The Use of 3D Optical Coherence Tomography to Analyze the Architecture of Cyanobacterial Biofilms Formed on a Carbon Nanotube Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surface Preparation
2.2. Surface Characterization
2.2.1. Water Contact Angle Measurements
2.2.2. Atomic Force Microscopy (AFM)
2.2.3. Scanning Electron Microscopy (SEM)
2.3. Organism and Inoculum Preparation
2.4. Biofilm Formation
2.5. Biofilm Analysis
2.5.1. Optical Coherence Tomography (OCT)
- Contour Coefficient
- Total Biofilm Volume
- Biovolume
- Porosity
- Average Size of Non-Connected Pores
2.5.2. Wet Weight Determination
2.5.3. Scanning Electron Microscopy (SEM)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Surface Characterization
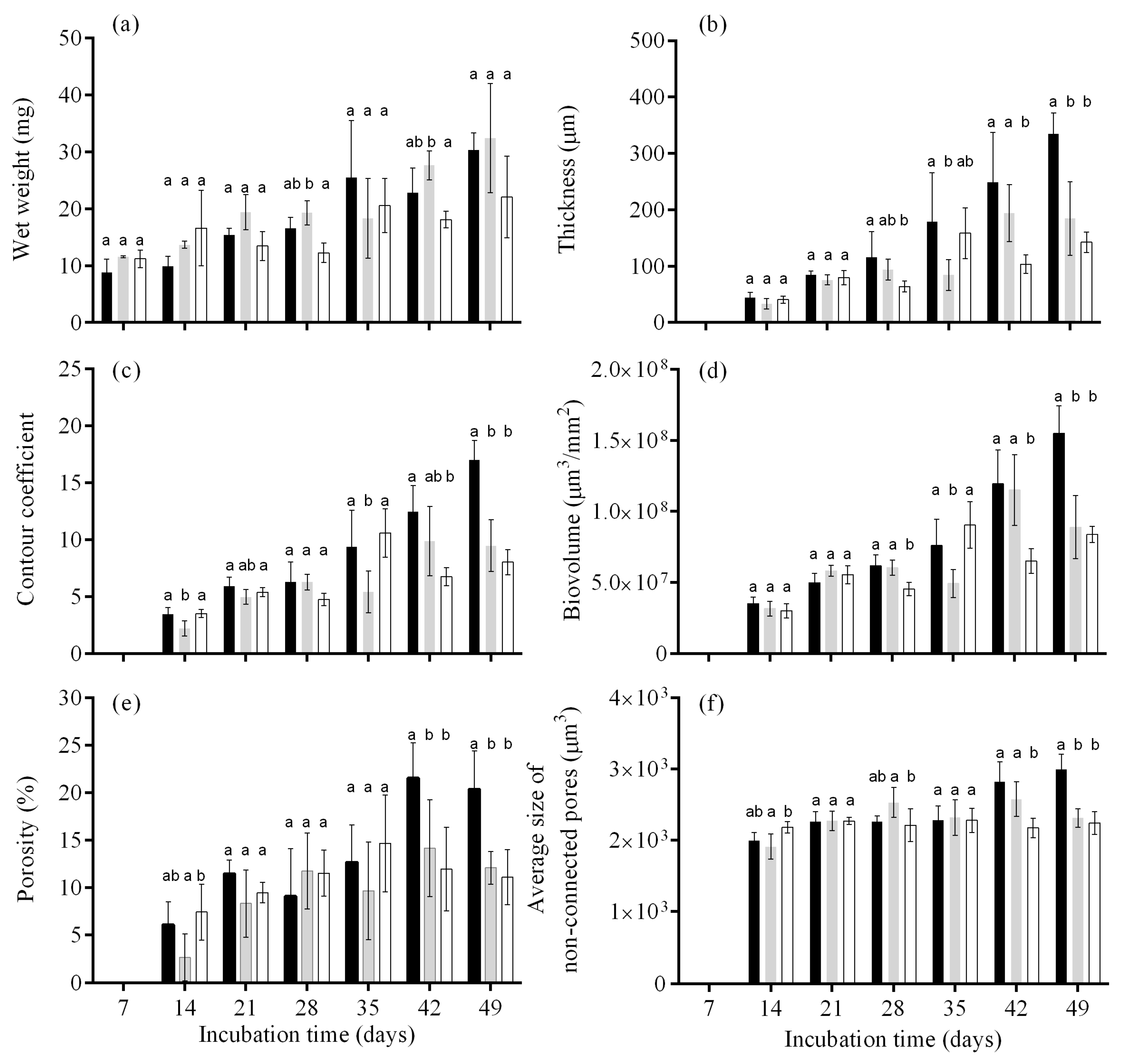
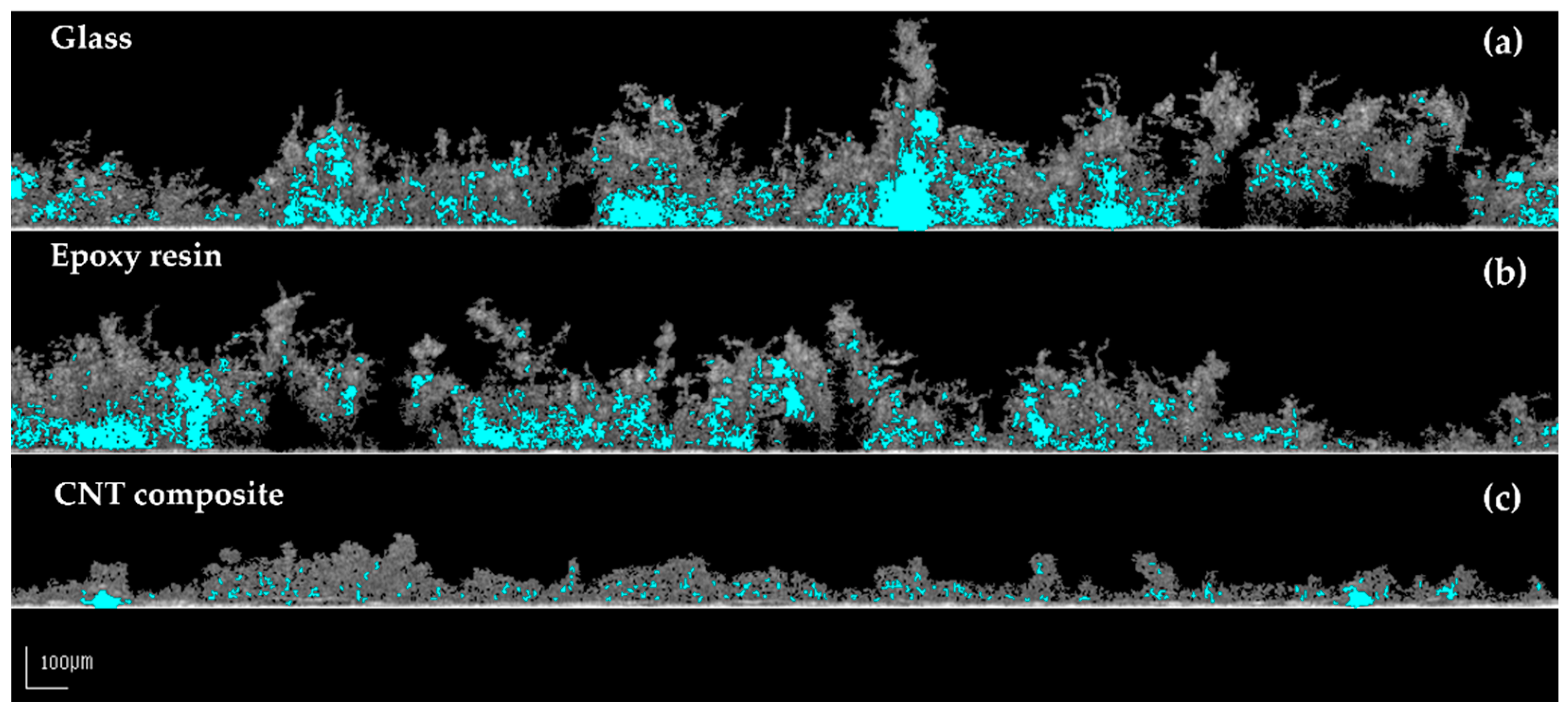
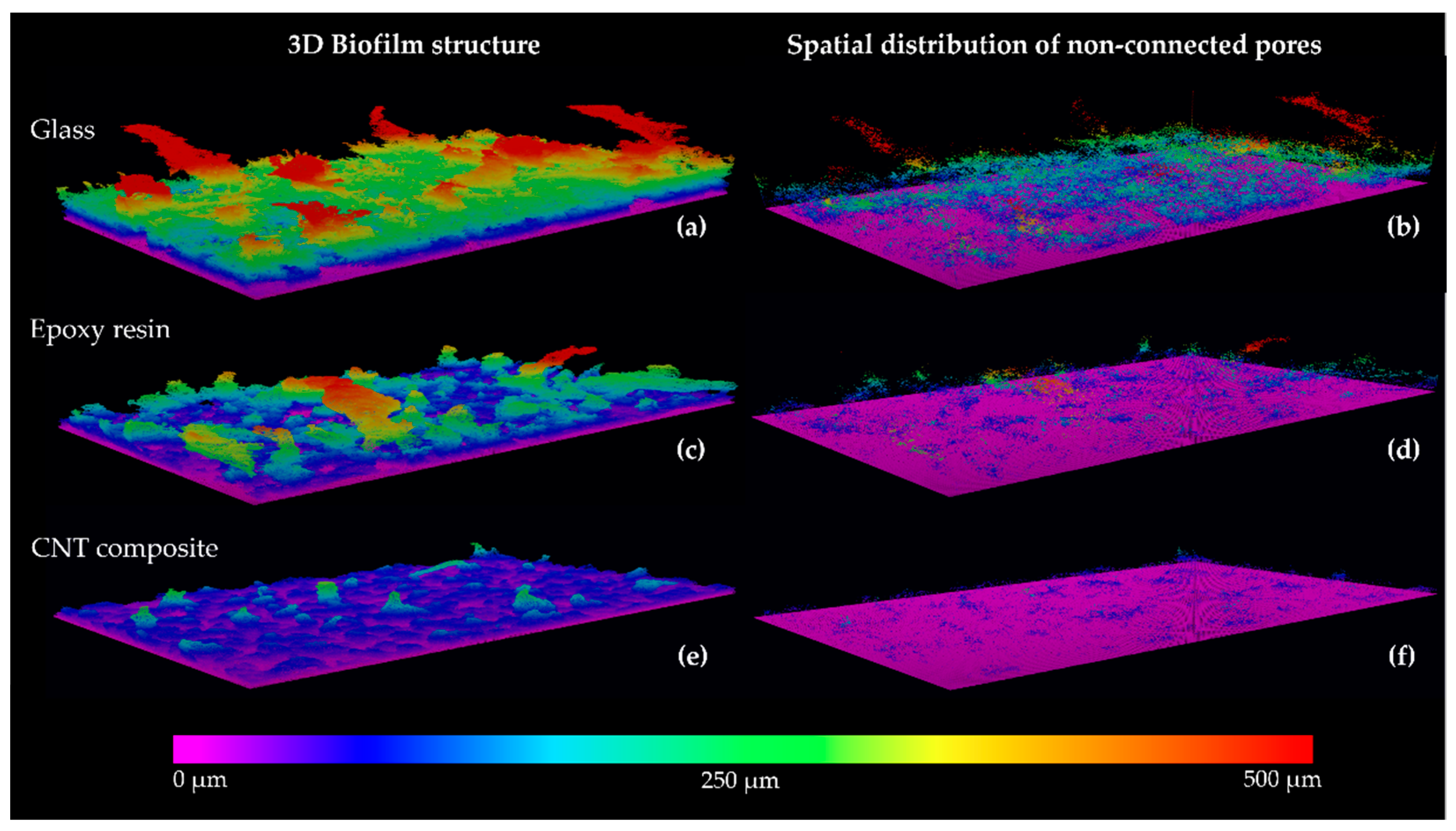
3.2. Biofilm Formation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M.V. Review on the Antimicrobial Properties of Carbon Nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef]
- Teixeira-Santos, R.; Gomes, M.; Gomes, L.C.; Mergulhão, F.J. Antimicrobial and Anti-Adhesive Properties of Carbon Nanotube-Based Surfaces for Medical Applications: A Systematic Review. iScience 2021, 24, 102001. [Google Scholar] [CrossRef]
- Venkataraman, A.; Amadi, E.V.; Chen, Y.; Papadopoulos, C. Carbon Nanotube Assembly and Integration for Applications. Nanoscale Res. Lett. 2019, 14, 220. [Google Scholar] [CrossRef]
- Ba, M.; Zhang, Z.; Qi, Y. The Influence of MWCNTs-OH on the Properties of the Fouling Release Coatings Based on Polydimethylsiloxane with the Incorporation of Phenylmethylsilicone Oil. Prog. Org. Coat. 2019, 130, 132–143. [Google Scholar] [CrossRef]
- Ji, Y.; Sun, Y.; Lang, Y.; Wang, L.; Liu, B.; Zhang, Z. Effect of CNT/PDMS Nanocomposites on the Dynamics of Pioneer Bacterial Communities in the Natural Biofilms of Seawater. Materials 2018, 11, 902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukowiak, A.; Kedziora, A.; Strek, W. Antimicrobial Graphene Family Materials: Progress, Advances, Hopes and Fears. Adv. Colloid Interface Sci. 2016, 236, 101–112. [Google Scholar] [CrossRef]
- Lejars, M.; Margaillan, A.; Bressy, C. Fouling Release Coatings: A Nontoxic Alternative to Biocidal Antifouling Coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef] [PubMed]
- Dustebek, J.; Kandemir-Cavas, C.; Nitodas, S.F.; Cavas, L. Effects of Carbon Nanotubes on the Mechanical Strength of Self-Polishing Antifouling Paints. Prog. Org. Coat. 2016, 98, 18–27. [Google Scholar] [CrossRef]
- Smith, A.M.; Callow, J.A. Biological Adhesives; Springer: Berlin/Heidelberg, Germany, 2006; Volume 23, ISBN 978-354-031-048-8. [Google Scholar]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial Effects of Carbon Nanotubes: Size Does Matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef]
- Gomes, M.; Gomes, L.C.; Teixeira-Santos, R.; Pereira, M.F.R.; Soares, O.S.G.P.; Mergulhão, F.J. Optimizing Cnt Loading in Antimicrobial Composites for Urinary Tract Application. Appl. Sci. 2021, 11, 4038. [Google Scholar] [CrossRef]
- Dong, X.; Yang, L. Inhibitory effects of single-walled carbon nanotubes on biofilm formation from Bacillus anthracis spores. Biofouling 2014, 30, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, J.D.; Elimelech, M. Antibacterial activity of electrospun polymer mats with incorporated narrow diameter single-walled carbon nanotubes. ACS Appl. Mater. Interfaces 2011, 3, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Saleemi, M.A.; Fouladi, M.H.; Yong, P.V.C.; Wong, E.H. Elucidation of Antimicrobial Activity of Non-Covalently Dispersed Carbon Nanotubes. Materials 2020, 13, 1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, F.; Santos, C.M.; Vergara, R.A.M.V.; Tria, M.C.R.; Advincula, R.; Rodrigues, D.F. Antimicrobial applications of electroactive PVK-SWNT nanocomposites. Environ. Sci. Technol. 2012, 46, 1804–1810. [Google Scholar] [CrossRef]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical Review on Biofilm Methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef] [Green Version]
- Wagner, M.; Horn, H. Optical Coherence Tomography in Biofilm Research: A Comprehensive Review. Biotechnol. Bioeng. 2017, 114, 1386–1402. [Google Scholar] [CrossRef]
- Hou, J.; Wang, C.; Rozenbaum, R.T.; Gusnaniar, N.; de Jong, E.D.; Woudstra, W.; Geertsema-Doornbusch, G.I.; Atema-Smit, J.; Sjollema, J.; Ren, Y.; et al. Bacterial Density and Biofilm Structure Determined by Optical Coherence Tomography. Sci. Rep. 2019, 9, 9794. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Beyenal, H.; Harkin, G.; Lewandowski, Z. Quantifying Biofilm Structure Using Image Analysis. J. Microbiol. Methods 2000, 39, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Heydorn, A.; Nielsen, A.T.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersbøll, B.K.; Molin, S. Quantification of Biofilm Structures by the Novel Computer Program COMSTAT. Microbiology 2000, 146 Pt 10, 2395–2407. [Google Scholar] [CrossRef] [Green Version]
- Mueller, L.N.; de Brouwer, J.F.C.; Almeida, J.S.; Stal, L.J.; Xavier, J.B. Analysis of a Marine Phototrophic Biofilm by Confocal Laser Scanning Microscopy Using the New Image Quantification Software PHLIP. BMC Ecol. 2006, 6, 1. [Google Scholar] [CrossRef]
- Chávez de Paz, L.E. Image Analysis Software Based on Color Segmentation for Characterization of Viability and Physiological Activity of Biofilms. Appl. Environ. Microbiol. 2009, 75, 1734–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daims, H.; Lücker, S.; Wagner, M. Daime, a Novel Image Analysis Program for Microbial Ecology and Biofilm Research. Environ. Microbiol. 2006, 8, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Claessen, D.; Rozen, D.E.; Kuipers, O.P.; Søgaard-Andersen, L.; van Wezel, G.P. Bacterial Solutions to Multicellularity: A Tale of Biofilms, Filaments and Fruiting Bodies. Nat. Rev. Microbiol. 2014, 12, 115–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern Approaches to Marine Antifouling Coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef] [Green Version]
- Palmer, J.; Flint, S.; Brooks, J. Bacterial Cell Attachment, the Beginning of a Biofilm. J. Ind. Microbiol. Biotechnol. 2007, 34, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Mostafaei, A.; Nasirpouri, F. Preparation and Characterization of a Novel Conducting Nanocomposite Blended with Epoxy Coating for Antifouling and Antibacterial Applications. J. Coat. Technol. Res. 2013, 10, 679–694. [Google Scholar] [CrossRef]
- Hoge, J.; Leach, C. Epoxy Resin Infused Boat Hulls. Reinf. Plast. 2016, 60, 221–223. [Google Scholar] [CrossRef]
- Vagos, M.R.; Gomes, M.; Moreira, J.M.R.; Soares, O.S.G.P.; Pereira, M.F.R.; Mergulhão, F.J. Carbon Nanotube/Poly(Dimethylsiloxane) Composite Materials to Reduce Bacterial Adhesion. Antibiotics 2020, 9, 434. [Google Scholar] [CrossRef]
- Soares, O.; Gonçalves, A.G.; Delgado, J.J.; Órfão, J.J.M.; Pereira, M.F.R. Modification of Carbon Nanotubes by Ball-Milling to Be Used as Ozonation Catalysts. Catal. Today 2015, 249, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Faria, S.I.; Teixeira-Santos, R.; Gomes, L.C.; Silva, E.R.; Morais, J.; Vasconcelos, V.; Mergulhão, F.J.M. Experimental Assessment of the Performance of Two Marine Coatings to Curb Biofilm Formation of Microfoulers. Coatings 2020, 10, 893. [Google Scholar] [CrossRef]
- Luan, Y.; Van Der Mei, H.C.; Dijk, M.; Geertsema-Doornbusch, G.I.; Atema-Smit, J.; Ren, Y.; Chen, H.; Busscher, H.J. Polarization of Macrophages, Cellular Adhesion, and Spreading on Bacterially Contaminated Gold Nanoparticle-Coatings In Vitro. ACS Biomater. Sci. Eng. 2020, 6, 933–945. [Google Scholar] [CrossRef] [Green Version]
- Alves, P.; Gomes, L.C.; Vorobii, M.; Rodriguez-Emmenegger, C.; Mergulhão, F.J. The Potential Advantages of Using a Poly(HPMA) Brush in Urinary Catheters: Effects on Biofilm Cells and Architecture. Colloids Surf. B Biointerfaces 2020, 191, 110976. [Google Scholar] [CrossRef] [PubMed]
- Ramos, V.; Morais, J.; Castelo-Branco, R.; Pinheiro, Â.; Martins, J.; Regueiras, A.; Pereira, A.L.; Lopes, V.R.; Frazão, B.; Gomes, D.; et al. Cyanobacterial Diversity Held in Microbial Biological Resource Centers as a Biotechnological Asset: The Case Study of the Newly Established LEGE Culture Collection. J. Appl. Phycol. 2018, 30, 1437–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotai, J. Instructions for the Preparation of Modified Nutrient Solution Z8 for Algae; Norwegian Institute for Water Research: Blindern, Norway, 1972; p. 5. [Google Scholar]
- Romeu, M.J.; Alves, P.; Morais, J.; Miranda, J.M.; de Jong, E.D.; Sjollema, J.; Ramos, V.; Vasconcelos, V.; Mergulhão, F.J.M. Biofilm Formation Behaviour of Marine Filamentous Cyanobacterial Strains in Controlled Hydrodynamic Conditions. Environ. Microbiol. 2019, 21, 4411–4424. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.N.; Kelble, C.R.; Ortner, P.B.; Rudnick, D.T. Phytoplankton Bloom Status: Chlorophyll a Biomass as an Indicator of Water Quality Condition in the Southern Estuaries of Florida, USA. Ecol. Indic. 2009, 9, 56–67. [Google Scholar] [CrossRef]
- Porra, R.; Thompson, W.; Kriedemann, P. Determination of Accurate Extinction Coefficients and Simultaneous Equations for Assaying Chlorophylls a and b Extracted with Four Different Solvents: Verification of the Concentration of Chlorophyll Standards by Atomic Absorption Spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Bakker, D.P.; Van Der Plaats, A.; Verkerke, G.J.; Busscher, H.J.; Mei, H.C. Van Der Comparison of Velocity Profiles for Different Flow Chamber Designs Used in Studies of Microbial Adhesion to Surfaces. Appl. Environ. Microbiol. 2003, 69, 6280–6287. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.R.; Tulcidas, A.V.; Ferreira, O.; Bayón, R.; Igartua, A.; Mendoza, G.; Mergulhão, F.J.M.; Faria, S.I.; Gomes, L.C.; Carvalho, S.; et al. Assessment of the Environmental Compatibility and Antifouling Performance of an Innovative Biocidal and Foul-Release Multifunctional Marine Coating. Environ. Res. 2021, 198, 111219. [Google Scholar] [CrossRef]
- Faria, S.; Teixeira-Santos, R.; Romeu, M.J.; Morais, J.; de Jong, E.; Sjollema, J.; Vasconcelos, V.; Mergulhão, F.J. Unveiling the Antifouling Performance of Different Marine Surfaces and Their Effect on the Development and Structure of Cyanobacterial Biofilms. Microorganisms 2021, 9, 1102. [Google Scholar] [CrossRef]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man. Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Romeu, M.J.; Domínguez-Pérez, D.; Almeida, D.; Morais, J.; Araújo, M.J.; Osório, H.; Campos, A.; Vasconcelos, V.; Mergulhão, F.J. Quantitative Proteomic Analysis of Marine Biofilms Formed by Filamentous Cyanobacterium. Environ. Res. 2021, 111566. [Google Scholar] [CrossRef]
- Alves, P.; Gomes, L.C.; Rodríguez-Emmenegger, C.; Mergulhão, F.J. Efficacy of A Poly(MeOEGMA) Brush on the Prevention of Escherichia Coli Biofilm Formation and Susceptibility. Antibiotics 2020, 9, 216. [Google Scholar] [CrossRef]
- Fan, F.X.; Zheng, Y.M.; Ba, M.; Wang, Y.F.; Kong, J.J.; Liu, J.H.; Wu, Q. Long Time Super-Hydrophobic Fouling Release Coating with the Incorporation of Lubricant. Prog. Org. Coat. 2021, 152, 106136. [Google Scholar] [CrossRef]
- Selim, M.S.; El-Safty, S.A.; Fatthallah, N.A.; Shenashen, M.A. Silicone/Graphene Oxide Sheet-Alumina Nanorod Ternary Composite for Superhydrophobic Antifouling Coating. Prog. Org. Coat. 2018, 121, 160–172. [Google Scholar] [CrossRef]
- Mahltig, B. Smart Hydrophobic and Soil-Repellent Protective Composite Coatings for Textiles and Leather. In Smart Composite Coatings and Membranes; Montemor, M.F., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 261–292. ISBN 978-178-242-283-9. [Google Scholar]
- Leonardi, A.K.; Ober, C.K. Polymer-Based Marine Antifouling and Fouling Release Surfaces: Strategies for Synthesis and Modification. Annu. Rev. Chem. Biomol. Eng. 2019, 10, 241–264. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J.F.; Aldred, N.; Callow, M.E.; Finlay, J.A.; Callow, J.A.; Clare, A.S.; Brennan, A.B. Species-Specific Engineered Antifouling Topographies: Correlations between the Settlement of Algal Zoospores and Barnacle Cyprids. Biofouling 2007, 23, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Yandi, W.; Mieszkin, S.; Martin-Tanchereau, P.; Callow, M.E.; Callow, J.A.; Tyson, L.; Liedberg, B.; Ederth, T. Hydration and Chain Entanglement Determines the Optimum Thickness of Poly(HEMA-Co-PEG10MA) Brushes for Effective Resistance to Settlement and Adhesion of Marine Fouling Organisms. ACS Appl. Mater. Interfaces 2014, 6, 11448–11458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scardino, A.J.; Guenther, J.; de Nys, R. Attachment Point Theory Revisited: The Fouling Response to a Microtextured Matrix. Biofouling 2008, 24, 45–53. [Google Scholar] [CrossRef]
- Brady, R.F.; Singer, I.L. Mechanical Factors Favoring Release from Fouling Release Coatings. Biofouling 2000, 15, 73–81. [Google Scholar] [CrossRef]
- Moreira, J.M.R.; Gomes, L.C.; Whitehead, K.A.; Lynch, S.; Tetlow, L.A.; Mergulhão, F.J. Effect of Surface Conditioning with Cellular Extracts on Escherichia Coli Adhesion and Initial Biofilm Formation. Food Bioprod. Process. 2017, 104, 1–12. [Google Scholar] [CrossRef]
- Bas, S.; Kramer, M.; Stopar, D. Biofilm Surface Density Determines Biocide Effectiveness. Front. Microbiol. 2017, 8, 2443. [Google Scholar] [CrossRef] [PubMed]
- Laspidou, C.S.; Aravas, N. Variation in the Mechanical Properties of a Porous Multi-Phase Biofilm under Compression Due to Void Closure. Water Sci. Technol. 2007, 55, 447–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, B.W.; van der Mei, H.C.; Sjollema, J.; Busscher, H.J.; Sharma, P.K.; Chapman, M.; Hultgren, S.J. A Distinguishable Role of EDNA in the Viscoelastic Relaxation of Biofilms. MBio 2021, 4, e00497-13. [Google Scholar] [CrossRef] [Green Version]
- Peterson, B.W.; He, Y.; Ren, Y.; Zerdoum, A.; Libera, M.R.; Sharma, P.K.; van Winkelhoff, A.J.; Neut, D.; Stoodley, P.; van der Mei, H.C.; et al. Viscoelasticity of Biofilms and Their Recalcitrance to Mechanical and Chemical Challenges. FEMS Microbiol. Rev. 2015, 39, 234–245. [Google Scholar] [CrossRef] [Green Version]
- Delauney, L.; Compare, C.; Lehaitre, M. Biofouling Protection for Marine Environmental Sensors. Ocean Sci. 2010, 6, 503–511. [Google Scholar] [CrossRef] [Green Version]
- Sjollema, J.; Rustema-Abbing, M.; van der Mei, H.C.; Busscher, H.J. Generalized Relationship between Numbers of Bacteria and Their Viability in Biofilms. Appl. Environ. Microbiol. 2011, 77, 5027–5029. [Google Scholar] [CrossRef] [Green Version]
- Milferstedt, K.; Pons, M.-N.; Morgenroth, E. Analyzing Characteristic Length Scales in Biofilm Structures. Biotechnol. Bioeng. 2009, 102, 368–379. [Google Scholar] [CrossRef]
- Pons, M.-N.; Milferstedt, K.; Morgenroth, E. Biofilm Monitoring on Rotating Discs by Image Analysis. Biotechnol. Bioeng. 2009, 103, 105–116. [Google Scholar] [CrossRef]
- de Carvalho, C.C.C.R.; da Fonseca, M.M.R. Assessment of Three-Dimensional Biofilm Structure Using an Optical Microscope. Biotechniques 2007, 42, 618–620. [Google Scholar] [CrossRef] [Green Version]
- Luef, B.; Neu, T.R.; Zweimüller, I.; Peduzzi, P. Structure and Composition of Aggregates in Two Large European Rivers, Based on Confocal Laser Scanning Microscopy and Image and Statistical Analyses. Appl. Environ. Microbiol. 2009, 75, 5952–5962. [Google Scholar] [CrossRef]
- Rodríguez, S.J.; Bishop, P.L. Three-Dimensional Quantification of Soil Biofilms Using Image Analysis. Environ. Eng. Sci. 2006, 24, 96–103. [Google Scholar] [CrossRef]
- Sousa-Cardoso, F.; Teixeira-Santos, R.; Mergulhão, F.J.M. Antifouling Performance of Carbon-Based Coatings for Marine Applications: A Systematic Review. Antibiotics 2022, 11, 1102. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; Suffredini, M.; Galli, G.; Glisenti, A.; Pettitt, M.E.; Callow, M.E.; Callow, J.A.; Williams, D.; Lyall, G. Amphiphilic Block Copolymer/Poly(Dimethylsiloxane) (PDMS) Blends and Nanocomposites for Improved Fouling-Release. Biofouling 2011, 27, 529–541. [Google Scholar] [CrossRef]
- Xie, M.; Zhao, W.; Wu, Y. Preventing Algae Biofilm Formation via Designing Long-Term Oil Storage Surfaces for Excellent Antifouling Performance. Appl. Surf. Sci. 2021, 554, 149612. [Google Scholar] [CrossRef]
- Sun, Y.; Lang, Y.; Yan, Z.; Wang, L.; Zhang, Z. High-Throughput Sequencing Analysis of Marine Pioneer Surface-Biofilm Bacteria Communities on Different PDMS-Based Coatings. Colloids Surf. B Biointerfaces 2020, 185, 110538. [Google Scholar] [CrossRef]
- Sun, Y.; Lang, Y.; Sun, T.; Liu, Q.; Pan, Y.; Qi, Z.; Ling, N.; Feng, Y.; Yu, M.; Ji, Y.; et al. Antifouling Potential of Multi-Walled Carbon Nanotubes-Modified Chlorinated Rubber-Based Composites on the Colonization Dynamics of Pioneer Biofilm-Forming Eukaryotic Microbes. Int. Biodeterior. Biodegrad. 2020, 149, 104921. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z. New Anti-Biofouling Carbon Nanotubes-Filled Polydimethylsiloxane Composites against Colonization by Pioneer Eukaryotic Microbes. Int. Biodeterior. Biodegrad. 2016, 110, 147–154. [Google Scholar] [CrossRef]
- Yang, J.L.; Li, Y.F.; Guo, X.P.; Liang, X.; Xu, Y.F.; Ding, D.W.; Bao, W.Y.; Dobretsov, S. The Effect of Carbon Nanotubes and Titanium Dioxide Incorporated in PDMS on Biofilm Community Composition and Subsequent Mussel Plantigrade Settlement. Biofouling 2016, 32, 763–777. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Z.; Wu, G.; Yang, Z.; Cui, Y.; Li, H.; Zhang, Y. Fluorinated Carbon Nanotube Superamphiphobic Coating for High-Efficiency and Long-Lasting Underwater Antibiofouling Surfaces. ACS Appl. Bio Mater. 2021, 4, 6351–6360. [Google Scholar] [CrossRef]
- Mountcastle, S.E.; Vyas, N.; Villapun, V.M.; Cox, S.C.; Jabbari, S.; Sammons, R.L.; Shelton, R.M.; Walmsley, A.D.; Kuehne, S.A. Biofilm Viability Checker: An Open-Source Tool for Automated Biofilm Viability Analysis from Confocal Microscopy Images. NPJ Biofilms Microbiomes 2021, 7, 44. [Google Scholar] [CrossRef]
- Van den Driessche, F.; Rigole, P.; Brackman, G.; Coenye, T. Optimization of Resazurin-Based Viability Staining for Quantification of Microbial Biofilms. J. Microbiol. Methods 2014, 98, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Tian, L.; Bing, W.; Zhao, J.; Ren, L. Bioinspired Marine Antifouling Coatings: Status, Prospects, and Future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]




| Symbol | Description |
|---|---|
| x | Pixel position on the horizontal axis (width) |
| y | Pixel position on the vertical axis (height) |
| z | Pixel position on the perpendicular axis (depth) |
| i | Index of x,z position in the horizontal plane |
| N | Number of voxels in the horizontal plane of the region of interest (ROI) |
| Volume of a voxel (µm3) | |
| Biofilm thickness at a given position i (µm) | |
| Average biofilm thickness (µm) | |
| Total area of the ROI (mm2) | |
| Number of connected voxels identified as belonging to biofilm matrix/bacteria in the biofilm (biovolume) in a horizontal plane at position y | |
| Number of non-biofilm-connected voxels without an open connection to the environment (biofilm holes) in a horizontal plane at position y | |
| Number of voxels identified as belonging to biofilm matrix/bacteria in the biofilm (biovolume) in column i (vertical line of voxels at position i) and connected to the environment (including corner voxels) |
| Surface | Water Contact Angle | Roughness |
|---|---|---|
| Glass | 40.9 ± 7.4 | 6.3 |
| Epoxy resin | 76.3 ± 2.5 | 13.1 |
| CNT composite | 68.9 ± 4.9 | 644 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romeu, M.J.; Lima, M.; Gomes, L.C.; Jong, E.D.d.; Morais, J.; Vasconcelos, V.; Pereira, M.F.R.; Soares, O.S.G.P.; Sjollema, J.; Mergulhão, F.J. The Use of 3D Optical Coherence Tomography to Analyze the Architecture of Cyanobacterial Biofilms Formed on a Carbon Nanotube Composite. Polymers 2022, 14, 4410. https://doi.org/10.3390/polym14204410
Romeu MJ, Lima M, Gomes LC, Jong EDd, Morais J, Vasconcelos V, Pereira MFR, Soares OSGP, Sjollema J, Mergulhão FJ. The Use of 3D Optical Coherence Tomography to Analyze the Architecture of Cyanobacterial Biofilms Formed on a Carbon Nanotube Composite. Polymers. 2022; 14(20):4410. https://doi.org/10.3390/polym14204410
Chicago/Turabian StyleRomeu, Maria J., Marta Lima, Luciana C. Gomes, Ed. D. de Jong, João Morais, Vítor Vasconcelos, Manuel F. R. Pereira, Olívia S. G. P. Soares, Jelmer Sjollema, and Filipe J. Mergulhão. 2022. "The Use of 3D Optical Coherence Tomography to Analyze the Architecture of Cyanobacterial Biofilms Formed on a Carbon Nanotube Composite" Polymers 14, no. 20: 4410. https://doi.org/10.3390/polym14204410
APA StyleRomeu, M. J., Lima, M., Gomes, L. C., Jong, E. D. d., Morais, J., Vasconcelos, V., Pereira, M. F. R., Soares, O. S. G. P., Sjollema, J., & Mergulhão, F. J. (2022). The Use of 3D Optical Coherence Tomography to Analyze the Architecture of Cyanobacterial Biofilms Formed on a Carbon Nanotube Composite. Polymers, 14(20), 4410. https://doi.org/10.3390/polym14204410










