. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, E.; Sasaki, M.; Nabil, A.; Iijima, M.; Ebara, M. Temperature responsive polymer conjugate prepared by “grafting from” proteins toward the adsorption and removal of uremic toxin. Molecules 2022, 27, 1051. [Google Scholar]
- Marassi, V.; Giordani, S.; Reschiglian, P.; Roda, B.; Zattoni, A. Tracking heme-protein interactions in healthy and pathological human serum in native conditions by miniaturized FFF-multidetection. Appl. Sci. 2022, 12, 6762. [Google Scholar]
- Pich, A.; Richtering, W. Microgels by precipitation polymerization: Synthesis, characterization, and functionalization. Adv. Polym. Sci. 2010, 234, 1–37. [Google Scholar]
- Eum, J.Y.; Sang Youn, H.; Ju, Y.; Shim, J.M.; Piao, Y.; Lee, J.; Kim, H.S.; Kim, J. A highly sensitive immunoassay using antibody-conjugated spherical mesoporous silica with immobilized enzymes. Chem. Commun. 2014, 50, 3546–3548. [Google Scholar]
- Gao, Y.; Zhou, Y.; Chandrawati, R. Metal and metal oxide nanoparticles to enhance the performance of enzyme-linked immunosorbent assay (ELISA). ACS Appl. Nano Mater. 2020, 3, 1–21. [Google Scholar] [CrossRef]
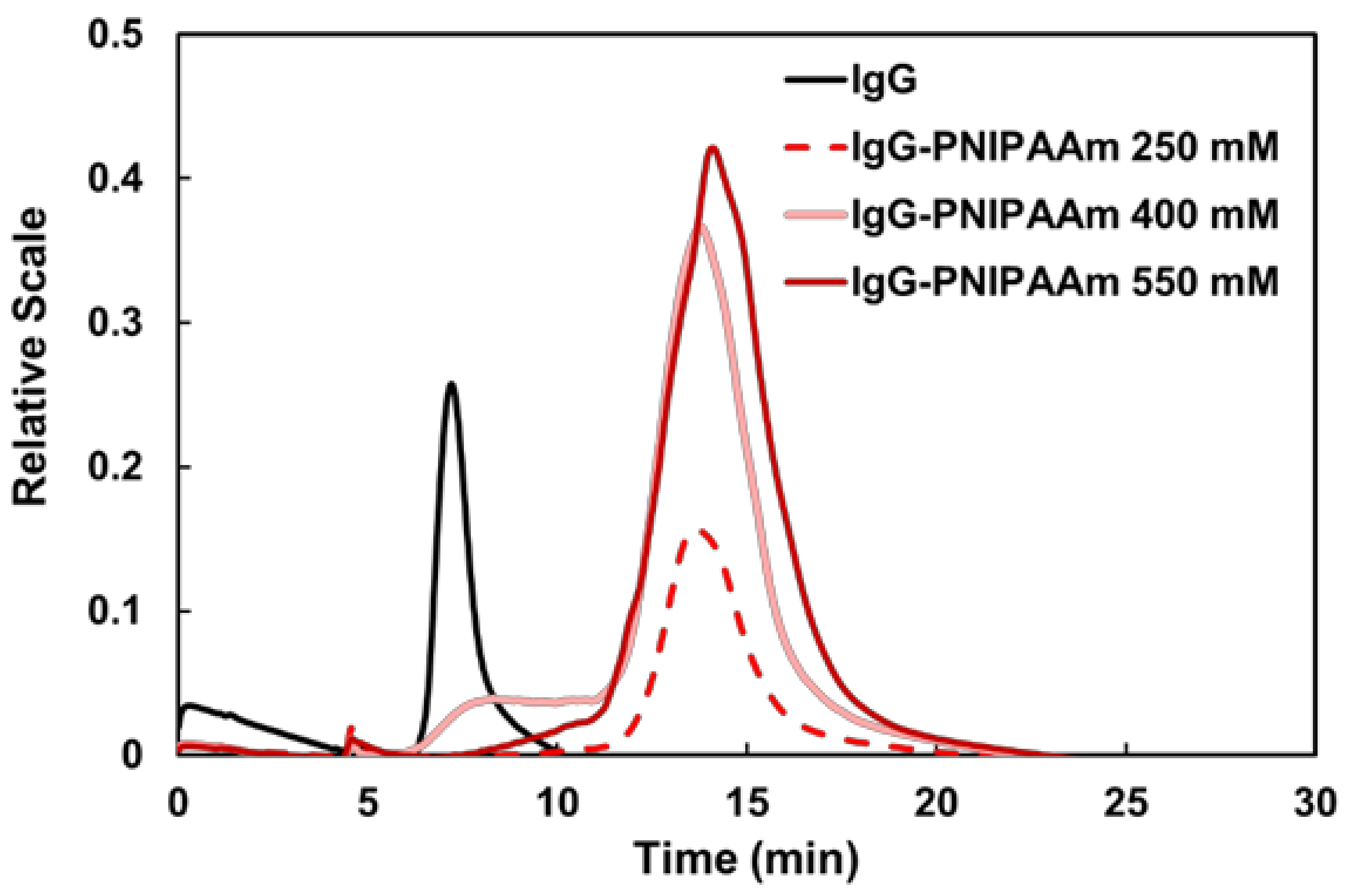


| Monomer Concentration in Feed (mM) | Molecular Weight (g/mol) | Radius of Inertia (nm) |
|---|---|---|
| 250 | 9.9 × 10⁶ | 104.2 |
| 400 | 1.3 × 10⁷ | 129.9 |
| 550 | 1.4 × 10⁷ | 132.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshihara, E.; Nabil, A.; Mochizuki, S.; Iijima, M.; Ebara, M. Preparation of Temperature-Responsive Antibody–Nanoparticles by RAFT-Mediated Grafting from Polymerization. Polymers 2022, 14, 4584. https://doi.org/10.3390/polym14214584
Yoshihara E, Nabil A, Mochizuki S, Iijima M, Ebara M. Preparation of Temperature-Responsive Antibody–Nanoparticles by RAFT-Mediated Grafting from Polymerization. Polymers. 2022; 14(21):4584. https://doi.org/10.3390/polym14214584
Chicago/Turabian StyleYoshihara, Erika, Ahmed Nabil, Shinichi Mochizuki, Michihiro Iijima, and Mitsuhiro Ebara. 2022. "Preparation of Temperature-Responsive Antibody–Nanoparticles by RAFT-Mediated Grafting from Polymerization" Polymers 14, no. 21: 4584. https://doi.org/10.3390/polym14214584
APA StyleYoshihara, E., Nabil, A., Mochizuki, S., Iijima, M., & Ebara, M. (2022). Preparation of Temperature-Responsive Antibody–Nanoparticles by RAFT-Mediated Grafting from Polymerization. Polymers, 14(21), 4584. https://doi.org/10.3390/polym14214584






